Genome-Wide Identification and Expression Profiling of Heat Shock Protein 20 Gene Family in Sorbus pohuashanensis (Hance) Hedl under Abiotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Identification of HSP20 Genes in the S. pohuashanensis Genome
2.3. Phylogenetic Analysis of SpHSP20 Genes
2.4. Analysis of Amino Acid Sequence, Gene Structure, and Conserved Motif of SpHSP20 Genes
2.5. Chromosomal Location and Synteny Analyses
2.6. Analysis of Cis-Elements in SpHSP20 Gene Promoters
2.7. Quantitative Real-Time PCR (qRT-PCR) and Expression Patterns Analysis
3. Results
3.1. Identification of HSP20 Genes in S. pohuashanensis
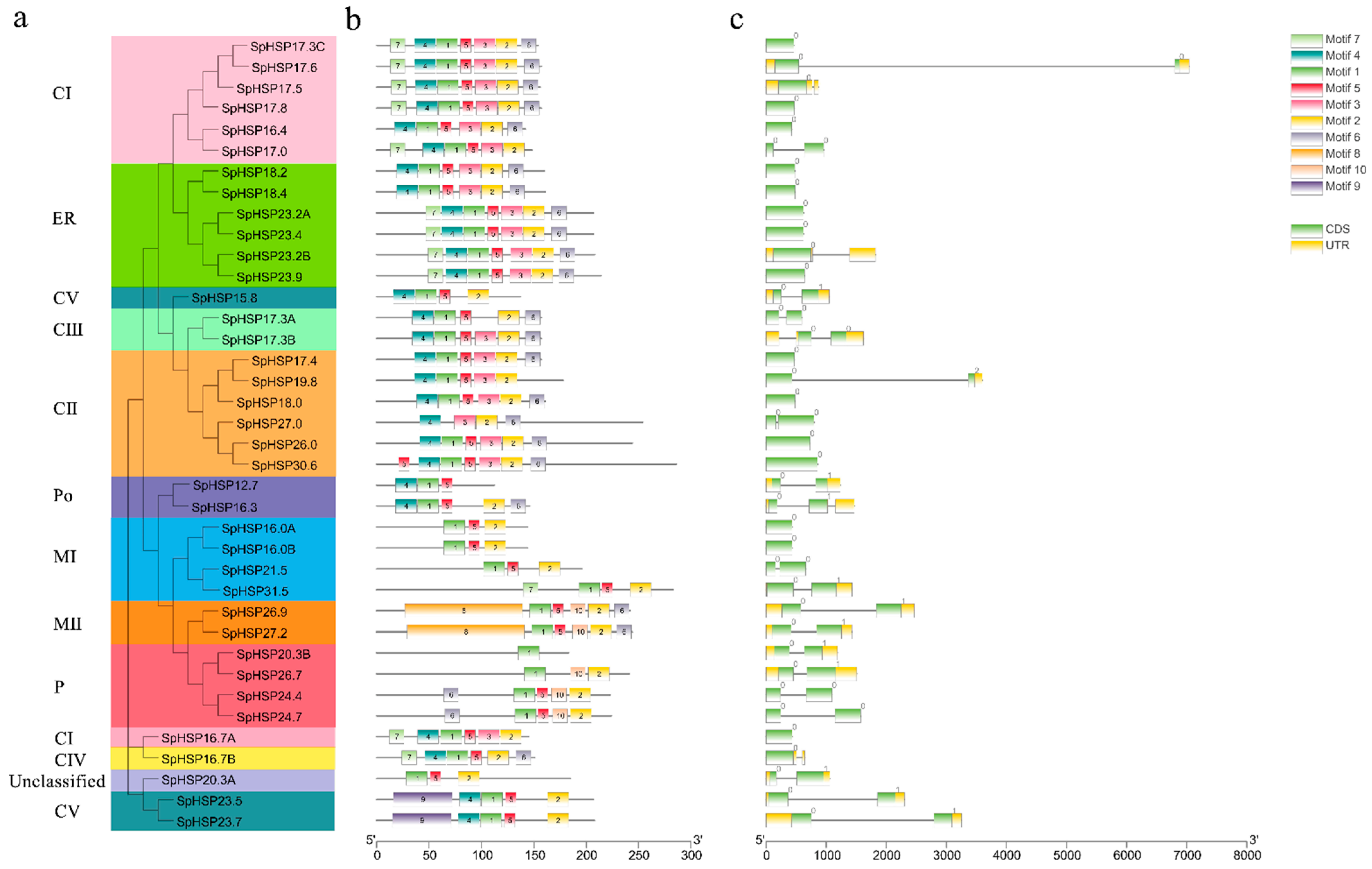
3.2. Phylogenetic Analysis of SpHSP20 Genes
3.3. Conserved Motifs and Gene Structure Analysis of SpHSP20 Genes
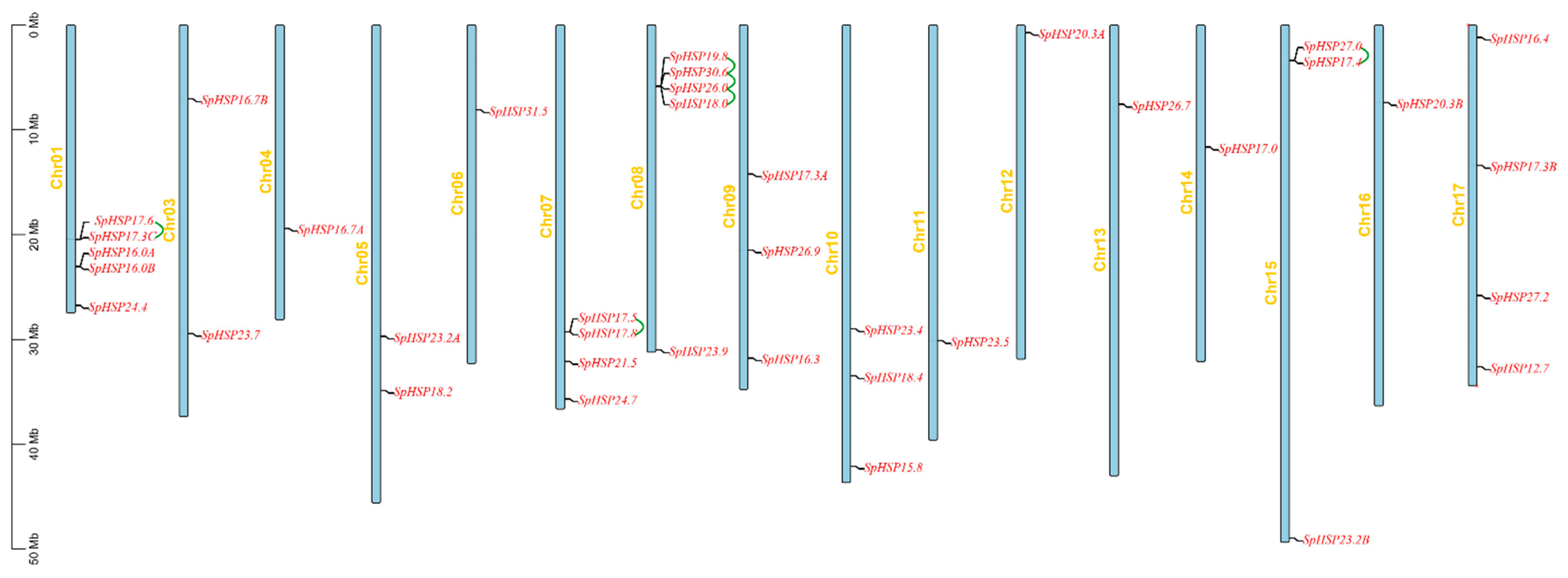
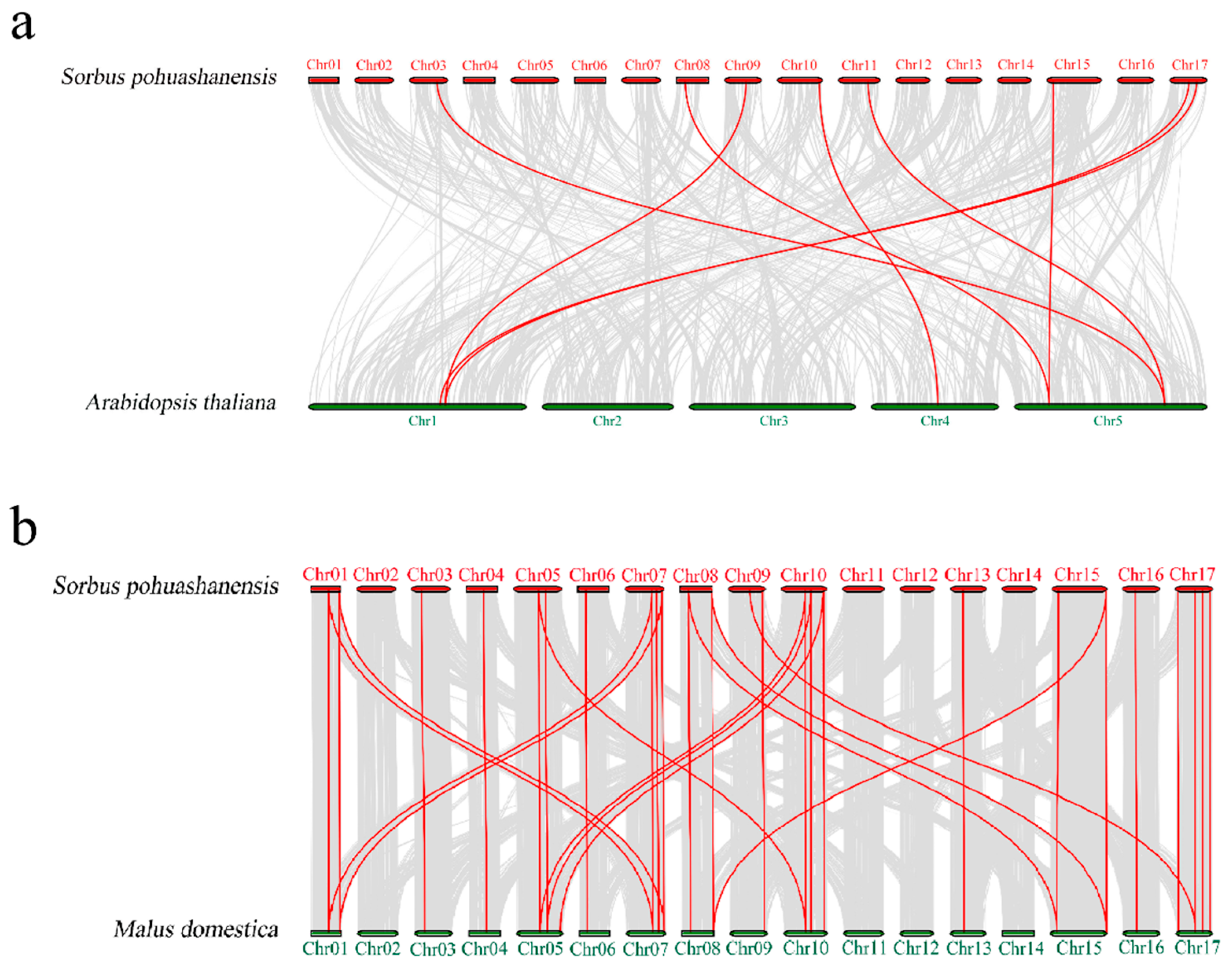
3.4. Chromosomal Location, Gene Duplication, and Synteny Analyses
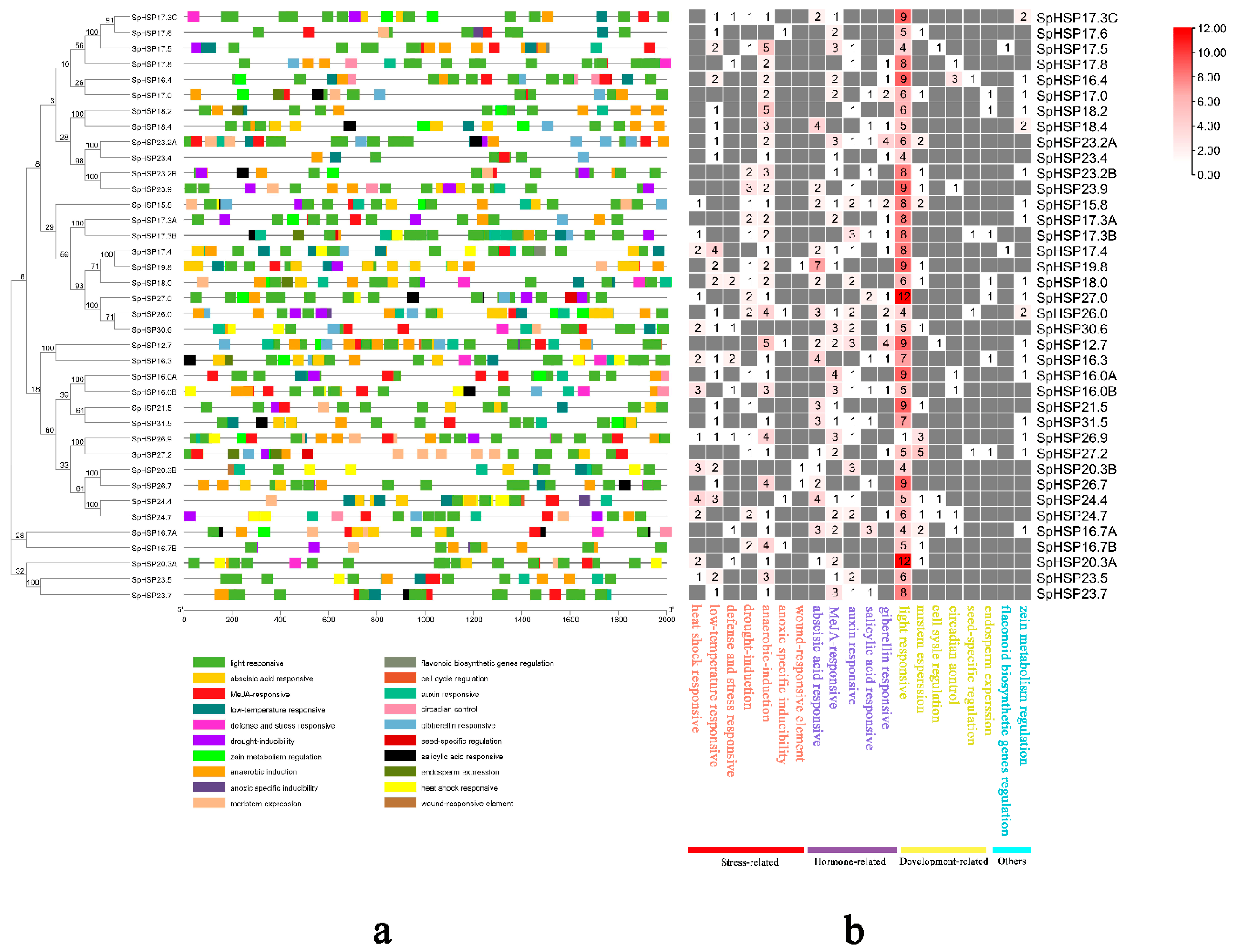
3.5. Analysis of Cis-Elements in SpHSP20 Gene Promoters
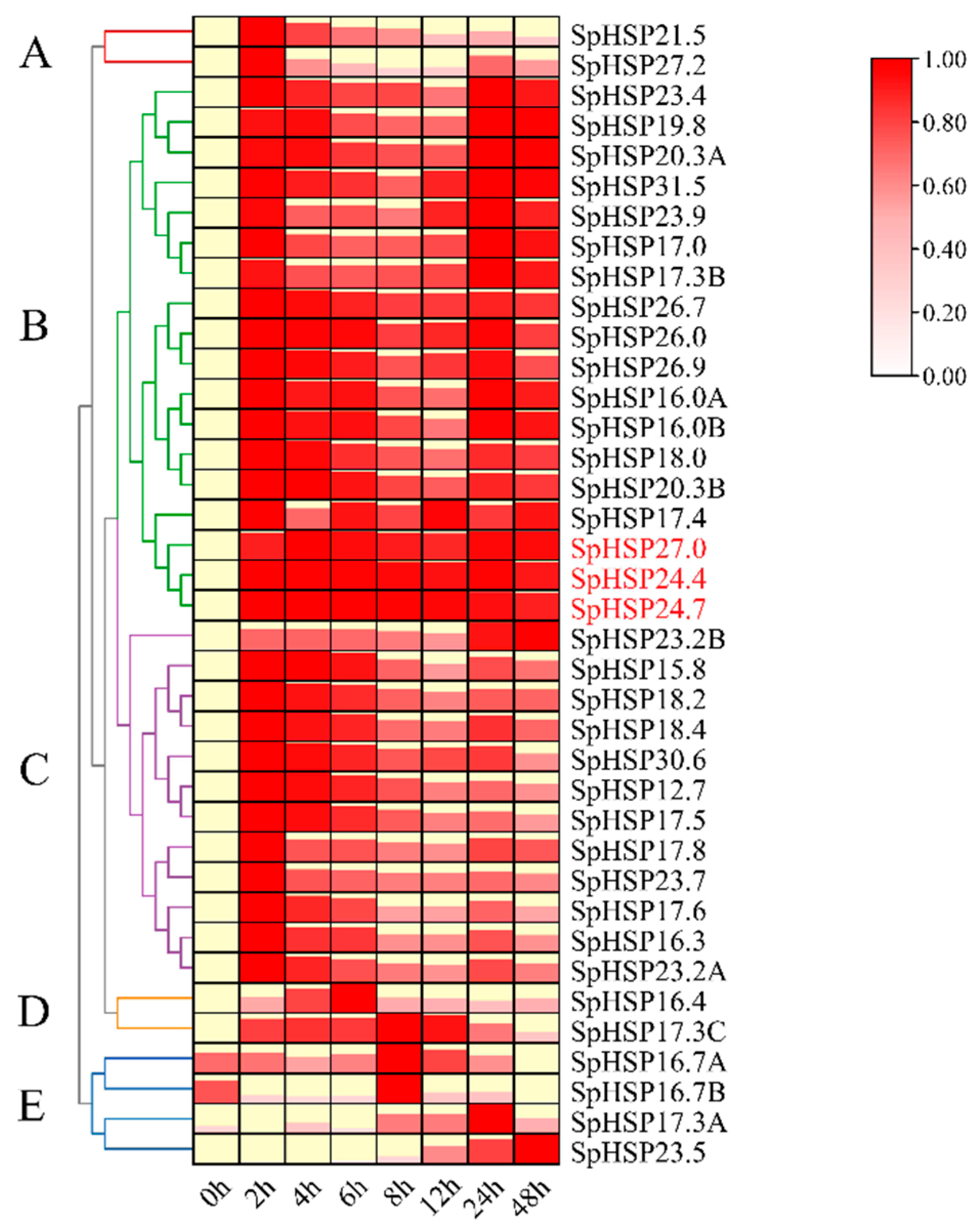
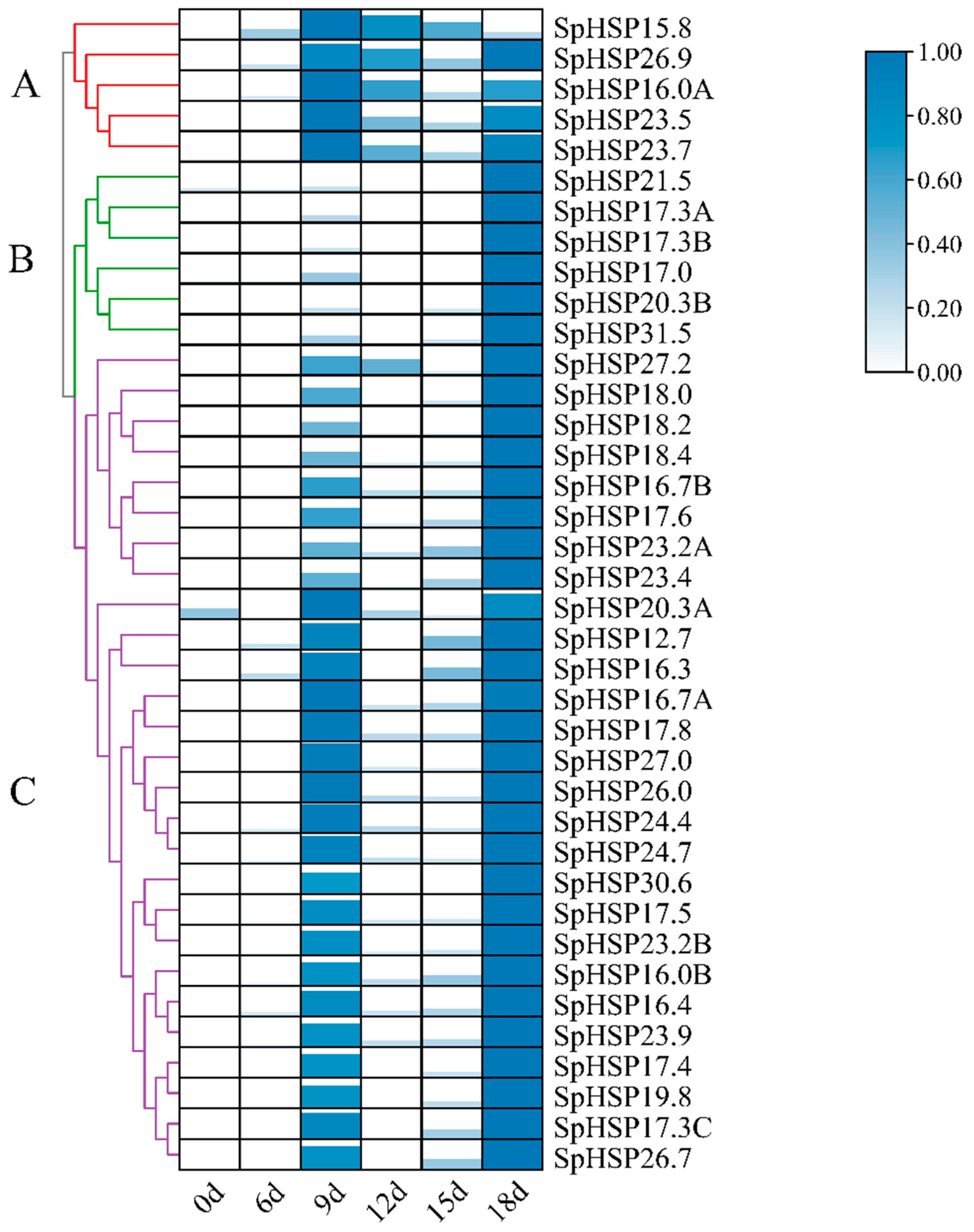
3.6. Expression Patterns of SpHSP20 Genes under Heat, Salt, and Drought Stress
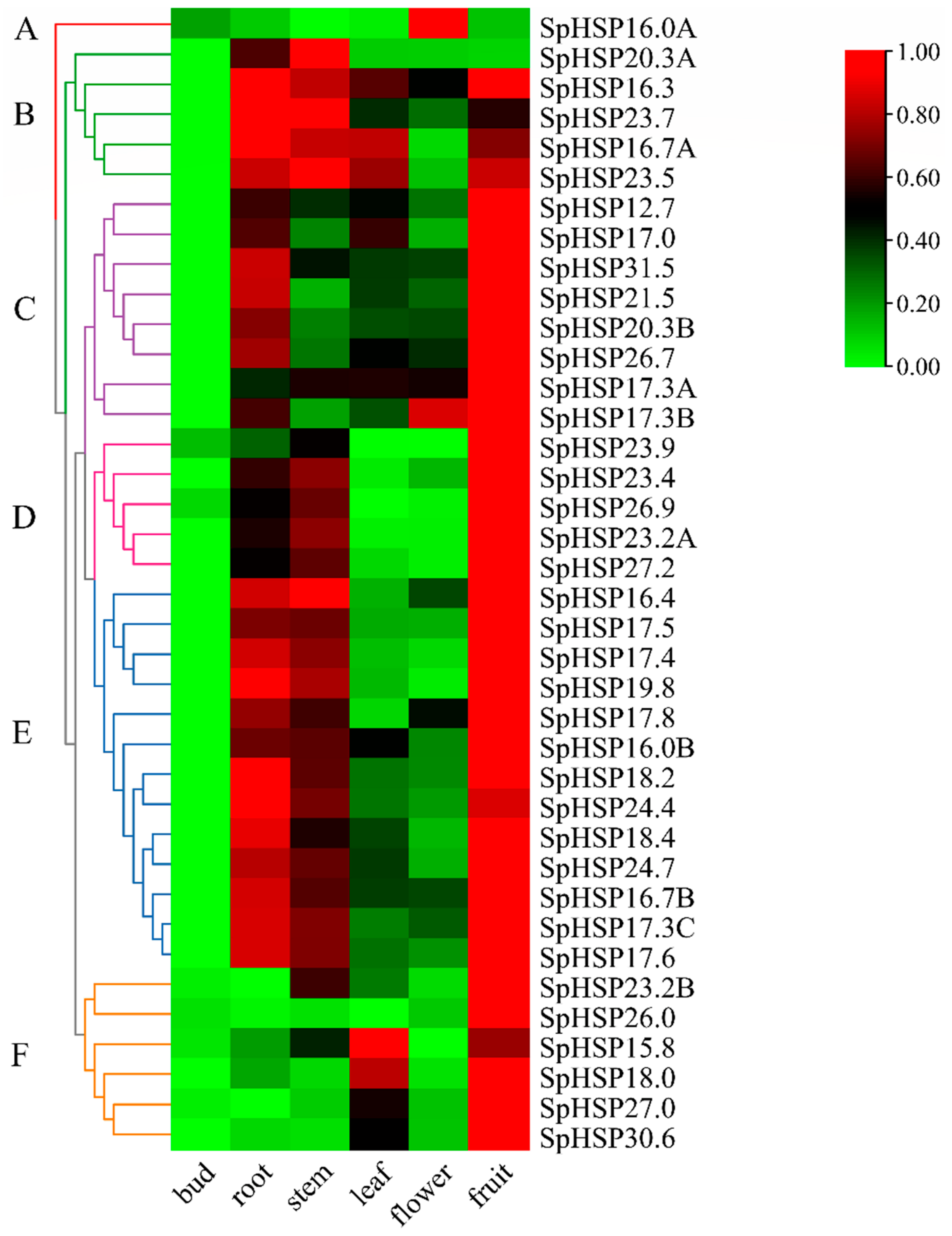
3.7. Tissue-Specific Expression of SpHSP20 Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayaz, A.; Huang, H.; Zheng, M.; Zaman, W.; Li, D.; Saqib, S.; Zhao, H.; Lü, S. Molecular Cloning and Functional Analysis of GmLACS2-3 Reveals Its Involvement in Cutin and Suberin Biosynthesis along with Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 9175. [Google Scholar] [CrossRef] [PubMed]
- Liaquat, F.; Qunlu, L.; Arif, S.; Haroon, U.; Saqib, S.; Zaman, W.; Jianxin, S.; Shengquan, C.; Li, L.X.; Akbar, M.; et al. Isolation and characterization of pathogen causing brown rot in lemon and its control by using ecofriendly botanicals. Physiol. Mol. Plant Pathol. 2021, 114, 101639. [Google Scholar] [CrossRef]
- Khan, M.; Ali, S.; Manghwar, H.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. Melatonin Function and Crosstalk with Other Phytohormones under Normal and Stressful Conditions. Genes 2022, 13, 1699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jian, S.; Wang, Z. Comprehensive Analysis of the Hsp20 Gene Family in Canavalia rosea Indicates Its Roles in the Response to Multiple Abiotic Stresses and Adaptation to Tropical Coral Islands. Int. J. Mol. Sci. 2022, 23, 6405. [Google Scholar] [CrossRef]
- Deryng, D.; Conway, D.; Ramankutty, N.; Price, J.; Warren, R. Global crop yield response to extreme heat stress under multiple climate change futures. Environ. Res. Lett. 2014, 9, 034011. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef]
