Genome-Wide Association Studies for Flesh Color and Intramuscular Fat in (Duroc × Landrace × Large White) Crossbred Commercial Pigs
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals and Phenotypic Measurements
2.3. Genotyping and Quality Control
2.4. Single-Locus Genome-Wide Association Study
2.5. Linkage Disequilibrium and Gene Annotation
3. Result and Discussion
3.1. Phenotypic Statistical Analysis
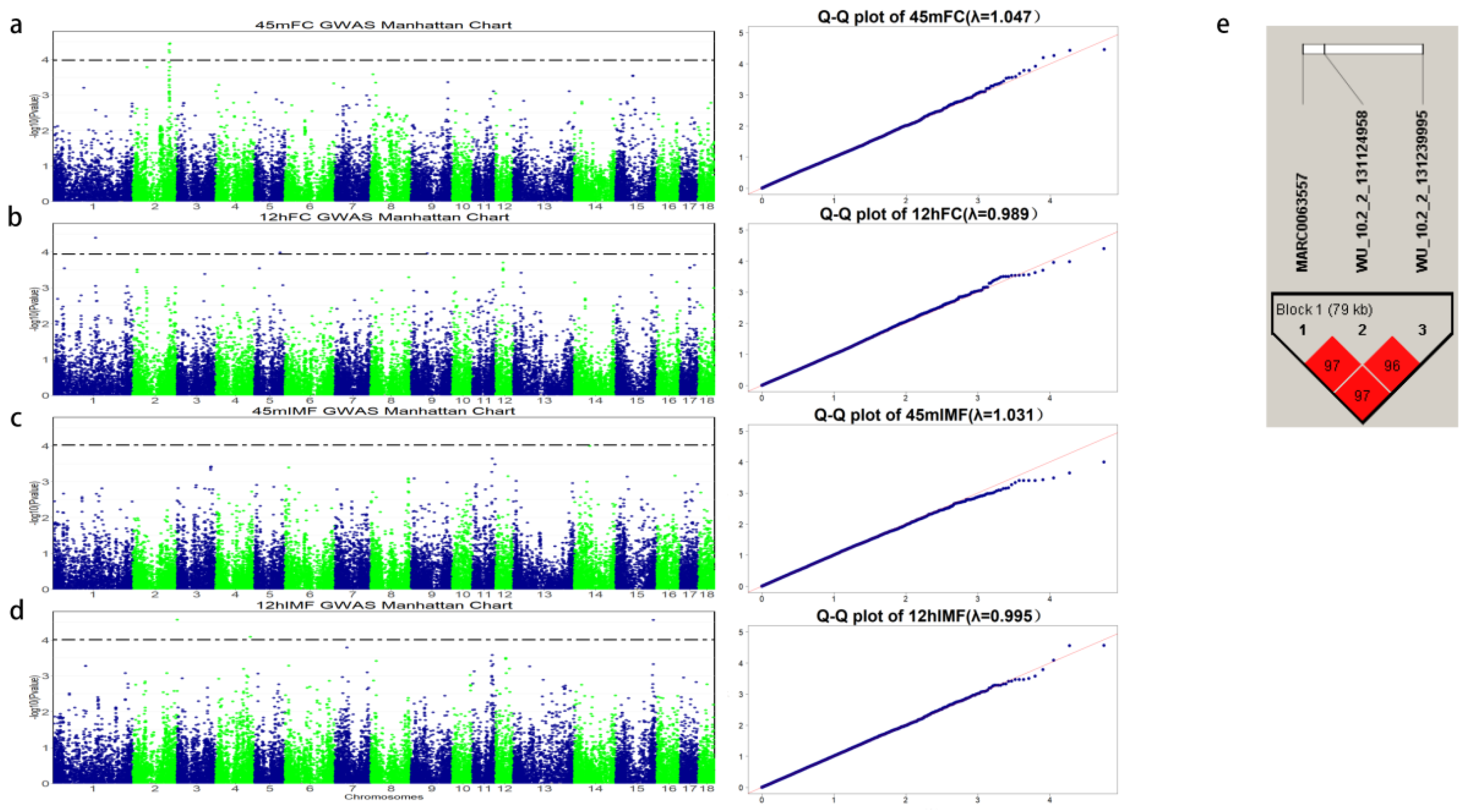
3.2. Flesh Color GWAS
3.3. IMF GWAS
3.4. Pathway Enrichment Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omana, D.; Goddard, E.; Plastow, G.; Janz, J.; Ma, L.; Anders, S.; Moore, S.; Bruce, H. Influence of on-farm production practices on sensory and technological quality characteristics of pork loin. Meat Sci. 2014, 96, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Gee, A.; Polkinghorne, R.; Porter, M. Consumer assessment of eating quality-development of protocols for Meat Standards Australia (MSA) testing. Aust. J. Exp. Agric. 2008, 48, 1360–1367. [Google Scholar] [CrossRef]
- Ji, J.; Zhou, L.; Huang, Y.; Zheng, M.; Liu, X.; Zhang, Y.; Huang, C.; Peng, S.; Zeng, Q.; Zhong, L.; et al. A whole-genome sequence based association study on pork eating quality traits and cooking loss in a specially designed heterogeneous F6 pig population. Meat Sci. 2018, 146, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Mandal, P.K. Chapter 1—Current perspectives of meat quality evaluation: Techniques, technologies, and challenges. In Meat Quality Analysis; Biswas, A.K., Mandal, P.K., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 3–17. ISBN 978-0-12-819233-7. [Google Scholar]
- Wood, J.; Nute, G.; Richardson, R.; Whittington, F.; Southwood, O.; Plastow, G.; Mansbridge, R.; da Costa, N.; Chang, K. Effects of breed, diet and muscle on fat deposition and eating quality in pigs. Meat Sci. 2004, 67, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Hocquette, J.F.; Gondret, F.; Baéza, E.; Médale, F.; Jurie, C.; Pethick, D.W. Intramuscular fat content in meat-producing animals: Development, genetic and nutritional control, and identification of putative markers. Animal 2010, 4, 303–319. [Google Scholar] [CrossRef]
- Sellier, P. Genetics of meat and carcass traits. In Genetics on Pig; CABI: Wallingford, UK, 1998. [Google Scholar]
- Franco, D.; Vazquez, J.A.; Lorenzo, J.M. Growth performance, carcass and meat quality of the Celta pig crossbred with Duroc and Landrance genotypes. Meat Sci. 2014, 96, 195–202. [Google Scholar] [CrossRef]
- Miar, Y.; Plastow, G.S.; Moore, S.S.; Manafiazar, G.; Charagu, P.; Kemp, R.A.; Van Haandel, B.; Huisman, A.E.; Zhang, C.Y.; McKay, R.M.; et al. Genetic and phenotypic parameters for carcass and meat quality traits in commercial crossbred pigs1. J. Anim. Sci. 2014, 92, 2869–2884. [Google Scholar] [CrossRef]
- Schwab, C.R.; Baas, T.J.; Stalder, K.J.; Mabry, J.W. Effect of long-term selection for increased leanness on meat and eating quality traits in Duroc swine1. J. Anim. Sci. 2006, 84, 1577–1583. [Google Scholar] [CrossRef]
- Hu, Z.-L.; Park, C.A.; Reecy, J.M. Developmental progress and current status of the Animal QTLdb. Nucleic Acids Res. 2015, 44, D827–D833. [Google Scholar] [CrossRef]
