The HD-ZIP Gene Family in Watermelon: Genome-Wide Identification and Expression Analysis under Abiotic Stresses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification and Biochemical Characterization of HD-ZIP Genes in Watermelon
2.2. Phylogenetic and Syntenic Analyses of ClHDZs
2.3. Prediction of Gene Structure, Conserved Motifs, and cis-Regulatory Elements
2.4. Plant Materials and Treatments
2.5. RNA Extraction and Quantitative Real-Time RT-PCR
3. Results
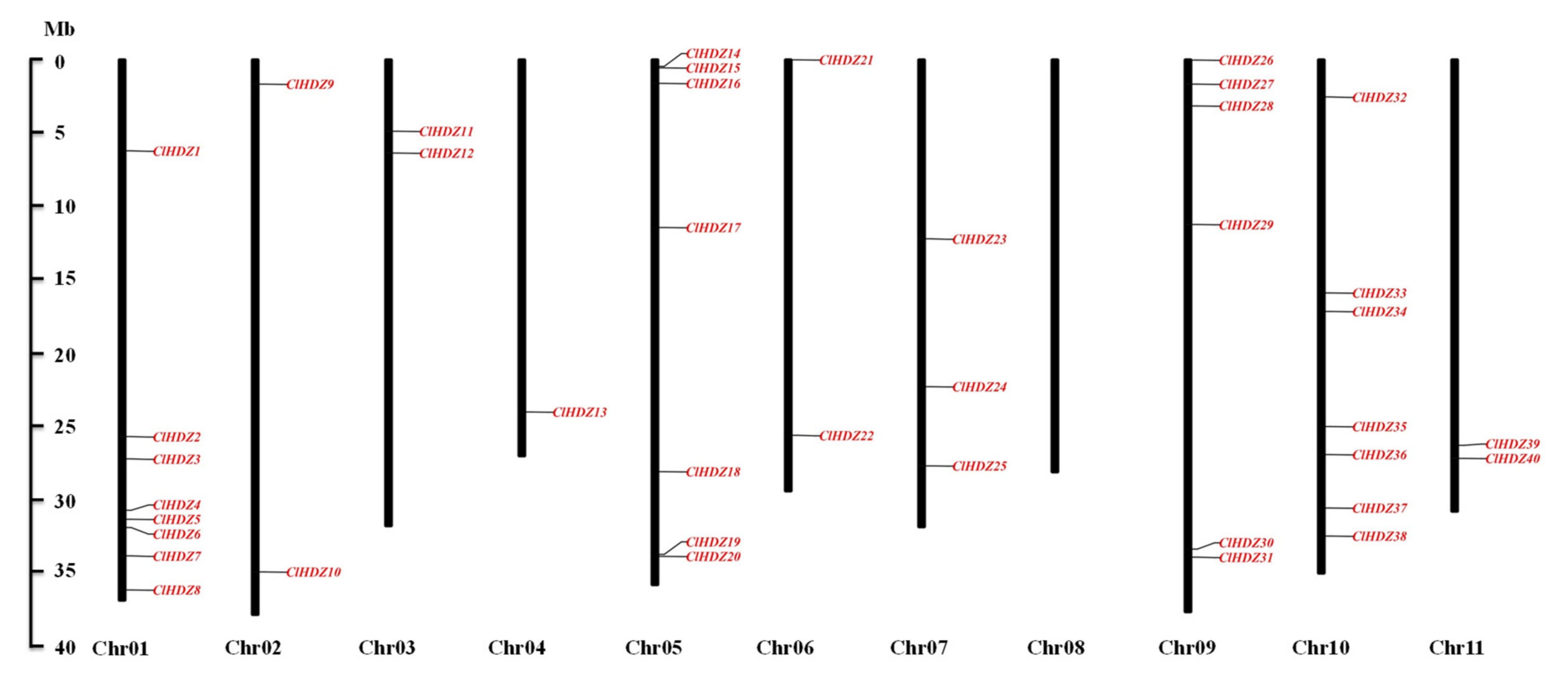
3.1. Genome-Wide Identification of HD-ZIP Genes in Watermelon
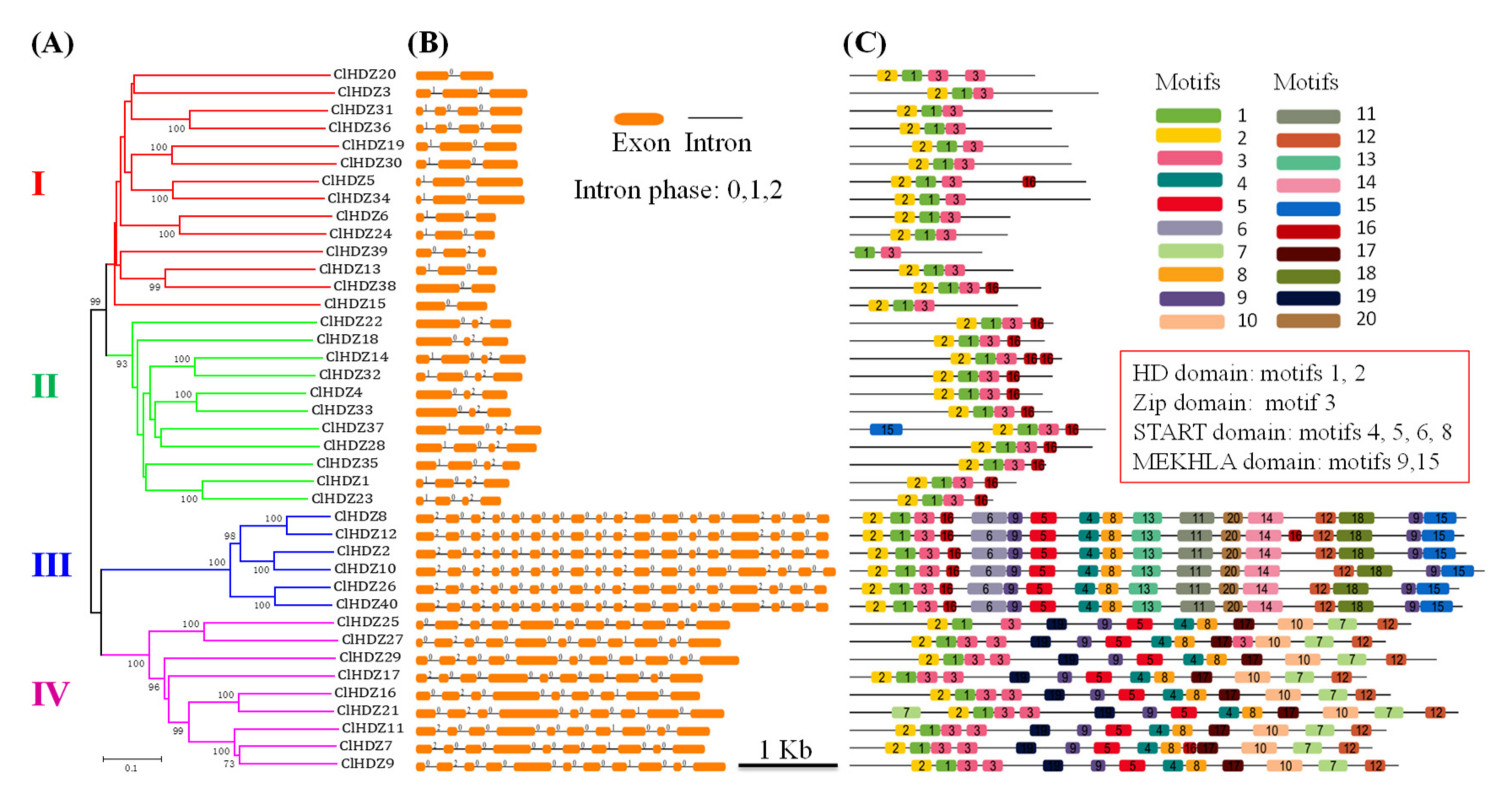
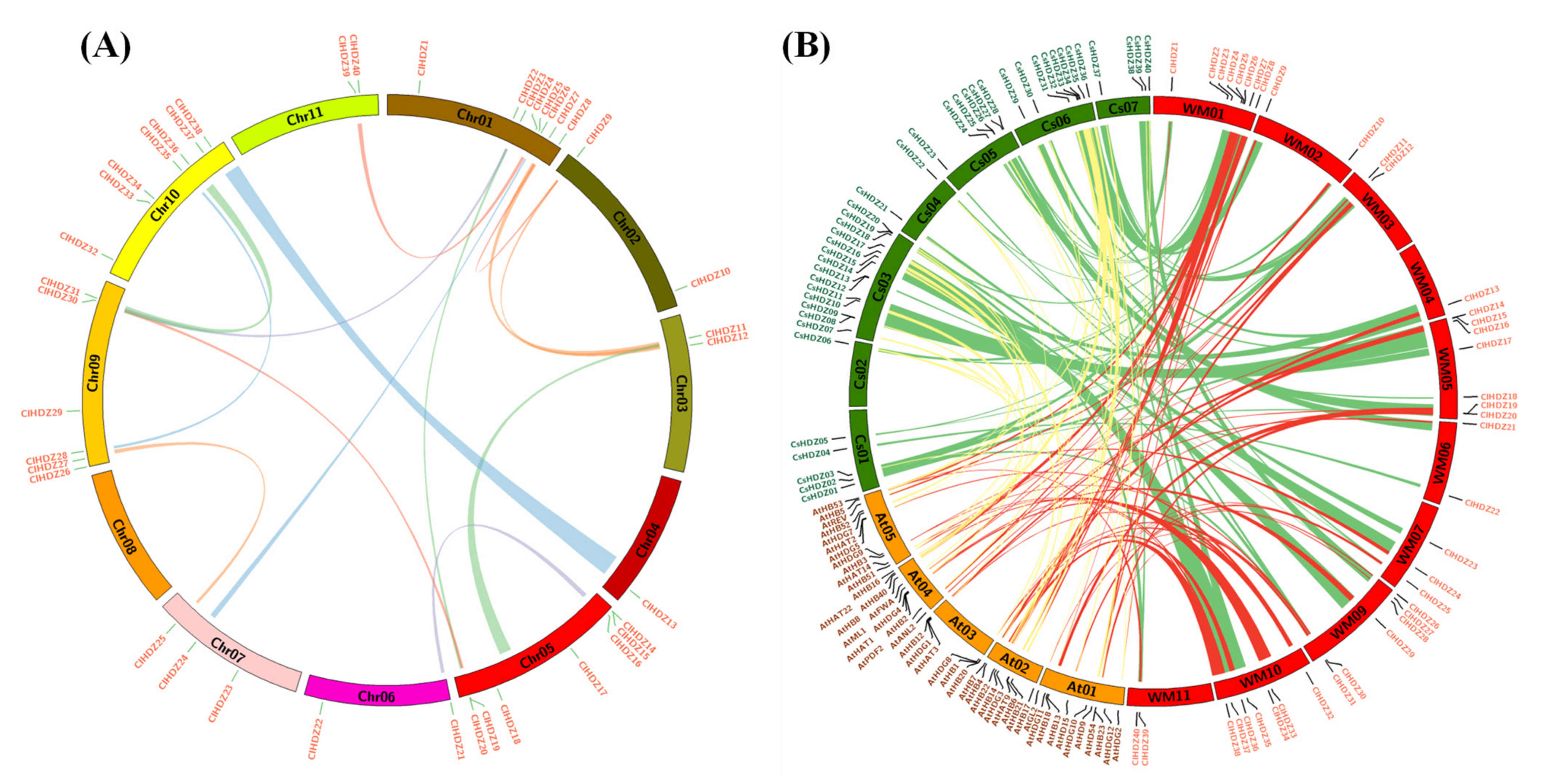
3.2. Evolutionary and Syntenic Analyses of HD-ZIP Gene Family
3.3. Gene Structure and Conserved Motif of ClHDZs
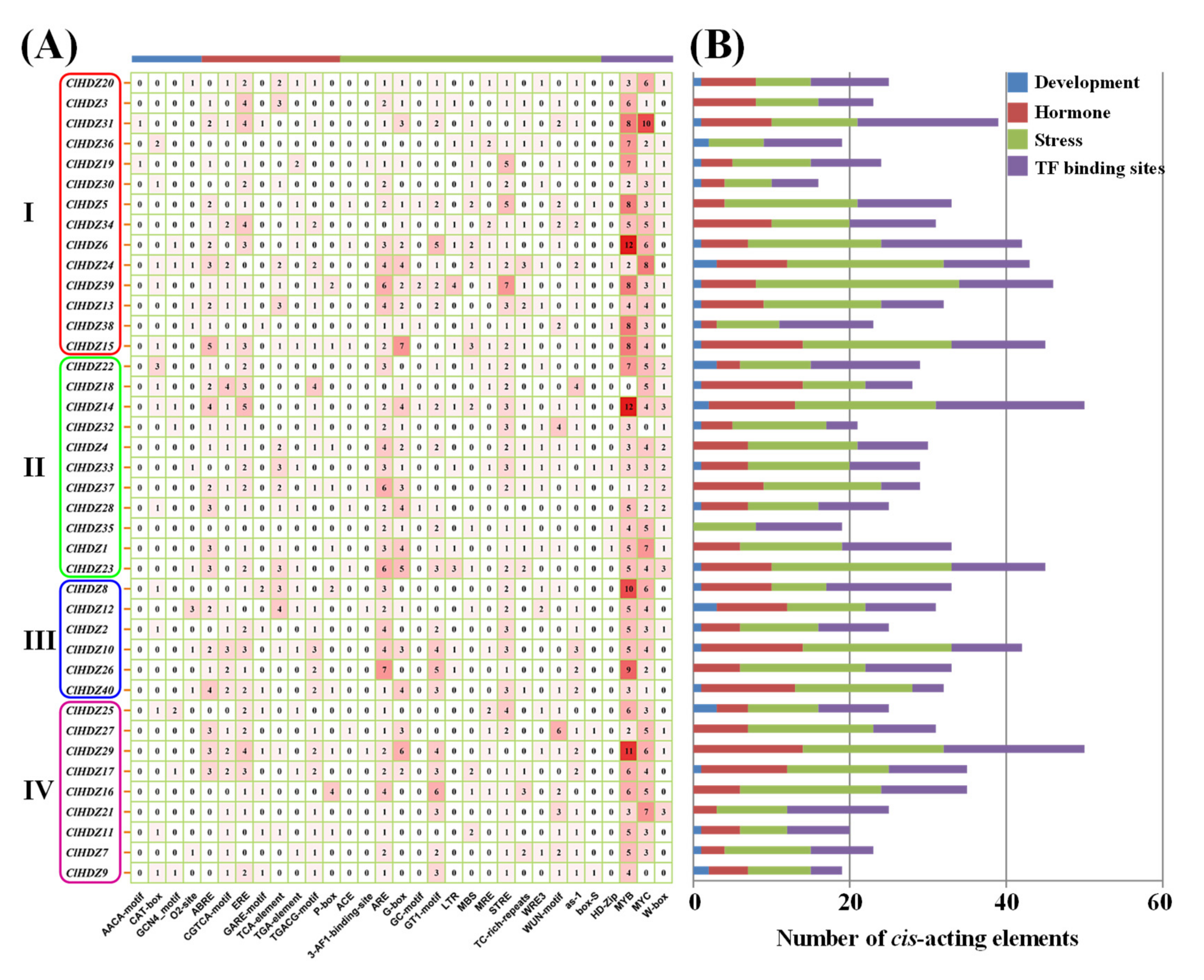
3.4. Prediction of cis-Regulatory Elements in ClHDZs
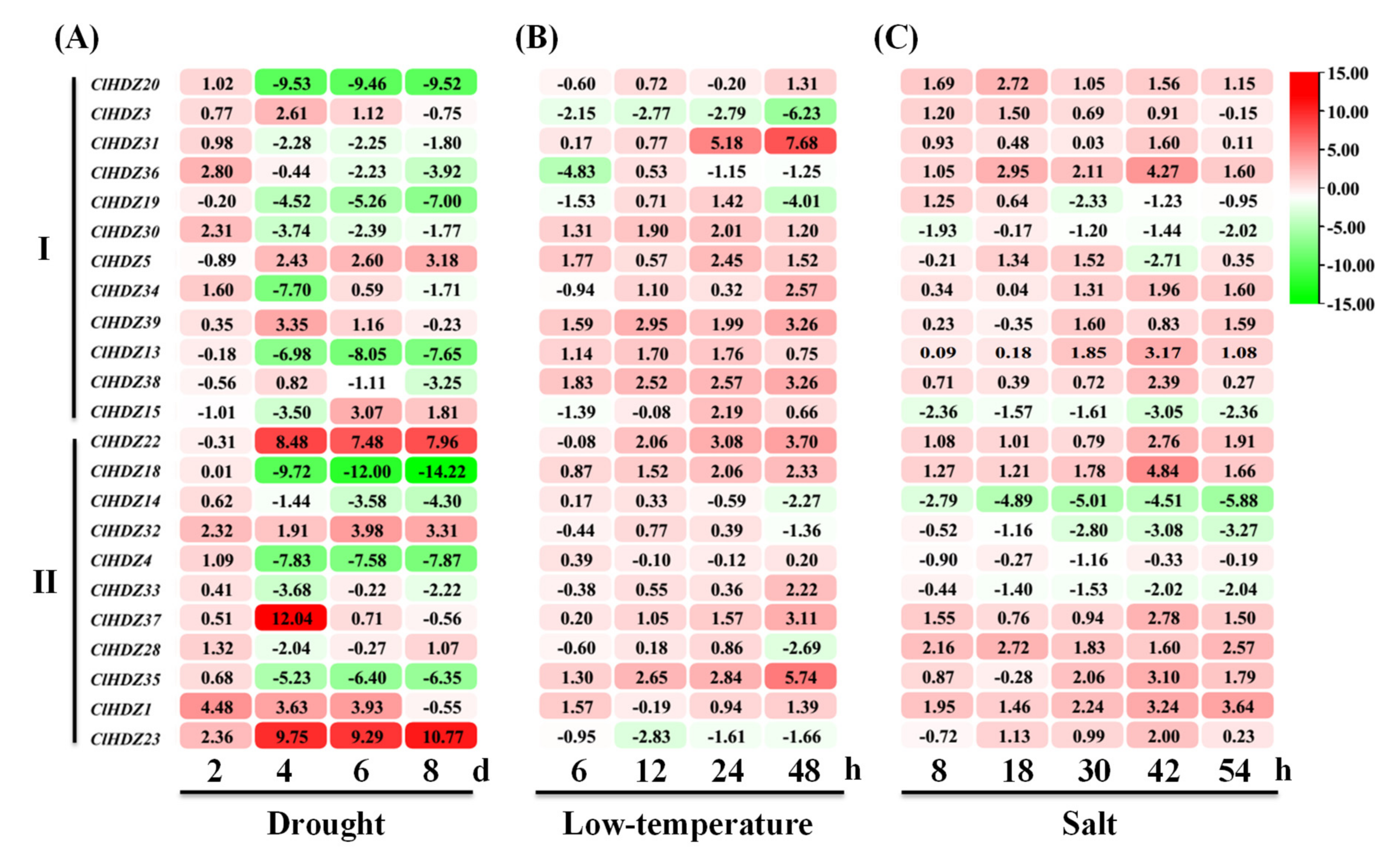
3.5. Expression Analysis of ClHDZs from Subfamilies I and II under Drought, Cold and Salt Stresses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ariel, F.D.; Manavella, P.A.; Dezar, C.A.; Chan, R.L. The true story of the HD-Zip family. Trends Plant Sci. 2007, 12, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Raza, A.; Chen, P.; Li, Y.; El-Ballat, E.M.; Rauf, A.; Hano, C.; El-Esawi, M.A. HD-ZIP gene family: Potential roles in improving plant growth and regulating stress-responsive mechanisms in plants. Genes 2021, 12, 1256. [Google Scholar] [CrossRef]