- Mittler, R.; Finka, A.; Goloubinoff, P. How do plants feel the heat? Trends Biochem. Sci. 2012, 37, 118–125. [Google Scholar] [CrossRef]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- Neta-Sharir, I.; Isaacson, T.; Lurie, S.; Weiss, D. Dual role for tomato heat shock protein 21: Protecting photosystem II from oxidative stress and promoting color changes during fruit maturation. Plant Cell 2005, 17, 1829–1838. [Google Scholar] [CrossRef]
- Ahuja, I.; de Vos, R.C.; Bones, A.M.; Hall, R.D. Plant molecular stress responses face climate change. Trends Plant Sci. 2010, 15, 664–674. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Liu, B.; Zhang, L.; Chen, J.; Lu, M. Genome-wide analysis of the Populus Hsp90 gene family reveals differential expression patterns, localization, and heat stress responses. BMC Genom. 2013, 14, 532. [Google Scholar] [CrossRef]
- Waters, E.R. The evolution, function, structure, and expression of the plant sHSPs. J. Exp. Bot. 2013, 64, 391–403. [Google Scholar] [CrossRef]
- Sarkar, N.K.; Kim, Y.-K.; Grover, A. Rice sHsp genes: Genomic organization and expression profiling under stress and development. BMC Genom. 2009, 10, 393. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Shoseyov, O.; Altman, A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004, 9, 244–252. [Google Scholar] [CrossRef]
- Ouyang, Y.; Chen, J.; Xie, W.; Wang, L.; Zhang, Q. Comprehensive sequence and expression profile analysis of Hsp20 gene family in rice. Plant Mol. Biol. 2009, 70, 341–357. [Google Scholar] [CrossRef]
- Haslbeck, M.; Vierling, E. A first line of stress defense: Small heat shock proteins and their function in protein homeostasis. J. Mol. Biol. 2015, 427, 1537–1548. [Google Scholar] [CrossRef]
- Bondino, H.G.; Valle, E.M.; Ten Have, A. Evolution and functional diversification of the small heat shock protein/α-crystallin family in higher plants. Planta 2012, 235, 1299–1313. [Google Scholar] [CrossRef]
- Jaya, N.; Garcia, V.; Vierling, E. Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proc. Natl. Acad. Sci. USA 2009, 106, 15604–15609. [Google Scholar] [CrossRef]
- Siddique, M.; Gernhard, S.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. The plant sHSP superfamily: Five new members in Arabidopsis thaliana with unexpected properties. Cell Stress Chaperones 2008, 13, 183–197. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, Y.; Feng, K.; Ruan, M.; Ye, Q.; Wang, R.; Li, Z.; Zhou, G.; Yao, Z.; Yang, Y.; et al. Genome-Wide Identification and Expression Profiling of Tomato Hsp20 Gene Family in Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2016, 7, 1215. [Google Scholar] [CrossRef]
