Molecular and Physiological Evaluation of Bread Wheat (Triticum aestivum L.) Genotypes for Stay Green under Drought Stress
Abstract
:1. Introduction
2. Methods and Materials
2.1. Greenhouse Experiment
2.2. Physiological Characterization
2.3. Molecular Characterization of Wheat Genotypes
- ACCGCCAGCTCTTCCACCCT (Tubulin-F); TCACTGGGGCATAGGAGGAA ((Tubulin-R);
- TCAGCGACCTCACCGTCA (TaLhcb6-F); CCCCAAAGAAGTCACGGACA (TaLhcb6-R);
- AAAGGCCGAGGAGGACAA (TaLhcb4-F); CCACCGACCACTTAAGAGG (TaLhcb4-R);
- GGAGAACACACAATACACC (TaLhcb1-F); CCCATTATGTGTGCAGTTC (TaLhcb1-R);
- CCTCACCAGCCTCAAGTTCC (TaLhca3-F); CGCACGCTCACGTTTCC (TaLhca3- R);
- CCCCAACCGCAAGAACC (TaLhca2-F); CCGACGAAGGCGAGCAT (TaLhca2-R);
- CAACCTGCCGACCATCCTG (TaLhca1-F) and CAGCCGCCCGTTCTTGAT (TaLhca1-R).
2.3.1. Total RNA Extraction and cDNA Synthesis
2.3.2. Real-Time PCR and Data Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Relative Water Content (RWC)
3.2. Membrane Stability Index (MSI)
3.3. Proline Contents
3.4. Chlorophyll Contents
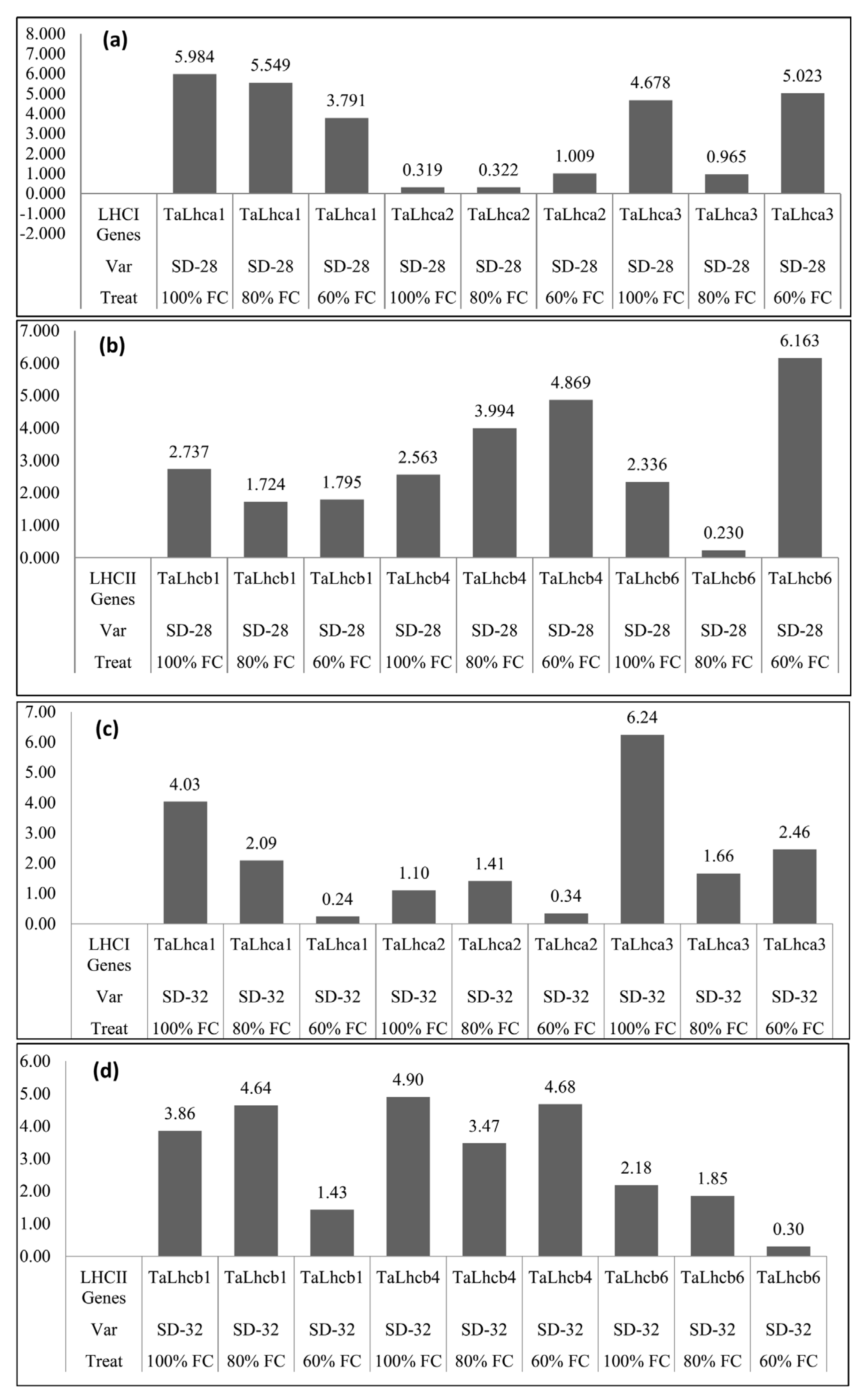
3.5. Expression Levels of Genes Involved in LHCI and LHCII in Response to Drought Stress
3.6. Discussion
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ullah, M.I.; Mahpara, S.; Bibi, R.; Shah, R.U.; Ullah, R.; Abbas, S.; Ullah, M.I.; Hassan, A.M.; El-Shehawi, A.M.; Brestic, M. Grain yield and correlated traits of bread wheat lines: Implications for yield improvement. Saudi J. Biol. Sci. 2021, 28, 5714–5719. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Neumann, K.; Friedel, S.; Kilian, B.; Chen, M.; Altmann, T.; Klukas, C. Dissecting the phenotypic components of crop plant growth and drought responses based on high-throughput image analysis. Plant Cell 2014, 26, 4636–4655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Ali, A.; Ullah, Z.; Ali, I.; Kaushik, P.; Alyemeni, M.N.; Rasheed, A.; Sher, H. Exploiting the drought tolerance of wild Elymus species for bread wheat improvement. Front. Plant Sci. 2022, 13, 982844. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Ali, A.; Afridi, M.S.; Ali, S.; Ullah, Z. Agrobiodiversity: Effect of drought stress on the eco-physiology and morphology of wheat. In Biodiversity, Conservation and Sustainability in Asia; Springer: Cham, Switzerland, 2022; pp. 597–618. [Google Scholar]
- Karatayev, M.; Clarke, M.; Salnikov, V.; Bekseitova, R.; Nizamova, M. Monitoring climate change, drought conditions and wheat production in Eurasia: The case study of Kazakhstan. Heliyon 2022, 8, e08660. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, Z.U.R.; Ayub, M.A.; ur Rehman, M.Z.; Sohail, M.I.; Usman, M.; Khalid, H.; Naz, K. Regulation of drought stress in plants. In Plant Life under Changing Environment; Academic Press: Cambridge, MA, USA, 2020; pp. 77–104. [Google Scholar]
