The Roles of SNHG Family in Osteoblast Differentiation
Abstract
:1. Introduction
2. SNHGs Display Aberrant Expression in Osteoblast Differentiation
3. The Mechanism of SNHGs Regulating Osteoblast Differentiation
3.1. SNHG1
3.2. GAS5 (Alias SNHG2)
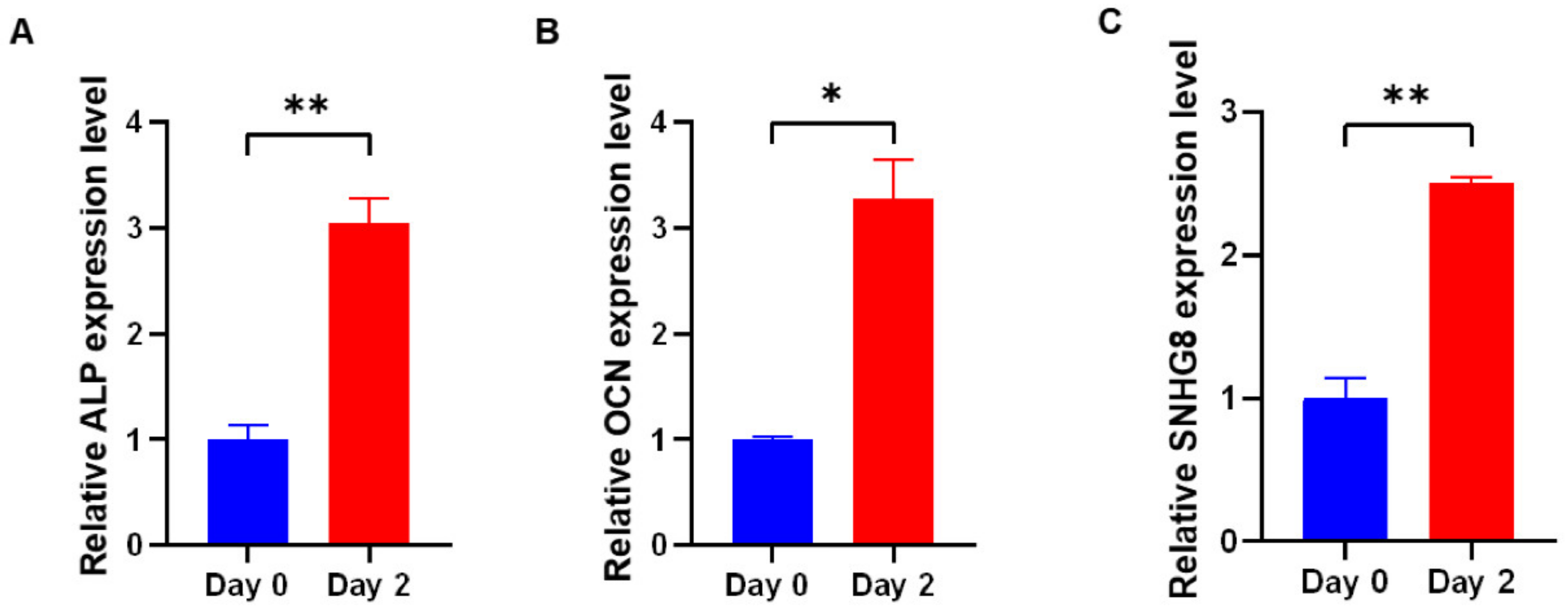
3.3. SNHG5
3.4. SNHG7
3.5. DANCR (Alias SNHG13)
3.6. SNHG14
4. Prospect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shibli, J.A.; Nagay, B.E.; Suárez, L.J.; Urdániga Hung, C.; Bertolini, M.; Barão, V.A.R.; Souza, J.G.S. Bone Tissue Engineering Using Osteogenic Cells: From the Bench to the Clinical Application. Tissue Eng. Part C Methods 2022, 28, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, G.; Moncrieff, L.; Dompe, C.; Janowicz, K.; Sibiak, R.; Bryja, A.; Jankowski, M.; Mozdziak, P.; Bukowska, D.; Antosik, P.; et al. Bone Regeneration, Reconstruction and Use of Osteogenic Cells; from Basic Knowledge, Animal Models to Clinical Trials. J. Clin. Med. 2020, 9, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, P.; Ghosh, S.; Wang, B.; Heyns, M.; Graham, K.; Mackey, J.R.; Kovalchuk, O.; Damaraju, S. Profiling of Small Nucleolar RNAs by Next Generation Sequencing: Potential New Players for Breast Cancer Prognosis. PLoS ONE 2016, 11, e0162622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, J.; Li, Y.; Liu, C.; Xiang, Y.; Li, C.; Ye, Y.; Zhang, Z.; Hawke, D.H.; Park, P.K.; Diao, L.; et al. A Pan-Cancer Analysis of the Expression and Clinical Relevance of Small Nucleolar RNAs in Human Cancer. Cell Rep. 2017, 21, 1968–1981. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Sun, W.; Wang, Z.; Dong, W.; He, L.; Zhang, T.; Zhang, H. Long Non-Coding Small Nucleolar RNA Host Genes (SNHGs) in Endocrine-Related Cancers. Onco. Targets Ther. 2020, 13, 7699–7717. [Google Scholar] [CrossRef]
- Yu, X.; Rong, P.-Z.; Song, M.-S.; Shi, Z.-W.; Feng, G.; Chen, X.-J.; Shi, L.; Wang, C.-H.; Pang, Q.-J. LncRNA SNHG1 Induced by SP1 Regulates Bone Remodeling and Angiogenesis via Sponging MiR-181c-5p and Modulating SFRP1/Wnt Signaling Pathway. Mol. Med. 2021, 27, 141. [Google Scholar] [CrossRef]
- Du, M.; Wu, B.; Fan, S.; Liu, Y.; Ma, X.; Fu, X. SNHG14 Induces Osteogenic Differentiation of Human Stromal (Mesenchymal) Stem Cells in Vitro by Downregulating MiR-2861. BMC Musculoskelet. Disord. 2020, 21, 525. [Google Scholar] [CrossRef]
- Tong, X.; Gu, P.; Xu, S.; Lin, X. Long Non-Coding RNA-DANCR in Human Circulating Monocytes: A Potential Biomarker Associated with Postmenopausal Osteoporosis. Biosci. Biotechnol. Biochem. 2015, 79, 732–737. [Google Scholar] [CrossRef]
- Wang, C.-G.; Hu, Y.-H.; Su, S.-L.; Zhong, D. LncRNA DANCR and MiR-320a Suppressed Osteogenic Differentiation in Osteoporosis by Directly Inhibiting the Wnt/β-Catenin Signaling Pathway. Exp. Mol. Med. 2020, 52, 1310–1325. [Google Scholar] [CrossRef]