- Hong, Y.; Liu, Y.; Zhang, Y.; Jia, L.; Yang, X.; Zhang, X.; Liu, J.; Luan, Y. Genome-wide characterization of homeobox-leucine zipper gene family in tomato (Solanum lycopersicum) and functional analysis of SlHDZ34 (III sub-family member) under salinity stress. Environ. Exp. Bot. 2021, 192, 104652. [Google Scholar] [CrossRef]
- Wei, M.; Liu, A.; Zhang, Y.; Zhou, Y.; Li, D.; Dossa, K.; Zhou, R.; Zhang, X.; You, J. Genome-wide characterization and expression analysis of the HD-Zip gene family in response to drought and salinity stresses in sesame. BMC Genom. 2019, 20, 748. [Google Scholar] [CrossRef] [Green Version]
- Sharif, R.; Xie, C.; Wang, J.; Cao, Z.; Zhang, H.; Chen, P.; Yuhong, L. Genome wide identification, characterization and expression analysis of HD-ZIP gene family in Cucumis sativus L. under biotic and various abiotic stresses. Int. J. Biol. Macromol. 2020, 158, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Fu, L.; Yan, Y.; Tie, W.; Xia, Z.; Wang, W.; Peng, M.; Hu, W.; Zhang, J. Genome-wide characterization and expression profiling of HD-Zip gene family related to abiotic stress in cassava. PLoS ONE 2017, 12, e0173043. [Google Scholar] [CrossRef] [Green Version]
- Jain, M.; Tyagi, A.K.; Khurana, J.P. Genome-wide identification, classification, evolutionary expansion and expression analyses of homeobox genes in rice. FEBS J. 2008, 275, 2845–2861. [Google Scholar] [CrossRef]
- Gong, S.; Ding, Y.; Hu, S.; Ding, L.; Chen, Z.; Zhu, C. The role of HD-Zip class I transcription factors in plant response to abiotic stresses. Physiol. Plantarum. 2019, 167, 516–525. [Google Scholar] [CrossRef]