- Lopes-Caitar, V.S.; de Carvalho, M.C.; Darben, L.M.; Kuwahara, M.K.; Nepomuceno, A.L.; Dias, W.P.; Abdelnoor, R.V.; Marcelino-Guimarães, F.C. Genome-wide analysis of the Hsp20 gene family in soybean: Comprehensive sequence, genomic organization and expression profile analysis under abiotic and biotic stresses. BMC Genom. 2013, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liu, J.-H.; Liu, J.-P.; Zhai, Y.-F.; Wang, H.; Gong, Z.-H.; Wang, S.-B.; Lu, M.-H. Genome-wide analysis of the CaHsp20 gene family in pepper: Comprehensive sequence and expression profile analysis under heat stress. Front. Plant Sci. 2015, 6, 806. [Google Scholar] [CrossRef] [PubMed]
- Scharf, K.-D.; Siddique, M.; Vierling, E. The expanding family of Arabidopsis thaliana small heat stress proteins and a new family of proteins containing α-crystallin domains (Acd proteins). Cell Stress Chaperones 2001, 6, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Elicker, K.S.; Hutson, L.D. Genome-wide analysis and expression profiling of the small heat shock proteins in zebrafish. Gene 2007, 403, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, J.; Jia, H.; Li, Y.; Xu, X.; Wang, L.; Lu, M. The Populus trichocarpa PtHSP17.8 involved in heat and salt stress tolerances. Plant Cell Rep. 2016, 35, 1587–1599. [Google Scholar] [CrossRef]
- Kim, K.-H.; Alam, I.; Kim, Y.-G.; Sharmin, S.A.; Lee, K.-W.; Lee, S.-H.; Lee, B.-H. Overexpression of a chloroplast-localized small heat shock protein OsHSP26 confers enhanced tolerance against oxidative and heat stresses in tall fescue. Biotechnol. Lett. 2012, 34, 371–377. [Google Scholar] [CrossRef]
- Waters, E.R.; Vierling, E. Plant small heat shock proteins—Evolutionary and functional diversity. New Phytol. 2020, 227, 24–37. [Google Scholar] [CrossRef]
- Sun, X.; Sun, C.; Li, Z.; Hu, Q.; Han, L.; Luo, H. AsHSP17, a creeping bentgrass small heat shock protein modulates plant photosynthesis and ABA-dependent and independent signalling to attenuate plant response to abiotic stress. Plant Cell Environ. 2016, 39, 1320–1337. [Google Scholar] [CrossRef]
- Yogendra, K.N.; Kumar, A.; Sarkar, K.; Li, Y.; Pushpa, D.; Mosa, K.A.; Duggavathi, R.; Kushalappa, A.C. Transcription factor StWRKY1 regulates phenylpropanoid metabolites conferring late blight resistance in potato. J. Exp. Bot. 2015, 66, 7377–7789. [Google Scholar] [CrossRef]
- Park, C.-J.; Seo, Y.-S. Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef]