- Risch, N.; Merikangas, K. The Future of Genetic Studies of Complex Human Diseases. Science 1996, 273, 1516–1517. [Google Scholar] [CrossRef]
- Qiao, R.; Gao, J.; Zhang, Z.; Li, L.; Xie, X.; Fan, Y.; Cui, L.; Ma, J.; Ai, H.; Ren, J.; et al. Genome-wide association analyses reveal significant loci and strong candidate genes for growth and fatness traits in two pig populations. Genet. Sel. Evol. 2015, 47, 17. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-L.; Park, C.A.; Reecy, J.M. Bringing the Animal QTLdb and CorrDB into the future: Meeting new challenges and providing updated services. Nucleic Acids Res. 2021, 50, D956–D961. [Google Scholar] [CrossRef] [PubMed]
- Salas, R.C.D.; Mingala, C.N. Genetic Factors Affecting Pork Quality: Halothane and Rendement Napole Genes. Anim. Biotechnol. 2016, 28, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Z.; Bruce, H.; Kemp, R.A.; Charagu, P.; Miar, Y.; Yang, T.; Plastow, G. Genome-wide association studies (GWAS) identify a QTL close to PRKAG3 affecting meat pH and colour in crossbred commercial pigs. BMC Genet. 2015, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Ding, R.R.; Quan, J.P.; Yang, L.X.; Yang, M.; Zheng, E.Q.; Liu, D.W.; Cai, G.Y.; Wu, Z.F.; Yang, J. Genome-wide association analysis reveals genetic loci and candidate genes associated with intramuscular fat in Duroc pigs. Front. Agric. Sci. Eng. 2017, 4, 335–341. [Google Scholar] [CrossRef]
- Davoli, R.; Luise, D.; Mingazzini, V.; Zambonelli, P.; Braglia, S.; Serra, A.; Russo, V. Genome-wide study on intramuscular fat in Italian Large White pig breed using the PorcineSNP60 BeadChip. J. Anim. Breed. Genet. 2015, 133, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Cheng, D.; Chen, S.; Wang, L.; Li, Y.; Ma, X.; Song, X.; Liu, X.; Li, W.; Liang, J.; et al. Genome-Wide Association Analysis of Meat Quality Traits in a Porcine Large White × Minzhu Intercross Population. Int. J. Biol. Sci. 2012, 8, 580–595. [Google Scholar] [CrossRef]
- Davoli, R.; Catillo, G.; Serra, A.; Zappaterra, M.; Zambonelli, P.; Zilio, D.M.; Steri, R.; Mele, M.; Buttazzoni, L.; Russo, V. Genetic parameters of backfat fatty acids and carcass traits in Large White pigs. Animal 2019, 13, 924–932. [Google Scholar] [CrossRef]
- Liu, X.; Xiong, X.; Yang, J.; Zhou, L.; Yang, B.; Ai, H.; Ma, H.; Xie, X.; Huang, Y.; Fang, S.; et al. Genome-wide association analyses for meat quality traits in Chinese Erhualian pigs and a Western Duroc × (Landrace × Yorkshire) commercial population. Genet. Sel. Evol. 2015, 47, 44. [Google Scholar] [CrossRef]
- Gao, G.; Gao, N.; Li, S.; Kuang, W.; Zhu, L.; Jiang, W.; Yu, W.; Guo, J.; Li, Z.; Yang, C.; et al. Genome-Wide Association Study of Meat Quality Traits in a Three-Way Crossbred Commercial Pig Population. Front. Genet. 2021, 12. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Zheng, Z.; Ma, T.; Liu, Y.; Long, H.; Cheng, H.; Fang, M.; Gong, J.; Li, X.; et al. Genome-wide analysis of expression QTL (eQTL) and allele-specific expression (ASE) in pig muscle identifies candidate genes for meat quality traits. Genet. Sel. Evol. 2020, 52, 59. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Chen, X.; Boerwinkle, E.; Xiong, M. Genome-Wide Causation Studies of Complex Diseases. J. Comput. Biol. 2022, 29, 908–931. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, K.; Hurley, N.E.; Davis, N.L.; Rueckert, R.R.; Fleissner, E. Structural Studies of Avian Myeloblastosis Virus: Comparison of Polypeptides in Virion and Core Component by Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis. J. Virol. 1974, 13, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Ozsensoy, Y.; Sahin, S. Comparison of different DNA isolation methods and use of dodecyle trimethyl ammonium bromide (DTAB) for the isolation of DNA from meat products. J. Adv. Veter.- Anim. Res. 2016, 3, 368. [Google Scholar] [CrossRef]
- Ding, R.; Yang, M.; Quan, J.; Li, S.; Zhuang, Z.; Zhou, S.; Zheng, E.; Hong, L.; Li, Z.; Cai, G.; et al. Single-Locus and Multi-Locus Genome-Wide Association Studies for Intramuscular Fat in Duroc Pigs. Front. Genet. 2019, 10, 619. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Ding, R.; Yang, M.; Wang, X.; Quan, J.; Zhuang, Z.; Zhou, S.; Li, S.; Xu, Z.; Zheng, E.; Cai, G.; et al. Genetic Architecture of Feeding Behavior and Feed Efficiency in a Duroc Pig Population. Front. Genet. 2018, 9, 220. [Google Scholar] [CrossRef]
- Zhuang, Z.; Ding, R.; Peng, L.; Wu, J.; Ye, Y.; Zhou, S.; Wang, X.; Quan, J.; Zheng, E.; Cai, G.; et al. Genome-wide association analyses identify known and novel loci for teat number in Duroc pigs using single-locus and multi-locus models. BMC Genom. 2020, 21, 344. [Google Scholar] [CrossRef]
- Yang, J.; Manolio, T.A.; Pasquale, L.R.; Boerwinkle, E.; Caporaso, N.; Cunningham, J.M.; de Andrade, M.; Feenstra, B.; Feingold, E.; Hayes, M.G.; et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat. Genet. 2011, 43, 519–525. [Google Scholar] [CrossRef]
- Zhou, X.; Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012, 44, 821–824. [Google Scholar] [CrossRef]
- Platt, R.W.; Leroux, B.G.; Breslow, N. Generalized linear mixed models for meta-analysis. Stat. Med. 1999, 18, 643–654. [Google Scholar] [CrossRef]
- Yu, J.; Pressoir, G.; Briggs, W.H.; Vroh Bi, I.; Yamasaki, M.; Doebley, J.F.; McMullen, M.D.; Gaut, B.S.; Nielsen, D.M.; Holland, J.B.; et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat. Genet. 2006, 38, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Hayes, B.J.; Goddard, M. Technical note: Prediction of breeding values using marker-derived relationship matrices. J. Anim. Sci. 2008, 86, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- Price, A.L.; Patterson, N.J.; Plenge, R.M.; Weinblatt, M.E.; Shadick, N.A.; Reich, D. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 2006, 38, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Stephens, M. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat. Methods 2014, 11, 407–409. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Johnson, R.C.; Nelson, G.W.; Troyer, J.L.; Lautenberger, J.A.; Kessing, B.D.; Winkler, C.A.; O’Brien, S.J. Accounting for multiple comparisons in a genome-wide association study (GWAS). BMC Genom. 2010, 11, 724. [Google Scholar] [CrossRef]