- Carabelli, M.; Possenti, M.; Sessa, G.; Ruzza, V.; Morelli, G.; Ruberti, I. Arabidopsis HD-Zip II proteins regulate the exit from proliferation during leaf development in canopy shade. J. Exp. Bot. 2018, 69, 5419–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Jung, J.H.; Reyes, J.L.; Kim, Y.S.; Kim, S.Y.; Chung, K.S.; Kim, J.A.; Lee, M.; Lee, Y.; Narry Kim, V.; et al. microRNA-directed cleavage of ATHB15 mRNA regulates vascular development in Arabidopsis inflorescence stems. Plant J. 2005, 42, 84–94. [Google Scholar] [CrossRef]
- Baima, S.; Possenti, M.; Matteucci, A.; Wisman, E.; Altamura, M.M.; Ruberti, I.; Morelli, G. The arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 2001, 126, 643–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubo, H.; Peeters, A.J.; Aarts, M.G.; Pereira, A.; Koornneef, M. ANTHOCYANINLESS2, a homeobox gene affecting anthocyanin distribution and root development in Arabidopsis. Plant Cell 1999, 11, 1217–1226. [Google Scholar] [CrossRef] [Green Version]
- Abe, M.; Katsumata, H.; Komeda, Y.; Takahashi, T. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 2003, 130, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Zhang, J.; Sun, H.; Salse, J.; Lucas, W.J.; Zhang, H.; Zheng, Y.; Mao, L.; Ren, Y.; Wang, Z.; et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nature Genet. 2013, 45, 51–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Zhao, S.; Sun, H.; Wang, X.; Wu, S.; Lin, T.; Ren, Y.; Gao, L.; Deng, Y.; Zhang, J.; et al. Resequencing of 414 cultivated and wild watermelon accessions identifies selection for fruit quality traits. Plant Physiol. 2019, 51, 1616–1623. [Google Scholar] [CrossRef] [Green Version]