- Sadiq, M.; Asghar, R.; Qureshi, R.; Ali, A. Study of polypeptides induced by drought stress in some local varieties of barley from Pakistan. Pak. J. Bot. 2013, 45, 1251–1254. [Google Scholar]
- Ali, Z.; Ali, B.; Mohammad, A.; Ahmad, M.; Ahmad, I.; Napar, A.A.; Kazi, A.G.; Ali, A.; Shah, S.S.; Mujeeb-Kazi, A. Combating water scarcity for global food security. In Agricultural Systems in the 21st Century; Nova: New York, NY, USA, 2013; pp. 1–30. [Google Scholar]
- Ali, A.; Ali, Z.; Quraishi, U.M.; Kazi, A.G.; Malik, R.N.; Sher, H.; Mujeeb-Kazi, A. Integrating physiological and genetic 394 approaches for improving drought tolerance in crops. In Emerging Technologies and Management of Crop Stress Tolerance; Academic Press: Cambridge, MA, USA, 2014; pp. 315–345, 395. [Google Scholar]
- Ali, A.; Arshad, M.; Naqvi, S.M.S.; Rasheed, A.; Sher, H.; Kazi, A.G.; Mujeeb-Kazi, A. Comparative assessment of synthetic-derived and conventional bread wheat advanced lines under osmotic stress and implications for molecular analysis. Plant Mol. Biol. Rep. 2015, 33, 1907–1917. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Hauptvogel, P.; Misheva, S.; Kocheva, K.; Yang, X.; Li, X.; Allakhverdiev, S.I. Wheat plant selection 422 for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature 423 conditions. Photosynth. Res. 2018, 136, 245–255. [Google Scholar] [CrossRef]
- Gaballah, M.M.; Metwally, A.M.; Skalicky, M.; Hassan, M.M.; Brestic, M.; El Sabagh, A.; Fayed, A.M. Genetic diversity 434 of selected rice genotypes under water stress conditions. Plants 2020, 10, 27. [Google Scholar] [CrossRef]
- Clarke, D.; Hess, T.M.; Haro-Monteagudo, D.; Semenov, M.A.; Knox, J.W. Assessing future drought risks and wheat yield losses in England. Agric. For. Meteorol. 2021, 297, 108248. [Google Scholar] [CrossRef]
- Halford, N.G.; Hey, S.J. Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem. J. 2009, 419, 247–259. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Salah, K.B.H.; Sher, H.; Ali, H.; Ullah, Z.; Ali, A.; Alam, N.; Shah, S.A.; Iqbal, J.; Ilyas, M.; et al. Drought stress enhances the efficiency of floral dip method of Agrobacterium-mediated transformation in Arabidopsis thaliana. Braz. J. Biol. 2022, 84, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.; Minhas, N.M.; Farrakh, A.G.; Farrakh, S.; Ali, A.; Bux, H.; Kazi, M. Phenotypic and genotypic characterization of wheat landraces of Pakistan. Emir. J. Food Agric. 2014, 26, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Khan, A.; Ali, A.; Ullah, Z.; Dai, D.Q.; Khan, N.; Khan, A.; Al-Tawaha, A.R.; Sher, H. Iron and Zinc micronutrients and soil inoculation of Trichoderma harzianum enhance wheat grain quality and yield. Front. Plant Sci. 2022, 13, 960948. [Google Scholar] [CrossRef]