- Centofanti, F.; Santoro, M.; Marini, M.; Visconti, V.V.; Rinaldi, A.M.; Celi, M.; D’Arcangelo, G.; Novelli, G.; Orlandi, A.; Tancredi, V.; et al. Identification of Aberrantly-Expressed Long Non-Coding RNAs in Osteoblastic Cells from Osteoporotic Patients. Biomedicines 2020, 8, 65. [Google Scholar] [CrossRef]
- Zheng, J.; Guo, H.; Qin, Y.; Liu, Z.; Ding, Z.; Zhang, L.; Wang, W. SNHG5/MiR-582-5p/RUNX3 Feedback Loop Regulates Osteogenic Differentiation and Apoptosis of Bone Marrow Mesenchymal Stem Cells. J. Cell. Physiol. 2020. Published online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. SNHG7 Promotes the Osteo/Dentinogenic Differentiation Ability of Human Dental Pulp Stem Cells by Interacting with Hsa-MiR-6512–3p in an Inflammatory Microenvironment. Biochem. Biophys. Res. Commun. 2021, 581, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Fu, H.; Xu, Z.; Fan, W.; Liu, F.; Chen, B. LncRNA SNHG1 Attenuates Osteogenic Differentiation via the MiR-101/DKK1 Axis in Bone Marrow Mesenchymal Stem Cells. Mol. Med. Rep. 2020, 22, 3715–3722. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, F.; Liang, Y.; Shen, M.; Chen, N. Human Amnion-Derived Mesenchymal Stem Cells Promote Osteogenic Differentiation in Human Bone Marrow Mesenchymal Stem Cells by Influencing the ERK1/2 Signaling Pathway. Stem Cells Int. 2016, 2016, 4851081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Miao, J.; Meng, X.; Chen, N.; Wang, Y. Expression of Long Non-Coding RNAs in Human Bone Marrow Mesenchymal Stem Cells Co-Cultured with Human Amnion-Derived Mesenchymal Stem Cells. Mol. Med. Rep. 2017, 16, 6683–6689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, W.; Di, S.; Xing, S.; Sun, Z.; Shen, Z.; Dou, X.; He, S.; Tang, H.; Min, J. Long Non-Coding RNA DANCR Modulates Osteogenic Differentiation by Regulating the MiR-1301-3p/PROX1 Axis. Mol. Cell Biochem. 2021, 476, 2503–2512. [Google Scholar] [CrossRef]
- Yang, Q.; Han, Y.; Liu, P.; Huang, Y.; Li, X.; Jia, L.; Zheng, Y.; Li, W. Long Noncoding RNA GAS5 Promotes Osteogenic Differentiation of Human Periodontal Ligament Stem Cells by Regulating GDF5 and P38/JNK Signaling Pathway. Front. Pharmacol. 2020, 11, 701. [Google Scholar] [CrossRef]
- Han, Y.; Yang, Q.; Huang, Y.; Jia, L.; Zheng, Y.; Li, W. Long Non-Coding RNA SNHG5 Promotes the Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells via the MiR-212-3p/GDF5/SMAD Pathway. Stem. Cell Res. Ther. 2022, 13, 130. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, D.; Zhu, Y.; Dong, Y.; Liu, Y. Long Non-Coding RNA GAS5 Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Regulating the MiR-135a-5p/FOXO1 Pathway. Mol. Cell. Endocrinol. 2019, 496, 110534. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, H.; Song, Z.; Yang, Y.; Zhang, S.; Wang, W.; Zhang, S. Long Noncoding RNA GAS5 Inhibits Osteogenic Differentiation through MicroRNA 382-3p/TAF1 Signaling. Mol. Cell Biol. 2022, 42, e00541-20. [Google Scholar] [CrossRef]