- Andersson, L. Genetic dissection of phenotypic diversity in farm animals. Nat. Rev. Genet. 2001, 2, 130–138. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The Structure of Haplotype Blocks in the Human Genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Peng, S.; Zhong, L.; Zhou, L.; Yan, G.; Xiao, S.; Ma, J.; Huang, L. Identification and validation of a regulatory mutation upstream of the BMP2 gene associated with carcass length in pigs. Genet. Sel. Evol. 2021, 53, 94. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Maltecca, C.; Schwab, C.; Gray, K.; Tiezzi, F. Genetic parameters of meat quality, carcass composition, and growth traits in commercial swine. J. Anim. Sci. 2019, 97, 3669–3683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Gong, H.; Cui, L.; Zhang, W.; Ma, J.; Chen, C.; Ai, H.; Xiao, S.; Huang, L.; et al. Genetic correlation of fatty acid composition with growth, carcass, fat deposition and meat quality traits based on GWAS data in six pig populations. Meat Sci. 2018, 150, 47–55. [Google Scholar] [CrossRef]
- Larzul, C.; Lefaucheur, L.; Ecolan, P.; Gogué, J.; Talmant, A.; Sellier, P.; Le Roy, P.; Monin, G. Phenotypic and genetic parameters for longissimus muscle fiber characteristics in relation to growth, carcass, and meat quality traits in large white pigs. J. Anim. Sci. 1997, 75, 3126–3137. [Google Scholar] [CrossRef]
- Buttle, L.; Crampton, V.; Williams, P. The effect of feed pigment type on flesh pigment deposition and colour in farmed Atlantic salmon, Salmo salar L. Aquac. Res. 2001, 32, 103–111. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Whittington, F.M.; Hughes, S.I. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Lehnert, S.J.; Christensen, K.A.; Vandersteen, W.E.; Sakhrani, D.; Pitcher, T.E.; Heath, J.W.; Koop, B.F.; Heath, D.D.; Devlin, R.H. Carotenoid pigmentation in salmon: Variation in expression at BCO2-l locus controls a key fitness trait affecting red coloration. Proc. R. Soc. B Boil. Sci. 2019, 286, 20191588. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Suman, S.P.; Joseph, P. Myoglobin Chemistry and Meat Color. Annu. Rev. Food Sci. Technol. 2013, 4, 79–99. [Google Scholar] [CrossRef]
- Yamamoto, K.; Gandin, V.; Sasaki, M.; McCracken, S.; Li, W.; Silvester, J.L.; Elia, A.J.; Wang, F.; Wakutani, Y.; Alexandrova, R.; et al. Largen: A Molecular Regulator of Mammalian Cell Size Control. Mol. Cell 2014, 53, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Jana, B.; Całka, J.; Miciński, B. Regulatory Influence of Galanin and GALR1/GALR2 Receptors on Inflamed Uterus Contractility in Pigs. Int. J. Mol. Sci. 2021, 22, 6415. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Tomoya, I.; Chengwei, D.; Jie, Y.; Yuqin, W.; Tomohiko, F.; Jianguo, G. ST3GAL3, ST3GAL4, and ST3GAL6 differ in their regulation of biological functions via the specificities for the α2,3-sialylation of target proteins. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 881–897. [Google Scholar]
- Andersson-Eklund, L.; Marklund, L.; Lundström, K.; Haley, C.S.; Andersson, K.; Hansson, I.; Moller, M.; Andersson, L. Mapping quantitative trait loci for carcass and meat quality traits in a wild boar x Large White intercross. J. Anim. Sci. 1998, 76, 694–700. [Google Scholar] [CrossRef]