- Afzal, F.; Ali, A.; Ullah, Z.; Sher, H.; Gul, A.; Mujeeb-Kazi, A.; Arshad, M. Terminal drought stress adaptability in synthetic derived bread wheat is explained by alleles of major adaptability genes and superior phenology. Int. J. Agric. Biol. 2018, 20, 1623–1631. [Google Scholar]
- Afzal, F.; Li, H.; Gul, A.; Subhani, A.; Ali, A.; Mujeeb-Kazi, A.; Ogbonnaya, F.; Trethowan, R.; Xia, X.; He, Z.; et al. Genome-wide analyses reveal footprints of divergent selection and drought adaptive traits in synthetic-derived wheats. G3 Genes Genomes Genet. 2019, 9, 1957–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amanuel, M.; Gebre, D.; Debele, T. Performance of bread wheat genotypes under different environment in lowland irrigated areas of Afar Region, Ethiopia. Afr. J. Agric. Res. 2018, 13, 927–933. [Google Scholar]
- Nehe, A.; Akin, B.; Sanal, T.; Evlice, A.K.; Unsal, R.; Dinçer, N.; Demir, L.; Geren, H.; Sevim, I.; Orhan, Ş. Genotype x environment interaction and genetic gain for grain yield and grain quality traits in Turkish spring wheat released between 1964 and 2010. PLoS ONE 2019, 14, e0219432. [Google Scholar] [CrossRef] [Green Version]
- Spano, G.; Di Fonzo, N.; Perrotta, C.; Platani, C.; Ronga, G.; Lawlor, D.W.; Napier, J.A.; Shewry, P.R. Physiological characterization of ‘stay green’mutants in durum wheat. J. Exp. Bot. 2003, 54, 1415–1420. [Google Scholar] [CrossRef] [Green Version]
- Thomas, H.; Howarth, C.J. Five ways to stay green. J. Exp. Bot. 2000, 51, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Foulkes, M.J.; Snape, J.W.; Shearman, V.J.; Reynolds, M.P.; Gaju, O.; Sylvester-Bradley, R. Genetic progress in yield potential in wheat: Recent advances and future prospects. J. Agric. Sci. 2007, 145, 17. [Google Scholar] [CrossRef]
- Billah, M.; Aktar, S.; Brestic, M.; Zivcak, M.; Khaldun, A.B.M.; Uddin, M.; Bagum, S.A.; Yang, X.; Skalicky, M.; Mehari, T.G.; et al. Progressive genomic approaches to explore drought-and salt-induced oxidative stress responses in plants under changing climate. Plants 2021, 10, 1910. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Harikrishna; Barman, D.; Ghimire, O.P.; Gurumurthy, S.; Chinnusamy, V.; Singh, P.K.; Padaria, J.C.; Arora, A. tay-green trait serves as yield stability attribute under combined heat and drought stress in wheat (Triticum aestivum L.). Plant Growth Regul. 2022, 96, 67–78. [Google Scholar] [CrossRef]
- Allen, J.F.; Forsberg, J. Molecular recognition in thylakoid structure and function. Trends Plant Sci. 2001, 6, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Bagherikia, S.; Pahlevani, M.; Yamchi, A.; Zaynalinezhad, K.; Mostafaie, A. Transcript profiling of genes encoding fructan and sucrose metabolism in wheat under terminal drought tress. J. Plant Growth Regul. 2019, 38, 148–163. [Google Scholar] [CrossRef]