- Tian, M.; Gong, W.; Guo, J. Long Non-Coding RNA SNHG1 Indicates Poor Prognosis and Facilitates Disease Progression in Acute Myeloid Leukemia. Biol. Open 2019, 8, bio046417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long Non-Coding RNA SNHG1 Activates Glycolysis to Promote Hepatocellular Cancer Progression through the miR-326/PKM2 Axis. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/jgm.3440 (accessed on 31 August 2022).
- Ma, J.; Lin, X.; Chen, C.; Li, S.; Zhang, S.; Chen, Z.; Li, D.; Zhao, F.; Yang, C.; Yin, C.; et al. Circulating MiR-181c-5p and MiR-497-5p Are Potential Biomarkers for Prognosis and Diagnosis of Osteoporosis. J. Clin. Endocrinol. Metab. 2020, 105, 1445–1460. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, W.; Jiao, G.; Chen, Y.; Liu, H. LncRNA SNHG1 Modulates P38 MAPK Pathway through Nedd4 and Thus Inhibits Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells. Life Sci. 2019, 228, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S.K.; Xiao, K.; Venkataramanan, V.; Snyder, P.M.; Freedman, N.J.; Weissman, A.M. Nedd4 Mediates Agonist-Dependent Ubiquitination, Lysosomal Targeting, and Degradation of the Β2-Adrenergic Receptor. J. Biol. Chem. 2008, 283, 22166–22176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, A.; Hou, Q.; Jarzylo, L.; Amato, S.; Gilbert, J.; Shang, F.; Man, H.-Y. Nedd4-Mediated AMPA Receptor Ubiquitination Regulates Receptor Turnover and Trafficking. J. Neurochem. 2011, 119, 27–39. [Google Scholar] [CrossRef]
- Grimsey, N.J.; Narala, R.; Rada, C.C.; Mehta, S.; Stephens, B.S.; Kufareva, I.; Lapek, J.; Gonzalez, D.J.; Handel, T.M.; Zhang, J.; et al. A Tyrosine Switch on NEDD4-2 E3 Ligase Transmits GPCR Inflammatory Signaling. Cell Rep. 2018, 24, 3312–3323.e5. [Google Scholar] [CrossRef] [Green Version]
- Robaszkiewicz, A.; Valkó, Z.; Kovács, K.; Hegedűs, C.; Bakondi, E.; Bai, P.; Virág, L. The Role of P38 Signaling and Poly(ADP-Ribosyl)Ation-Induced Metabolic Collapse in the Osteogenic Differentiation-Coupled Cell Death Pathway. Free Radic. Biol. Med. 2014, 76, 69–79. [Google Scholar] [CrossRef] [Green Version]
- Ba, P.; Duan, X.; Fu, G.; Lv, S.; Yang, P.; Sun, Q. Differential Effects of P38 and Erk1/2 on the Chondrogenic and Osteogenic Differentiation of Dental Pulp Stem Cells. Mol. Med. Rep. 2017, 16, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Yavropoulou, M.; Yovos, J. The Role of the Wnt Signaling Pathway in Osteoblast Commitment and Differentiation. Hormones 2007, 6, 279–294. [Google Scholar] [CrossRef]
- Albers, J.; Keller, J.; Baranowsky, A.; Beil, F.T.; Catala-Lehnen, P.; Schulze, J.; Amling, M.; Schinke, T. Canonical Wnt Signaling Inhibits Osteoclastogenesis Independent of Osteoprotegerin. J. Cell Biol. 2013, 200, 537–549. [Google Scholar] [CrossRef]