- Qing, X.; Jie, H.; Jianhui, D.; Xiaojin, H.; Zhang, X. Genomic survey and expression profiling of the MYB gene family in watermelon. Hortic. Plant J. 2018, 4, 1–15. [Google Scholar]
- Yang, X.; Li, H.; Yang, Y.; Wang, Y.; Mo, Y.; Zhang, R.; Zhang, Y.; Ma, J.; Wei, C.; Zhang, X. Identification and expression analyses of WRKY genes reveal their involvement in growth and abiotic stress response in watermelon (Citrullus lanatus). PLoS ONE 2018, 13, e0191308. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools—An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic. Acids. Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Zhang, R.; Yang, X.; Zhu, C.; Li, H.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Comparative analysis of calcium-dependent protein kinase in Cucurbitaceae and expression studies in watermelon. Int. J. Mol. Sci. 2019, 20, 2527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, C.; Zhu, C.; Yang, L.; Zhao, W.; Ma, R.; Li, H.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. A point mutation resulting in a 13 bp deletion in the coding sequence of Cldf leads to a GA-deficient dwarf phenotype in watermelon. Hortic Res 2019, 6, 132. [Google Scholar] [CrossRef]
- Yue, Z.; Ma, R.; Cheng, D.; Yan, X.; He, Y.; Wang, C.; Pan, X.; Yin, L.; Zhang, X.; Wei, C. Candidate gene analysis of watermelon stripe pattern locus ClSP ongoing recombination suppression. Theor. Appl. Genet. 2021, 134, 3263–3277. [Google Scholar] [CrossRef]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roodbarkelari, F.; Groot, E.P. Regulatory function of homeodomain-leucine zipper (HD-ZIP) family proteins during embryogenesis. New Phytol. 