- Grieco, M.; Roustan, V.; Dermendjiev, G.; Rantala, S.; Jain, A.; Leonardelli, M.; Neumann, K.; Berger, V.; Engelmeier, D.; Bachmann, G.; et al. Adjustment of photosynthetic activity to drought and fluctuating light in wheat. Plant Cell Environ. 2020, 43, 1484–1500. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Gong, J.; Zhang, J.; Zhang, M.; Wang, G.; Li, A.; Wang, W. Enhanced stability of thylakoid membrane proteins and antioxidant competence contribute to drought stress resistance in the tasg1 wheat stay-green mutant. J. Exp. Bot. 2013, 64, 1509–1520. [Google Scholar] [CrossRef] [Green Version]
- Standfuss, J.; Terwisscha van Scheltinga, A.C.; Lamborghini, M.; Kühlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 2005, 24, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Morita, R.; Nishimura, M.; Yamaguchi, H.; Kusaba, M. Mendel’s green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 14169–14174. [Google Scholar] [CrossRef] [Green Version]
- Cackett, L.; Luginbuehl, L.H.; Schreier, T.B.; Lopez-Juez, E.; Hibberd, J.M. Chloroplast development in green plant tissues: The interplay between light, hormone, and transcriptional regulation. New Phytol. 2022, 233, 2000–2016. [Google Scholar] [CrossRef]
- Jiang, X.J.; Chen, C.; Zhu, X.; Zakari, S.; Singh, A.K.; Zhang, W.; Zeng, H.; Yuan, Z.Q.; He, C.; Yu, S.; et al. Use of dye infiltration experiments and HYDRUS-3D to interpret preferential flow in soil in a rubber-based agroforestry systems in Xishuangbanna, China. Catena 2019, 178, 120–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Jiang, J.; Zhang, J.; Zhang, Z.; Zhang, M. Application of soil quality index to determine the effects of different vegetation types on soil quality in the Yellow River Delta wetland. Ecol. Indic. 2022, 141, 109116. [Google Scholar] [CrossRef]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R. Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regul. 1994, 14, 173–181. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Xu, W.; Rosenow. D.T.; Nguyen, H.T. Stay green trait in grain sorghum: Relationship between visual rating and leaf chlorophyll concentration. Plant Breed 2000, 119, 365–367. [Google Scholar] [CrossRef]
- Silva, S.A.; Carvallo, F.I.F.; Caetano, V.R.; Oliveira, A.C.; Coimbra, J.L.M.; Vasconcellos, N.J.S.; Lorencetti, C. Genetic basis of stay green trait. J. New Seeds 2000, 2, 55–68. [Google Scholar] [CrossRef]