- Malek, M.; Dekkers, J.C.; Lee, H.K.; Baas, T.J.; Prusa, K.; Huff-Lonergan, E.; Rothschild, M.F. A molecular genome scan analysis to identify chromosomal regions influencing economic traits in the pig. II. Meat and muscle composition. Mamm. Genome 2001, 12, 637–645. [Google Scholar] [CrossRef]
- Rohrer, G.A.; Thallman, R.M.; Shackelford, S.; Wheeler, T.; Koohmaraie, M. A genome scan for loci affecting pork quality in a Duroc-Landrace F2 population. Anim. Genet. 2005, 37, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, M. Promotion of intramuscular fat accumulation in porcine muscle by nutritional regulation. Anim. Sci. J. 2011, 82, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Plastow, G.; Carrión, D.; Gil, M.; García-Regueiro, J.; Furnols, M.F.; Gispert, M.; Oliver, M.; Velarde, A.; Guàrdia, M.; Hortós, M.; et al. Quality pork genes and meat production. Meat Sci. 2005, 70, 409–421. [Google Scholar] [CrossRef]
- Chang, B.H.-J.; Li, L.; Saha, P.; Chan, L. Absence of adipose differentiation related protein upregulates hepatic VLDL secretion, relieves hepatosteatosis, and improves whole body insulin resistance in leptin-deficient mice. J. Lipid Res. 2010, 51, 2132–2142. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Wang, Z.; Bruce, H.L.; Janz, J.; Goddard, E.; Moore, S.; Plastow, G.S. Associations between single nucleotide polymorphisms in 33 candidate genes and meat quality traits in commercial pigs. Anim. Genet. 2014, 45, 508–516. [Google Scholar] [CrossRef]
- Stewart, S.; Gardner, G.; McGilchrist, P.; Pethick, D.; Polkinghorne, R.; Thompson, J.; Tarr, G. Prediction of consumer palatability in beef using visual marbling scores and chemical intramuscular fat percentage. Meat Sci. 2021, 181, 108322. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ning, C.; Wang, C.; Guo, J.; Wang, J.; Wu, Y. Genome-wide association study for intramuscular fat content in Chinese Lulai black pigs. Asian-Australasian J. Anim. Sci. 2019, 32, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Fontanesi, L.; Schiavo, G.; Galimberti, G.; Calò, D.G.; Scotti, E.; Martelli, P.L.; Buttazzoni, L.; Casadio, R.; Russo, V. A genome wide association study for backfat thickness in Italian Large White pigs highlights new regions affecting fat deposition including neuronal genes. BMC Genom. 2012, 13, 583. [Google Scholar] [CrossRef] [PubMed]
- Mattheakis, L.C.; Shen, W.H.; Collier, R.J. DPH5, a methyltransferase gene required for diphthamide biosynthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992, 12, 4026–4037. [Google Scholar] [PubMed]
- Kong, F.; You, H.; Zheng, K.; Tang, R.; Zheng, C. The crosstalk between pattern-recognition receptor signaling and calcium signaling. Int. J. Biol. Macromol. 2021, 192, 745–756. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, J.; Zhang, C.; Li, X. Cardioprotection Effects of Sevoflurane by Regulating the Pathway of Neuroactive Ligand-Receptor Interaction in Patients Undergoing Coronary Artery Bypass Graft Surgery. Comput. Math. Method. Med. 2017, 2017, 3618213. [Google Scholar] [CrossRef]