- Pei, X.; Zhang, Y.; Zhu, L.; Zhao, D.; Lu, Y.; Zheng, J. Physiological and transcriptomic analyses characterized high temperature stress response mechanisms in Sorbus pohuashanensis. Sci. Rep. 2021, 11, 10117. [Google Scholar] [CrossRef]
- Peng, S.; Zheng, Y.-Q.; Ma, M.; Zhang, C.-H.; Du, X.-J.; Li, T.; Ni, Y.-S. Physiological Adaptation of Sorbus pohuashanensis Seedlings to Heat Stress. For. Res. 2011, 24, 602–608. [Google Scholar]
- Zhao, D.; Qi, X.; Zhang, Y.; Zhang, R.; Wang, C.; Sun, T.; Zheng, J.; Lu, Y. Genome-wide analysis of the heat shock transcription factor gene family in Sorbus pohuashanensis (Hance) Hedl identifies potential candidates for resistance to abiotic stresses. Plant Physiol. Biochem. 2022, 175, 68–80. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Y.; Lu, Y.; Fan, L.; Zhang, Z.; Chai, M.; Zheng, J. Genome sequence and transcriptome of Sorbus pohuashanensis provide insights into population evolution and leaf sunburn response. J. Genet. Genom. 2022, 49, 547–558. [Google Scholar] [CrossRef]
- Zhang, Z.; Pei, X.; Zhang, R.; Lu, Y.; Zheng, J.; Zheng, Y. Molecular characterization and expression analysis of small heat shock protein 17.3 gene from Sorbus pohuashanensis (Hance) Hedl. in response to abiotic stress. Mol. Biol. Rep. 2020, 47, 9325–9335. [Google Scholar] [CrossRef]
- Zhang, Z.; Pei, X.; Lu, Y.; Chang, J.; Zheng, J. Cloning and expression analysis on small heat shock protein 23.8 gene (SpHSP23.8) in Sorbus pohuashanensis. J. Plant Resour. Environ. 2020, 29, 9–20. [Google Scholar]
- Zhao, D.; Liu, C.; Lu, Y.; Chang, J.; Zheng, J. Cloning and Expression Analysis of Heat Shock Protein SpHSP70-2 Gene in Sorbus pohuashanensis. J. Northwest For. Univ. 2021, 36, 42–50. [Google Scholar]
- Liu, C.; Zhang, Z.; Guan, X.; Pei, X.; Zheng, J. Cloning and Expression Analysis of Heat Shock Protein 70 Gene in Sorbus pohuashanensis. Mol. Plant Breed. 2019, 17, 6276–6286. [Google Scholar]
- Vandesompele, J.; Preter, K.D.; Pattyn, F.; Poppe, B.; Roy, N.V.; Paepe, A.D.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Waters, E.R.; Aevermann, B.D.; Sanders-Reed, Z. Comparative analysis of the small heat shock proteins in three angiosperm genomes identifies new subfamilies and reveals diverse evolutionary patterns. Cell Stress Chaperones 2008, 13, 127–142. [Google Scholar] [CrossRef]