- Kianersi, F.; Azarm, D.A.; Fatemi, F.; Pour-Aboughadareh, A.; Poczai, P. Methyl jasmonate induces genes involved in linalool accumulation and increases the content of phenolics in two Iranian coriander (Coriandrum sativum L.) Ecotypes. Genes 2022, 13, 1717. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Kianersi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Rasheed, F. Identification and tissue-specific expression of rutin biosynthetic pathway genes in Capparis spinosa elicited with salicylic acid and methyl jasmonate. Sci. Rep. 2020, 10, 8884. [Google Scholar] [CrossRef]
- Siddique, K.; Regan, K.; Tennant, D.; Thomson, B. Water use and water use efficiency of cool season grain legumes in low rainfall Mediterranean-type environments. Eur. J. Agron. 2001, 15, 267–280. [Google Scholar] [CrossRef]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of aquaporins in leaf physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeselmani, M.; Abdullah, F.; Hareri, F.; Naaesan, M.; Ammar, M.A.; ZuherKanbar, O. Effect of drought on different physiological characters and yield component in different varieties of Syrian durum wheat. J. Agric. Sci. 2011, 3, 127. [Google Scholar] [CrossRef] [Green Version]
- Kocheva, K.V.; Petrov, P.I.; Georgiev, G.I. Physiological and anatomical responses of wheat to induced dehydration and rehydration. Central Eur. J. Biol. 2013, 8, 499–503. [Google Scholar] [CrossRef] [Green Version]
- ElBasyoni, I.; Saadalla, M.; Baenziger, S.; Bockelman, H.; Morsy, S. Cell membrane stability and association mapping for drought and heat tolerance in a worldwide wheat collection. Sustainability 2017, 9, 1606. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, S. Membrane fluidity and compositional changes in response to high temperature stress in wheat. In Physiological, Molecular, and Genetic Perspectives of Wheat Improvement; Springer: Berlin/Heidelberg, Germany, 2021; pp. 115–123. [Google Scholar]
- Sayar, R.; Khemira, H.; Kameli, A.; Mosbahi, M. Physiological tests as predictive appreciation for drought tolerance in durum wheat (Triticum durum Desf.). Agronomy Res. 2008, 6, 79–90. [Google Scholar]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef]
- Ali, A.; Arshad, M.; Naqvi, S.M.; Ahmad, M.; Sher, H.; Fatima, S.; Kazi, A.G.; Rasheed, A.; Mujeeb-Kazi, A. Exploitation of synthetic-derived wheats through osmotic stress responses for drought tolerance improvement. Acta Physiol. Plant. 2014, 36, 2453–2465. [Google Scholar] [CrossRef]
- Chun, S.C.; Paramasivan, M.; Chandrasekaran, M. Proline accumulation influenced by osmotic stress in arbuscular mycorrhizal symbiotic plants. Front. Microbiol. 2018, 9, 2525. [Google Scholar] [CrossRef] [Green Version]
- Petrov, P.; Petrova, A.; Dimitrov, I.; Tashev, T.; Olsovska, K.; Brestic, M.; Misheva, S. Relationships between leaf morpho-anatomy, water status and cell membrane stability in leaves of wheat seedlings subjected to severe soil drought. J. Agron. Crop Sci. 2018, 204, 219–227. [Google Scholar] [CrossRef]