- Li, Y.; Lu, W.; King, T.D.; Liu, C.-C.; Bijur, G.N.; Bu, G. Dkk1 Stabilizes Wnt Co-Receptor LRP6: Implication for Wnt Ligand-Induced LRP6 Down-Regulation. PLoS ONE 2010, 5, e11014. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, J.; Han, X.; Wang, G. LncRNA SNHG1 Delayed Fracture Healing via Modulating MiR-181a-5p/PTEN Axis. J. Investig. Surg. 2022, 35, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; King, R.M.; Philipson, L. Genes Specifically Expressed at Growth Arrest of Mammalian Cells. Cell 1988, 54, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Park, S.J.; Jung, S.-H.; Kim, E.J.; Jogeswar, G.; Ajita, J.; Rhee, Y.; Kim, C.-H.; Lim, S.-K. MiR-182 Is a Negative Regulator of Osteoblast Proliferation, Differentiation, and Skeletogenesis through Targeting FoxO1. J. Bone Miner. Res. 2012, 27, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Miyazono, K. Regulation of TGF-β Family Signaling by Inhibitory Smads. Cold Spring Harb. Perspect. Biol. 2017, 9, a022095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Xie, Z.; Li, J.; Lin, J.; Zheng, G.; Liu, W.; Tang, S.; Cen, S.; Ye, G.; Li, Z.; et al. GAS5 Protects against Osteoporosis by Targeting UPF1/SMAD7 Axis in Osteoblast Differentiation. eLife 2020, 9, e59079. [Google Scholar] [CrossRef]
- Cho, H.; Li, Y.; Archacki, S.; Wang, F.; Yu, G.; Chakrabarti, S.; Guo, Y.; Chen, Q.; Wang, Q.K. Splice Variants of LncRNA RNA ANRIL Exert Opposing Effects on Endothelial Cell Activities Associated with Coronary Artery Disease. RNA Biol. 2020, 17, 1391–1401. [Google Scholar] [CrossRef]
- Wu, S.; Nitschke, K.; Worst, T.S.; Fierek, A.; Weis, C.-A.; Eckstein, M.; Porubsky, S.; Kriegmair, M.; Erben, P. Long Noncoding RNA MIR31HG and Its Splice Variants Regulate Proliferation and Migration: Prognostic Implications for Muscle Invasive Bladder Cancer. J. Exp. Clin. Cancer Res. 2020, 39, 288. [Google Scholar] [CrossRef]
- Gao, J.; Zeng, K.; Liu, Y.; Gao, L.; Liu, L. LncRNA SNHG5 Promotes Growth and Invasion in Melanoma by Regulating the MiR-26a-5p/TRPC3 Pathway. Onco. Targets Ther. 2018, 12, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Huang, Y.; Yang, Q.; Jia, L.; Zheng, Y.; Li, W. Long Non-Coding RNA SNHG5 Mediates Periodontal Inflammation through the NF-ΚB Signalling Pathway. J. Clin. Periodontol. 2022, 49, 1038–1051. [Google Scholar] [CrossRef]
- Ota, T.; Suzuki, Y.; Nishikawa, T.; Otsuki, T.; Sugiyama, T.; Irie, R.; Wakamatsu, A.; Hayashi, K.; Sato, H.; Nagai, K.; et al. Complete Sequencing and Characterization of 21,243 Full-Length Human CDNAs. Nat. Genet. 2004, 36, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Chen, J.; Li, C.; Yuan, Y.; Fang, S.; Liu, W.; Qian, Y.; Ma, J.; Chang, L.; Chen, F.; et al. Exosome-Mediated Transfer of SNHG7 Enhances Docetaxel Resistance in Lung Adenocarcinoma. Cancer Lett. 2022, 526, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Janssens, K.; ten Dijke, P.; Ralston, S.H.; Bergmann, C.; Van Hul, W. Transforming Growth Factor-Β1 Mutations in Camurati-Engelmann Disease Lead to Increased Signaling by Altering Either Activation or Secretion of the Mutant Protein. J. Biol. Chem. 2003, 278, 7718–7724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Liu, Z.; Shen, L.; Jiang, H. Long non-coding RNA SNHG7 promotes the fracture repair through negative modulation of miR-9. Am. J. Transl. Res. 2019, 11, 974–982. [Google Scholar] [PubMed]