- Yao, F.; Song, C.; Wang, H.; Song, S.; Jiao, J.; Wang, M.; Zheng, X.; Bai, T. Genome-Wide Characterization of the HSP20 Gene Family Identifies Potential Members Involved in Temperature Stress Response in Apple. Front. Genet. 2020, 11, 609184. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fan, M.; Sun, Y.; Li, L. Genome-Wide Analysis of Watermelon HSP20s and Their Expression Profiles and Subcellular Locations under Stresses. Int. J. Mol. Sci. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.-R.; Yu, Y.-H.; Ni, P.-Y.; Zhang, G.-H.; Guo, D.-L. Genome-wide identification of small heat-shock protein (HSP20) gene family in grape and expression profile during berry development. BMC Plant Biol. 2019, 19, 433. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zheng, D.; Vimolmangkang, S.; Khan, M.A.; Beever, J.E.; Korban, S.S. Integration of physical and genetic maps in apple confirms whole-genome and segmental duplications in the apple genome. J. Exp. Bot. 2011, 62, 5117–5130. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, Y.; Chen, B.; Zhi, C.; Qiao, L.; Liu, C.; Pan, Y.; Cheng, Z. Genome-wide identification of small heat shock protein (HSP20) homologs in three cucurbit species and the expression profiles of CsHSP20s under several abiotic stresses. Int. J. Biol. Macromol. 2021, 190, 827–836. [Google Scholar] [CrossRef]
- Jeffares, D.C.; Penkett, C.J.; Bähler, J. Rapidly regulated genes are intron poor. Trends Genet. 2008, 24, 375–378. [Google Scholar] [CrossRef]
- Li, S.; Yu, X.; Cheng, Z.; Zeng, C.; Li, W.; Zhang, L.; Peng, M. Large-scale analysis of the cassava transcriptome reveals the impact of cold stress on alternative splicing. J. Exp. Bot. 2020, 71, 422–434. [Google Scholar] [CrossRef]
- Rao, P.K.; Roxas, B.A.; Li, Q. Determination of global protein turnover in stressed mycobacterium cells using hybrid-linear ion trap-fourier transform mass spectrometry. Anal. Chem. 2008, 80, 396–406. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005, 10, 88–94. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, D.; Wang, R.; Kong, N.; Zhang, C.; Yang, C.; Wu, W.; Ma, H.; Chen, Q. Genome-wide analysis of the potato Hsp20 gene family: Identification, genomic organization and expression profiles in response to heat stress. BMC Genom. 2018, 19, 61. [Google Scholar] [CrossRef]
- Ren, X.-Y.; Vorst, O.; Fiers MW, E.J.; Stiekema, W.J.; Nap, J.-P. In plants, highly expressed genes are the least compact. Trends Genet. 2006, 22, 528–532. [Google Scholar] [CrossRef]
- Chung, B.Y.; Simons, C.; Firth, A.E.; Brown, C.M.; Hellens, R.P. Effect of 5’UTR introns on gene expression in Arabidopsis thaliana. BMC Genom. 2006, 7, 120. [Google Scholar] [CrossRef]