2017, 213, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, T.; Wang, X.; Wang, J.; Gu, K.; Yu, J.; Hu, D.; Hao, Y. Genome-wide identification and expression analyses of homeodomain-leucine zipper family genes reveal their involvement in stress response in apple (Malus × domestica). Hortic. Plant J. 2022, 8, 261–278. [Google Scholar] [CrossRef]
- Harris, J.C.; Hrmova, M.; Lopato, S.; Langridge, P. Modulation of plant growth by HD-Zip class I and II transcription factors in response to environmental stimuli. New Phytol. 2011, 190, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Chen, X.; Wang, Z.; Liu, Q.; Li, H.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Genetic mapping of the LOBED LEAF 1 (ClLL1) gene to a 127.6-kb region in watermelon (Citrullus lanatus L.). PLoS ONE 2017, 12, e0180741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.; Zhang, H.; Sun, Y.; Shen, X.; Amoo, O.; Wang, Y.; Fan, C.; Zhou, Y. BnA10.RCO, a homeobox gene, positively regulates leaf lobe formation in Brassica napus L. Theor. Appl. Genet. 2020, 133, 3333–3343. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, A.; Lang, L.; Wang, Y.; Liu, X.; Liang, F.; Zhang, B.; Qin, M.; Dalelhan, J.; Huang, Z. Genetic mapping of a lobed-leaf gene associated with salt tolerance in Brassica napus L. Plant Sci. 2018, 269, 75–84. [Google Scholar] [CrossRef]
| Gene Name | Gene ID | Chromosome Location | Subfamily | Protein Length (aa) | Exon Number | MW(Da) | Gravy | pI |
|---|---|---|---|---|---|---|---|---|