- Haq, I.; Binjawhar, D.N.; Ullah, Z.; Ali, A.; Sher, H.; Ali, I. Wild vicia species possess a drought tolerance system for faba bean improvement. Genes 2022, 13, 1877. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Dagaonkar, V.S.; Phalak, M.S.; Umalkar, G.; Aurangabadkar, L.P. Alterations in photosynthetic pigments, protein and osmotic components in cotton genotypes subjected to short-term drought stress followed by recovery. Plant Biotechnol. Rep. 2007, 1, 37–48. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Impact of exogenously applied ABA on proline metabolism conferring drought and salinity stress tolerance in wheat genotypes. Cereal Res. Commun. 2020, 48, 309–315. [Google Scholar] [CrossRef]
- Wasaya, A.; Manzoor, S.; Yasir, T.A.; Sarwar, N.; Mubeen, K.; Ismail, I.A.; Raza, A.; Rehman, A.; Hossain, A.; EL Sabagh, A. Evaluation of fourteen bread wheat (Triticum aestivum L.) genotypes by observing gas exchange parameters, relative water and chlorophyll content, and yield attributes under drought stress. Sustainability 2021, 13, 4799. [Google Scholar] [CrossRef]
- Khayatnezhad, M.; Gholamin, R. The effect of drought stress on the superoxide dismutase and Chlorophyll content in durum wheat genotypes. Adv. Life Sci. 2021, 8, 119–123. [Google Scholar]
- Arjenaki, F.G.; Jabbari, R.; Morshedi, A. Evaluation of drought stress on relative water content, chlorophyll content and mineral elements of wheat (Triticum aestivum L.) varieties. Int. J. Agric. Crop Sci. 2012, 4, 726–729. [Google Scholar]
- Kumar, S.; Sairam, R.; Prabhu, K. Physiological traits for high temperature stress tolerance in Brassica juncea. Indian J. Plant Physiol. 2013, 18, 89–93. [Google Scholar] [CrossRef]
- Saeidi, M.; Ardalani, S.; Jalali-Honarmand, S.; Ghobadi, M.E.; Abdoli, M. Evaluation of drought stress at vegetative growth stage on the grain yield formation and some physiological traits as well as fluorescence parameters of different bread wheat cultivars. Acta Biol. Szeged. 2015, 59, 35–44. [Google Scholar]
- Hussain, S.; Ulhassan, Z.; Brestic, M.; Zivcak, M.; Zhou, W.; Allakhverdiev, S.I.; Yang, X.; Safdar, M.E.; Yang, W.; Liu, W. Photosynthesis research under climate change. Photosynth. Res. 2021, 150, 5–19. [Google Scholar] [CrossRef]
- Ali, I.; Sardar, Z.; Rasheed, A.; Mahmood, T. Molecular characterization of the puroindoline-a and b alleles in synthetic hexaploid wheats and in silico functional and structural insights into Pina-D1. J. Theor. Biol. 2015, 376, 1–7. [Google Scholar] [CrossRef]
- Gul, A.; Rasheed, A.; Afzal, F.; Napar, A.A.; Ali, A.; Jamil, M.; Khalid, M.; Bux, H.; Mujeeb-Kazi, A. Characterization of synthetic hexaploids derived from same Aegilops tauschii accessions and different durum cultivars. Cytologia 2015, 80, 427–440. [Google Scholar] [CrossRef] [Green Version]