- Kotak, S.; Port, M.; Ganguli, A.; Bicker, F.; Koskull-Döring, P.V. Characterization of C-terminal domains of Arabidopsis heat stress transcription factors (Hsfs) and identification of a new signature combination of plant class A Hsfs with AHA and NES motifs essential for activator function and intracellular localization. Plant J. 2004, 39, 98–112. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, T.; Liu, Y.; Li, Y.; Wang, M.; Zhu, B.; Liao, D.; Yun, T.; Huang, W.; Zhang, W.; et al. Pumpkin (Cucurbita moschata) HSP20 Gene Family Identification and Expression under Heat Stress. Front. Genet. 2021, 12, 753953. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Jia, H.; Yue, Z.; Lu, M.; Xin, X.; Hu, J. Genome-Wide Characterization of the sHsp Gene Family in Salix suchowensis Reveals Its Functions under Different Abiotic Stresses. Int. J. Mol. Sci. 2018, 19, 3246. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, A.; Chen, J.; He, X.; Xu, B.; Xie, G.; Miao, Z.; Zhang, X.; Huang, L. Genome-Wide Analysis of the PvHsp20 Family in Switchgrass: Motif, Genomic Organization, and Identification of Stress or Developmental-Related Hsp20s. Front. Plant Sci. 2017, 8, 1024. [Google Scholar] [CrossRef]
- Cui, F.; Taier, G.; Wang, X.; Wang, K. Genome-Wide Analysis of the HSP20 Gene Family and Expression Patterns of HSP20 Genes in Response to Abiotic Stresses in Cynodon transvaalensis. Front. Genet. 2021, 12, 732812. [Google Scholar] [CrossRef]
- Gao, T.; Mo, Z.; Tang, L.; Yu, X.; Du, G.; Mao, Y. Heat Shock Protein 20 Gene Superfamilies in Red Algae: Evolutionary and Functional Diversities. Front. Plant Sci. 2022, 13, 817852. [Google Scholar] [CrossRef]
- Reddy, P.S.; Kishor, P.B.K.; Seiler, C.; Kuhlmann, M.; Eschen-Lippold, L.; Lee, J.; Reddy, M.K.; Sreenivasulu, N. Unraveling Regulation of the Small Heat Shock Proteins by the Heat Shock Factor HvHsfB2c in Barley: Its Implications in Drought Stress Response and Seed Development. PLoS ONE 2014, 9, e89125. [Google Scholar] [CrossRef]
- Gang, L.; Xia, Z.; Lu, Z.; Zhou, H. Cloning and Expression Analysis of Small Heat Shock Protein (Fa HSP17.4)Gene from Strawberry Fruit (Fragaria ananassa). Mol. Plant Breed. 2016, 6, 235–236. [Google Scholar]
- Naeem, M.; Shahzad, K.; Saqib, S.; Shahzad, A.; Nasrullah Younas, M.; Afridi, M.I. The Solanum melongena COP1LIKE manipulates fruit ripening and flowering time in tomato (Solanum lycopersicum). Plant Growth Regul. 2022, 96, 369–382. [Google Scholar] [CrossRef]
- Xu, M.; Yu, X.; Zheng, Y.; Zhang, T.; Xia, X.; Fu, Q.; Zhang, C. Study on Nutritive Substances and Medicinal Components of Sorbus pohuashanensis. For. Res. 2020, 33, 154–160. [Google Scholar]
- Yu, X.; Zhang, C.; Zheng, Y.; Xia, X.; Huang, L. Relationship between Carotenoid Component and Fruit Color of Two Sorbus Species. For. Res. 2021, 34, 71–79. [Google Scholar]