| ClHDZ3 | Cla97C01G013400 | Chr01:27240628_27242205 | I | 341 | 3 | 38,075.8 | −0.742 | 4.63 |
| ClHDZ5 | Cla97C01G017950 | Chr01:31344079_31345409 | I | 324 | 3 | 36,813.5 | −0.799 | 4.83 |
| ClHDZ6 | Cla97C01G018750 | Chr01:31916455_31918441 | I | 220 | 3 | 25,182.4 | −0.94 | 8.48 |
| ClHDZ13 | Cla97C04G076510 | Chr04:24039469_24041867 | I | 224 | 3 | 25,795.9 | −0.798 | 6.23 |
| ClHDZ15 | Cla97C05G080560 | Chr05:620815_621585 | I | 230 | 2 | 26,411.5 | −0.927 | 5.82 |
| ClHDZ19 | Cla97C05G106550 | Chr05:33731410_33732703 | I | 300 | 3 | 34,313.1 | −1.036 | 6.38 |
| ClHDZ20 | Cla97C05G106720 | Chr05:33865821_33867128 | I | 254 | 2 | 29,228.5 | −0.917 | 5.09 |
| ClHDZ24 | Cla97C07G135990 | Chr07:22316542_22319234 | I | 216 | 3 | 24,822.1 | −0.884 | 5.86 |
| ClHDZ30 | Cla97C09G179770 | Chr09:33367941_33370759 | I | 304 | 3 | 33,618.7 | −0.643 | 6.46 |
| ClHDZ31 | Cla97C09G180320 | Chr09:33927034_33929071 | I | 278 | 4 | 31,932.2 | −0.832 | 4.62 |
| ClHDZ34 | Cla97C10G191910 | Chr10:17204865_17206214 | I | 330 | 3 | 37,342.1 | −0.841 | 4.81 |
| ClHDZ36 | Cla97C10G197160 | Chr10:26936967_26941299 | I | 277 | 4 | 31,253.6 | −0.826 | 4.9 |
| ClHDZ38 | Cla97C10G202610 | Chr10:32490507_32492602 | I | 262 | 2 | 30,042.6 | −0.666 | 9.65 |
| ClHDZ39 | Cla97C11G220290 | Chr11:26319113_26320758 | I | 181 | 3 | 21,163 | −0.882 | 6.21 |
| ClHDZ1 | Cla97C01G006340 | Chr01:6268951_6272330 | II | 228 | 4 | 25,840.8 | −0.593 | 9.53 |
| ClHDZ4 | Cla97C01G017140 | Chr01:30736614_30737606 | II | 264 | 3 | 29,144.1 | −0.655 | 8.11 |