- Ingham, P.W.; Nakano, Y.; Seger, C. Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. Genet. 2011, 12, 393–406. [Google Scholar] [CrossRef]
- Song, S.; Cheng, H.; Jung, E.-Y.; Joo, S.-T.; Kim, G.-D. Muscle Fiber Characteristics on Chop Surface of Pork Loin (M. longissimus thoracis et lumborum) Associated with Muscle Fiber Pennation Angle and Their Relationships with Pork Loin Quality. Korean J. Food Sci. Anim. Resour. 2020, 40, 957–968. [Google Scholar] [CrossRef]
| Phenotype 1 | Traits 2 | Mean (SD) 3 | CV 4 | h2(se) 5 | Phenotypic Correlation 6 | P7 | |
|---|---|---|---|---|---|---|---|
| Flesh color | 45 mFC | 3.650 ± 1.006 | 0.276 | 0.112 ± 0.032 | 0.343 | 0.135 (45 m) 0.293 (12 h) | 2.2 × 10−16 |
| 12 hFC | 2.193 ± 0.925 | 0.422 | 0.217 ± 0.042 | 1.4 × 10−7 | |||
| Intramuscular fat | 45 mIMF | 1.746 ± 0.717 | 0.411 | 0.139 ± 0.041 | 0.238 | 2.2 × 10−16 | |
| 12 hIMF | 1.515 ± 0.638 | 0.421 | 0.178 ± 0.041 | 2.2 × 10−16 | |||
| Phenotype 1 | SSC 2 | SNP | Position (bp) 3 | P-Value 4 | Gene | Range 5 |
|---|---|---|---|---|---|---|
| 45 mFC | 2 | WU_10.2_2_131124958 | 125,961,057 | 3.527 × 10−5 | SNCAIP | −4195 |
| 45 mFC | 2 | ALGA0015705 | 124,966,025 | 3.695 × 10−5 | ENSSSCG00000041876 | within |
| 45 mFC | 2 | DRGA0003514 | 123,998,374 | 5.468 × 10−5 | PRR16 | +201,134 |
| 45 mFC | 2 | WU_10.2_2_130487175 | 125,006,692 | 6.348 × 10−5 | ENSSSCG00000041876 | within |
| 12 hFC | 1 | ASGA0004802 | 147,198,001 | 4.044 × 10−5 | GALR1 | +245,820 |
| 12 hFC | 5 | ALGA0033448 | 88,285,420 | 1.042 × 10−4 | ENSSSCG00000000905 | +7341 |
| 12 hFC | 9 | WU_10.2_9_59299494 | 53,460,098 | 1.106 × 10−4 | ST3GAL4 | within |
| 12 hIMF | 2 | H3GA0054148 | 150,317,009 | 2.728 × 10−5 | ABLIM3 | within |
| 12 hIMF | 4 | INRA0017040 | 117,256,570 | 8.211 × 10−5 | DPH5 | +12,483 |
| 12 hIMF | 15 | WU_10.2_15_140224592 | 126,725,046 | 2.778 × 10−5 | DOCK10 | −13,515 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Xu, C.; Meng, F.; Yao, Z.; Fan, Z.; Yang, Y.; Meng, X.; Zhan, Y.; Sun, Y.; Ma, F.; et al. Genome-Wide Association Studies for Flesh Color and Intramuscular Fat in (Duroc × Landrace × Large White) Crossbred Commercial Pigs. Genes 2022, 13, 2131. https://doi.org/10.3390/genes13112131
Li H, Xu C, Meng F, Yao Z, Fan Z, Yang Y, Meng X, Zhan Y, Sun Y, Ma F, et al. Genome-Wide Association Studies for Flesh Color and Intramuscular Fat in (Duroc × Landrace × Large White) Crossbred Commercial Pigs. Genes. 2022; 13(11):2131. https://doi.org/10.3390/genes13112131
Chicago/Turabian StyleLi, Hao, Cineng Xu, Fanming Meng, Zekai Yao, Zhenfei Fan, Yingshan Yang, Xianglun Meng, Yuexin Zhan, Ying Sun, Fucai Ma, and et al. 2022. "Genome-Wide Association Studies for Flesh Color and Intramuscular Fat in (Duroc × Landrace × Large White) Crossbred Commercial Pigs" Genes 13, no. 11: 2131. https://doi.org/10.3390/genes13112131
APA StyleLi, H., Xu, C., Meng, F., Yao, Z., Fan, Z., Yang, Y., Meng, X., Zhan, Y., Sun, Y., Ma, F., Yang, J., Yang, M., Yang, J., Wu, Z., Cai, G., & Zheng, E. (2022). Genome-Wide Association Studies for Flesh Color and Intramuscular Fat in (Duroc × Landrace × Large White) Crossbred Commercial Pigs. Genes, 13(11), 2131. https://doi.org/10.3390/genes13112131