| Gene Name | Gene ID | Chr | Chromosome Position | AA | MW (KDa) | pI | Stability Index | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| SpHSP12.7 | Sp17G024510.1 | 17 | 32,629,484–32,630,718 | 111 | 12.67843 | 5.93 | 54.41 | Peroxisomal |
| SpHSP15.8 | Sp10G029800.1 | 10 | 42,110,310–42,111,362 | 136 | 15.7539 | 5.37 | 73.02 | Cytoplasmic |
| SpHSP16.0A | Sp01G015920.1 | 01 | 23,033,251–23,033,682 | 143 | 16.00126 | 6.1 | 30.47 | Mitochondrial |
| SpHSP16.0B | Sp01G015940.1 | 01 | 23,051,500–23,051,931 | 143 | 16.03922 | 5.6 | 37.15 | Mitochondrial |
| SpHSP16.3 | Sp09G023920.1 | 09 | 31,776,066–31,777,923 | 145 | 16.31363 | 8.02 | 50.1 | Peroxisomal |
| SpHSP16.4 | Sp17G001570.1 | 17 | 1,170,468–1,170,893 | 141 | 16.39386 | 7.82 | 34.52 | Cytoplasmic |
| SpHSP16.7A | Sp04G012430.1 | 04 | 19,406,854–19,407,288 | 144 | 16.69707 | 6.71 | 50.81 | Nuclear |
| SpHSP16.7B | Sp03G006770.1 | 03 | 7,035,379–7,036,024 | 150 | 16.72572 | 4.71 | 57.84 | Nuclear |
| SpHSP17.0 | Sp14G008870.1 | 14 | 11,649,378–11,650,338 | 147 | 17.03848 | 9.56 | 51.98 | Nuclear |
| SpHSP17.3A | Sp09G015010.1 | 09 | 14,210,375–14,210,971 | 156 | 17.28986 | 7.72 | 35.56 | Nuclear |
| SpHSP17.3B | Sp17G013620.1 | 17 | 13,391,460–13,393,080 | 156 | 17.33689 | 7.73 | 41.83 | Cytoplasmic |
| SpHSP17.3C | Sp01G012780.1 | 01 | 20,465,985–20,466,446 | 153 | 17.34467 | 6.18 | 47.22 | Nuclear |
| SpHSP17.4 | Sp15G004370.1 | 15 | 3,391,645–3,392,115 | 156 | 17.43811 | 5.58 | 39.53 | Cytoplasmic |
| SpHSP17.5 | Sp07G019210.1 | 07 | 29,273,801–29,274,672 | 155 | 17.52473 | 5.56 | 52.97 | Cytoplasmic |
| SpHSP17.6 | Sp01G012770.1 | 01 | 20,440,281–20,450,225 | 156 | 17.62492 | 5.84 | 51.77 | Nuclear |
| SpHSP17.8 | Sp07G019220.1 | 07 | 29,289,942–29,290,412 | 156 | 17.81794 | 6.39 | 67.34 | Nuclear |
| SpHSP18.0 | Sp08G006240.1 | 08 | 5,860,244–5,860,726 | 160 | 18.00361 | 5.97 | 45.72 | Cytoplasmic |
| SpHSP18.2 | Sp05G022540.1 | 05 | 34,877,634–34,878,113 | 159 | 18.22156 | 5.72 | 45.73 | Chloroplast |
| SpHSP18.4 | Sp10G020960.1 | 10 | 33,482,466–33,482,948 | 160 | 18.42399 | 6.32 | 45.11 | Cytoplasmic |
| SpHSP19.8 | Sp08G006210.1 | 08 | 5,837,234–5,840,830 | 177 | 19.81869 | 6.62 | 51.03 | Cytoplasmic |
| SpHSP20.3A | Sp12G000650.1 | 12 | 703,223–704,282 | 184 | 20.31725 | 9.06 | 49.37 | None |
| SpHSP20.3B | Sp16G008980.1 | 16 | 7,399,445–7,400,631 | 182 | 20.34325 | 9.1 | 43.73 | None |
| SpHSP21.5 | Sp07G022390.1 | 07 | 32,111,598–32,112,257 | 195 | 21.45444 | 8.83 | 48.78 | Mitochondrial |
| SpHSP23.2A | Sp05G017700.1 | 05 | 29,709,434–29,710,054 | 206 | 23.1906 | 6.14 | 53.52 | Cytoplasmic |
| SpHSP23.2B | Sp15G038330.1 | 15 | 48,982,836–48,984,657 | 207 | 23.20383 | 8.75 | 35.16 | Cytoplasmic |