| ClHDZ14 | Cla97C05G080370 | Chr05:505253_507284 | II | 291 | 4 | 32,306.1 | −0.806 | 7.06 |
| ClHDZ18 | Cla97C05G098910 | Chr05:28098057_28099506 | II | 267 | 3 | 29,247.4 | −0.588 | 8.46 |
| ClHDZ22 | Cla97C06G123330 | Chr06:25635223_25636830 | II | 279 | 3 | 32,054.7 | −1 | 7.67 |
| ClHDZ23 | Cla97C07G134460 | Chr07:12254360_12260018 | II | 196 | 4 | 22,589.9 | −0.786 | 8.68 |
| ClHDZ28 | Cla97C09G166050 | Chr09:3209878_3211125 | II | 333 | 4 | 36,909.1 | −0.823 | 5.69 |
| ClHDZ32 | Cla97C10G187080 | Chr10:2601999_2603584 | II | 278 | 4 | 31,111 | −0.801 | 7.66 |
| ClHDZ33 | Cla97C10G191810 | Chr10:15937459_15938526 | II | 278 | 3 | 30,745.9 | −0.676 | 9.09 |
| ClHDZ35 | Cla97C10G195530 | Chr10:25031280_25034315 | II | 269 | 4 | 30,517.1 | −0.933 | 6.68 |
| ClHDZ37 | Cla97C10G200510 | Chr10:30578990_30581007 | II | 351 | 4 | 38,288.9 | −0.574 | 5.9 |
| ClHDZ2 | Cla97C01G012720 | Chr01:25719610_25727010 | III | 847 | 12 | 92,620.4 | −0.079 | 5.69 |
| ClHDZ8 | Cla97C01G025160 | Chr01:36152833_36158141 | III | 847 | 18 | 92,756.8 | −0.142 | 5.85 |
| ClHDZ10 | Cla97C02G047220 | Chr02:34924600_34930198 | III | 872 | 18 | 95,349.8 | −0.067 | 5.79 |
| ClHDZ12 | Cla97C03G057570 | Chr03:6406462_6413316 | III | 844 | 18 | 92,949.2 | −0.11 | 5.81 |
| ClHDZ26 | Cla97C09G161990 | Chr09:85439_91313 | III | 837 | 18 | 92,308.8 | −0.137 | 6.03 |
| ClHDZ40 | Cla97C11G221100 | Chr11:27192123_27199004 | III | 842 | 18 | 92,544.7 | −0.149 | 5.74 |
| ClHDZ7 | Cla97C01G021770 | Chr01:33838063_33844699 | IV | 718 | 10 | 78,732.3 | −0.264 | 5.76 |
| ClHDZ9 | Cla97C02G028260 | Chr02:1723768_1729493 | IV | 754 | 11 | 82,236 | −0.314 | 5.85 |