- Kusaba, M.; Tanaka, A.; Tanaka, R. Stay-green plants: What do they tell us about the molecular mechanism of leaf senescence. Photosynth. Res. 2013, 117, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Gong, J.; Wang, G.; Wang, G.; Fan, Z.; Wang, W. Improved drought resistance in a wheat stay-green mutant tasg1 under field conditions. Biol. Plant. 2012, 56, 509–515. [Google Scholar] [CrossRef]
- Brestic, M.; Zivcak, M.; Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I. Photosystem II thermostability in situ: Environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol. Biochem. 2012, 57, 93–105. [Google Scholar] [CrossRef]
- Brestic, M.; Yang, X.; Li, X.; Allakhverdiev, S.I. Crop photosynthesis for the 21st century. Photosynth. Res. 2021, 150, 1–3. [Google Scholar] [CrossRef]
- Zhao, X.X.; Liang, C.; Fang, Y.; Wang, Y.Q.; Wang, W. Effect of glycinebetaine on function of thylakoid membranes in wheat flag leaves under drought stress. Biol. Plant. 2007, 51, 584–588. [Google Scholar] [CrossRef]
- Oksman-Caldentey, K.M.; Saito, K. Integrating genomics and metabolomics for engineering plant metabolic pathways. Curr. Opi. Biotechnol. 2005, 16, 174–179. [Google Scholar] [CrossRef]
- Reinders, J.; Sickmann, A. Modificomics: Posttranslational modifications beyond protein phosphorylation and glycosylation. Biomol. Eng. 2007, 24, 169–177. [Google Scholar] [CrossRef]

| Variables | Mean | SD | Variance | SE Mean | C. V. | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| Membrane Stability Index (%) | |||||||
| 100% FC | 55.23 | 3.55 | 12.62 | 1.03 | 6.43 | 49.37 | 59.38 |
| 80% FC | 60.69 | 1.98 | 3.91 | 0.57 | 3.26 | 57.21 | 62.83 |
| 60% FC | 54.11 | 5.01 | 25.12 | 1.45 | 9.26 | 43.94 | 62.26 |
| Proline Content (µmol/g) | |||||||
| 100% FC | 0.83 | 0.09 | 7.95 | 0.03 | 10.77 | 0.64 | 0.93 |
| 80% FC | 1.43 | 0.24 | 0.06 | 0.07 | 16.87 | 1.01 | 1.68 |
| 60% FC | 2.51 | 0.47 | 0.22 | 0.14 | 18.81 | 1.71 | 3.19 |
| Relative Water Content (%) | |||||||
| 100% FC | 94.58 | 2.51 | 6.28 | 0.72 | 2.65 | 91.25 | 98.32 |
| 80% FC | 81.89 | 2.37 | 5.69 | 0.69 | 2.91 | 77.63 | 86.61 |
| 60% FC | 73.66 | 2.87 | 8.24 | 0.83 | 3.88 | 70.34 | 79.34 |
| Chlorophyll a (mg/g) | |||||||
| 100% FC | 1.19 | 0.04 | 1.52 | 0.01 | 3.28 | 1.12 | 1.26 |
| 80% FC | 1.04 | 0.04 | 1.91 | 0.01 | 4.19 | 0.99 | 1.14 |
| 60% FC | 0.54 | 0.04 | 1.38 | 0.01 | 6.85 | 0.47 | 0.59 |
| Chlorophyll b (mg/g) | |||||||
| 100% FC | 1.11 | 0.17 | 0.03 | 0.05 | 15.72 | 0.85 | 1.34 |
| 80% FC | 0.93 | 0.16 | 0.02 | 0.04 | 16.79 | 0.72 | 1.18 |
| 60% FC | 0.56 | 0.04 | 1.24 | 0.01 | 6.29 | 0.52 | 0.62 |
| Total Chlorophyll (mg/g) | |||||||