| SpHSP23.4 | Sp10G016790.1 | 10 | 28,990,770–28,991,390 | 206 | 23.42879 | 6.03 | 51.74 | Cytoplasmic |
| SpHSP23.5 | Sp11G021010.1 | 11 | 30,127,432–30,129,736 | 206 | 23.53936 | 5.54 | 43.32 | Cytoplasmic |
| SpHSP23.7 | Sp03G019560.1 | 03 | 29,437,763–29,441,016 | 207 | 23.73253 | 5.19 | 46.64 | Cytoplasmic |
| SpHSP23.9 | Sp08G022710.1 | 08 | 31,018,189–31,018,830 | 213 | 23.87339 | 9.25 | 34.87 | Cytoplasmic |
| SpHSP24.4 | Sp01G020080.1 | 01 | 26,758,772–26,759,869 | 222 | 24.41663 | 9.02 | 46.29 | Chloroplast |
| SpHSP24.7 | Sp07G026110.1 | 07 | 35,681,144–35,682,720 | 223 | 24.69794 | 7.88 | 48.57 | Chloroplast |
| SpHSP26.0 | Sp08G006230.1 | 08 | 5,848,645–5,849,376 | 243 | 25.98482 | 6.01 | 57.56 | Cytoplasmic |
| SpHSP26.7 | Sp13G008820.1 | 13 | 7,558,891–7,560,398 | 240 | 26.73056 | 9.15 | 43.59 | None |
| SpHSP26.9 | Sp09G019280.1 | 09 | 21,477,023–21,479,489 | 241 | 26.92018 | 6.98 | 60.49 | Mitochondrial |
| SpHSP27.0 | Sp15G004360.1 | 15 | 3,382,832–3,383,631 | 253 | 27.0497 | 5.78 | 63.12 | Cytoplasmic |
| SpHSP27.2 | Sp17G019110.1 | 17 | 25,825,335–25,826,764 | 243 | 27.16939 | 7.77 | 52.43 | Mitochondrial |
| SpHSP30.6 | Sp08G006220.1 | 08 | 5,841,762–5,842,619 | 285 | 30.63445 | 5.42 | 51.72 | Cytoplasmic |
| SpHSP31.5 | Sp06G005610.1 | 06 | 8,090,079–8,091,509 | 282 | 31.53698 | 9.39 | 71.26 | Chloroplast |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Di, Z.; Li, Y.; Zhang, Z.; Guo, M.; Tong, B.; Lu, Y.; Zhang, Y.; Zheng, J. Genome-Wide Identification and Expression Profiling of Heat Shock Protein 20 Gene Family in Sorbus pohuashanensis (Hance) Hedl under Abiotic Stress. Genes 2022, 13, 2241. https://doi.org/10.3390/genes13122241
Qi X, Di Z, Li Y, Zhang Z, Guo M, Tong B, Lu Y, Zhang Y, Zheng J. Genome-Wide Identification and Expression Profiling of Heat Shock Protein 20 Gene Family in Sorbus pohuashanensis (Hance) Hedl under Abiotic Stress. Genes. 2022; 13(12):2241. https://doi.org/10.3390/genes13122241
Chicago/Turabian StyleQi, Xiangyu, Zexin Di, Yuyan Li, Zeren Zhang, Miaomiao Guo, Boqiang Tong, Yizeng Lu, Yan Zhang, and Jian Zheng. 2022. "Genome-Wide Identification and Expression Profiling of Heat Shock Protein 20 Gene Family in Sorbus pohuashanensis (Hance) Hedl under Abiotic Stress" Genes 13, no. 12: 2241. https://doi.org/10.3390/genes13122241
APA StyleQi, X., Di, Z., Li, Y., Zhang, Z., Guo, M., Tong, B., Lu, Y., Zhang, Y., & Zheng, J. (2022). Genome-Wide Identification and Expression Profiling of Heat Shock Protein 20 Gene Family in Sorbus pohuashanensis (Hance) Hedl under Abiotic Stress. Genes, 13(12), 2241. https://doi.org/10.3390/genes13122241