| ClHDZ11 | Cla97C03G056100 | Chr03:4929667_4937076 | IV | 737 | 10 | 81,747.9 | −0.314 | 6.03 |
| ClHDZ16 | Cla97C05G082240 | Chr05:1664314_1669186 | IV | 743 | 9 | 81,814.2 | −0.277 | 5.74 |
| ClHDZ17 | Cla97C05G093030 | Chr05:11485675_11489533 | IV | 710 | 10 | 79,039.1 | −0.25 | 6.25 |
| ClHDZ21 | Cla97C06G109300 | Chr06:66818_71658 | IV | 836 | 9 | 89,918.1 | −0.246 | 5.66 |
| ClHDZ25 | Cla97C07G139950 | Chr07:27706301_27714819 | IV | 771 | 11 | 86,037.3 | −0.528 | 6.4 |
| ClHDZ27 | Cla97C09G164060 | Chr09:1721143_1728584 | IV | 736 | 11 | 82,347.4 | −0.484 | 5.99 |
| ClHDZ29 | Cla97C09G174400 | Chr09:11280503_11288355 | IV | 806 | 11 | 89,745.7 | −0.391 | 5.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Yue, Z.; Pan, X.; Si, F.; Li, J.; Chen, X.; Li, X.; Luan, F.; Yang, J.; Zhang, X.; et al. The HD-ZIP Gene Family in Watermelon: Genome-Wide Identification and Expression Analysis under Abiotic Stresses. Genes 2022, 13, 2242. https://doi.org/10.3390/genes13122242
Yan X, Yue Z, Pan X, Si F, Li J, Chen X, Li X, Luan F, Yang J, Zhang X, et al. The HD-ZIP Gene Family in Watermelon: Genome-Wide Identification and Expression Analysis under Abiotic Stresses. Genes. 2022; 13(12):2242. https://doi.org/10.3390/genes13122242
Chicago/Turabian StyleYan, Xing, Zhen Yue, Xiaona Pan, Fengfei Si, Jiayue Li, Xiaoyao Chen, Xin Li, Feishi Luan, Jianqiang Yang, Xian Zhang, and et al. 2022. "The HD-ZIP Gene Family in Watermelon: Genome-Wide Identification and Expression Analysis under Abiotic Stresses" Genes 13, no. 12: 2242. https://doi.org/10.3390/genes13122242