| 100% FC | 2.29 | 0.18 | 0.03 | 0.052 | 7.84 | 2.04 | 2.57 |
| 80% FC | 1.97 | 0.14 | 0.02 | 0.041 | 0.04 | 1.79 | 2.20 |
| 60% FC | 1.10 | 0.05 | 2.85 | 0.015 | 4.85 | 0.99 | 1.20 |
| SOV | DF | MSI | Proline | RWC | Chla | Chlb | Total Chl |
|---|---|---|---|---|---|---|---|
| Treatments | 2 | 148.9 *** | 8.692 *** | 1333 *** | 1.371 *** | 0.938 *** | 4.563 *** |
| Wheats | 3 | 21.82 NS | 0.244 *** | 7.35 NS | 0.004 ** | 0.120 *** | 0.106 *** |
| Treatments * Wheats | 6 | 20.07 NS | 0.323 *** | 7.38 NS | 0.004 *** | 0.033 *** | 0.031 *** |
| Error | 22 | 11.980 | 0.022 | 7.380 | 0.001 | 0.002 | 0.031 |
| Treatment | Wheat | MSI | Proline | RWC | Chla | Chlb | TChl |
|---|---|---|---|---|---|---|---|
| 100% FC | SD-28 | 58.176 ab | 0.872 h | 93.701 a | 1.200 ab | 0.914 cd | 2.115 b |
| 100% FC | SD-32 | 50.679 c | 0.864 h | 96.477 a | 1.168 bc | 0.985 c | 2.153 b |
| 100% FC | Opata | 53.637 bc | 0.857 h | 93.010 a | 1.144 c | 1.285 a | 2.431 a |
| 100% FC | Chirya-1 | 58.415 ab | 0.719 h | 95.131 a | 1.230 a | 1.247 a | 2.477 a |
| 80% FC | SD-28 | 62.685 a | 1.148 g | 82.303 b | 1.017 e | 0.850 de | 1.869 c |
| 80% FC | SD-32 | 62.047 a | 1.556 ef | 80.193 bc | 1.099 d | 0.777 e | 1.876 c |
| 80% FC | Opata | 59.268 ab | 1.358 fg | 82.175 b | 1.034 e | 0.917 cd | 1.952 c |
| 80% FC | Chirya-1 | 58.764 ab | 1.667 e | 82.876 b | 1.014 e | 1.159 b | 2.173 b |
| 60% FC | SD-28 | 55.966 bc | 2.272 c | 73.396 de | 0.576 f | 0.562 f | 1.115 d |
| 60% FC | SD-32 | 53.740 bc | 1.971 d | 71.46 e | 0.547 f | 0.542 f | 1.090 d |
| 60% FC | Opata | 54.707 bc | 3.091 a | 73.659 de | 0.492 g | 0.562 f | 1.055 d |
| 60% FC | Chirya-1 | 52.009 c | 2.698 b | 76.111 cd | 0.550 f | 0.592 f | 1.143 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zada, A.; Ali, A.; Binjawhar, D.N.; Abdel-Hameed, U.K.; Shah, A.H.; Gill, S.M.; Hussain, I.; Abbas, Z.; Ullah, Z.; Sher, H.; et al. Molecular and Physiological Evaluation of Bread Wheat (Triticum aestivum L.) Genotypes for Stay Green under Drought Stress. Genes 2022, 13, 2261. https://doi.org/10.3390/genes13122261
Zada A, Ali A, Binjawhar DN, Abdel-Hameed UK, Shah AH, Gill SM, Hussain I, Abbas Z, Ullah Z, Sher H, et al. Molecular and Physiological Evaluation of Bread Wheat (Triticum aestivum L.) Genotypes for Stay Green under Drought Stress. Genes. 2022; 13(12):2261. https://doi.org/10.3390/genes13122261
Chicago/Turabian StyleZada, Ahmad, Ahmad Ali, Dalal Nasser Binjawhar, Usama K. Abdel-Hameed, Azhar Hussain Shah, Shahid Maqsood Gill, Irtiza Hussain, Zaigham Abbas, Zahid Ullah, Hassan Sher, and et al. 2022. "Molecular and Physiological Evaluation of Bread Wheat (Triticum aestivum L.) Genotypes for Stay Green under Drought Stress" Genes 13, no. 12: 2261. https://doi.org/10.3390/genes13122261