- Differential Expression of lncRNA/miRNA/mRNA and their Related Functional Networks during the Osteogenic/Odontogenic Differentiation of Dental Pulp Stem Cells. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/jcp.29223 (accessed on 2 August 2022).
- Yang, Z.; Feng, L.; Wang, H.; Li, Y.; Lo, J.H.T.; Zhang, X.; Lu, X.; Wang, Y.; Lin, S.; Tortorella, M.D.; et al. DANCR Mediates the Rescuing Effects of Sesamin on Postmenopausal Osteoporosis Treatment via Orchestrating Osteogenesis and Osteoclastogenesis. Nutrients 2021, 13, 4455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, M.; Liang, L.; Li, J.; Chen, Y.-X. Over-Expression of LncRNA DANCR Is Associated with Advanced Tumor Progression and Poor Prognosis in Patients with Colorectal Cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 11480–11484. [Google Scholar]
- Zhang, L.; Yang, C.; Chen, S.; Wang, G.; Shi, B.; Tao, X.; Zhou, L.; Zhao, J. Long Noncoding RNA DANCR Is a Positive Regulator of Proliferation and Chondrogenic Differentiation in Human Synovium-Derived Stem Cells. DNA Cell Biol. 2017, 36, 136–142. [Google Scholar] [CrossRef]
- Tang, Z.; Gong, Z.; Sun, X. LncRNA DANCR Involved Osteolysis after Total Hip Arthroplasty by Regulating FOXO1 Expression to Inhibit Osteoblast Differentiation. J. Biomed. Sci. 2018, 25, 4. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yin, Y.; Jiang, F.; Chen, N. Human Amnion Mesenchymal Stem Cells Promote Proliferation and Osteogenic Differentiation in Human Bone Marrow Mesenchymal Stem Cells. J. Mol. Hist. 2015, 46, 13–20. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Miao, Y.X.; Hirokazu, T.; Zhu, S.Z.; Lu, J.S. Effects of lncRNA DANCR on proliferation and differentiation of osteoblasts by regulating the Wnt/β-catenin pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5558–5566. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Q.; Han, Y.; Liu, H.; Wang, Y.; Huang, Y.; Zheng, Y.; Li, W. A Reduced Level of the Long Non-Coding RNA SNHG8 Activates the NF-KappaB Pathway by Releasing Functional HIF-1alpha in a Hypoxic Inflammatory Microenvironment. Stem. Cell Res. Ther 2022, 13, 229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Zhao, Z.; Han, X.; Chen, Y. Knockdown of DANCR Reduces Osteoclastogenesis and Root Resorption Induced by Compression Force via Jagged1. Cell Cycle 2019, 18, 1759–1769. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yang, L.; Wang, H.; Han, S. Silence of Long Noncoding RNA SNHG14 Alleviates Ischemia/Reperfusion-Induced Acute Kidney Injury by Regulating MiR-124-3p/MMP2 Axis. Biomed. Res. Int. 2021, 2021, 8884438. [Google Scholar] [CrossRef] [PubMed]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A CeRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paspaliaris, V.; Kolios, G. Stem Cells in Osteoporosis: From Biology to New Therapeutic Approaches. Stem Cells Int. 2019, 2019, 1730978. [Google Scholar] [CrossRef] [Green Version]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone Tissue Engineering via Growth Factor Delivery: From Scaffolds to Complex Matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]


| LncRNA | Cell | Expression Trend during Osteoblast Differentiation | References |
|---|---|---|---|
| SNHG1 | BMSC | Downregulation | [6,13] |
| SNHG2 (GAS5) | hDPSC/BMSC | Upregulation | [17,19] |
| GAS5 transcript variant 2 | hBMSC | Downregulation | [20] |
| SNHG5 | hBMSC | Upregulation | [11,18] |
| SNHG7 | hDPSC | Downregulation | [12] |
| SNHG13 (DANCR) | hBMSC (co-cultured with HAMSC) | Downregulation | [15] |
| SNHG13 (DANCR) | BMSC | Downregulation | [16] |
| LncRNA | Cell | Regulatory Axis | LncRNA’s Effect on Osteogenesis | References |
|---|---|---|---|---|
| SNHG1 | BMSC | SNHG1/miR-181c-5p/SFRP1/Wnt3a | Inhibitory | [6] |
| SNHG1 | BMSC | SNHG1/Nedd4/p38 | Inhibitory | [24] |
| SNHG1 | BMSC | SNHG1/miR101/DKK1/Wnt/ β-catenin | Inhibitory | [13] |
| SNHG1 | MC3T3-E1 | SNHG1/miR-181a- 5p/PTEN | Inhibitory | [33] |
| SNHG2 (GAS5) | hPDLSC | SNHG2/p38/JNK | Stimulative | [17] |
| SNHG2 (GAS5) | BMSC | SNHG2/miR-135a-5p/FOXO1 | Stimulative | [19] |
| SNHG2 (GAS5) | BMSC | SNHG2/UPF1/Smad7 | Stimulative | [37] |
| GAS5 transcript variant 2 | BMSC | SNHG2/miR-382-3p/TAF1 | Inhibitory | [20] |
| SNHG5 | BMSC | SNHG5/miR-582-5p/RUNX3 | Stimulative | [11] |
| SNHG5 | BMSC | YY1/SNHG5/miR-212-3p/GDF5/Smad | Stimulative | [18] |
| SNHG7 | MC3T3-E1 | SNHG7/miR-9/TGF-β | Stimulative | [45] |
| SNHG7 | hDPSC | SNHG7/miR-6512-3p/TNF-α | Stimulative | [12] |
| SNHG13 (DANCR) | MSC | SNHG13/FOXO1 | Inhibitory | [50] |
| SNHG13 (DANCR) | BMSC | SNHG13/miR-1301-3p/PROX1 | Inhibitory | [16] |
| SNHG13 (DANCR) | BMSC | SNHG13/miR-320a/CTNNB1/Wnt/β-catenin | Inhibitory | [9] |
| SNHG13 (DANCR) | MC3T3-E1 | SNHG13/Wnt/β-catenin | Inhibitory | [52] |
| SNHG14 | hMSC | SNHG14/miR-2861/AKT2 | Stimulative | [7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, A.-Q.; Zheng, Y.-F. The Roles of SNHG Family in Osteoblast Differentiation. Genes 2022, 13, 2268. https://doi.org/10.3390/genes13122268
Tan A-Q, Zheng Y-F. The Roles of SNHG Family in Osteoblast Differentiation. Genes. 2022; 13(12):2268. https://doi.org/10.3390/genes13122268
Chicago/Turabian StyleTan, An-Qi, and Yun-Fei Zheng. 2022. "The Roles of SNHG Family in Osteoblast Differentiation" Genes 13, no. 12: 2268. https://doi.org/10.3390/genes13122268





