Influence of Melatonin Treatment on Cellular Mechanisms of Redox Adaptation in K562 Erythroleukemic Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Identification of Erythroid Differentiation and Viability
2.3. Experimental Design
2.4. Administration of Melatonin (Protective Agent) and Hydrogen Peroxide (Stressing Agent)
2.5. Real-Time PCR
2.6. Statistical Analysis
3. Results
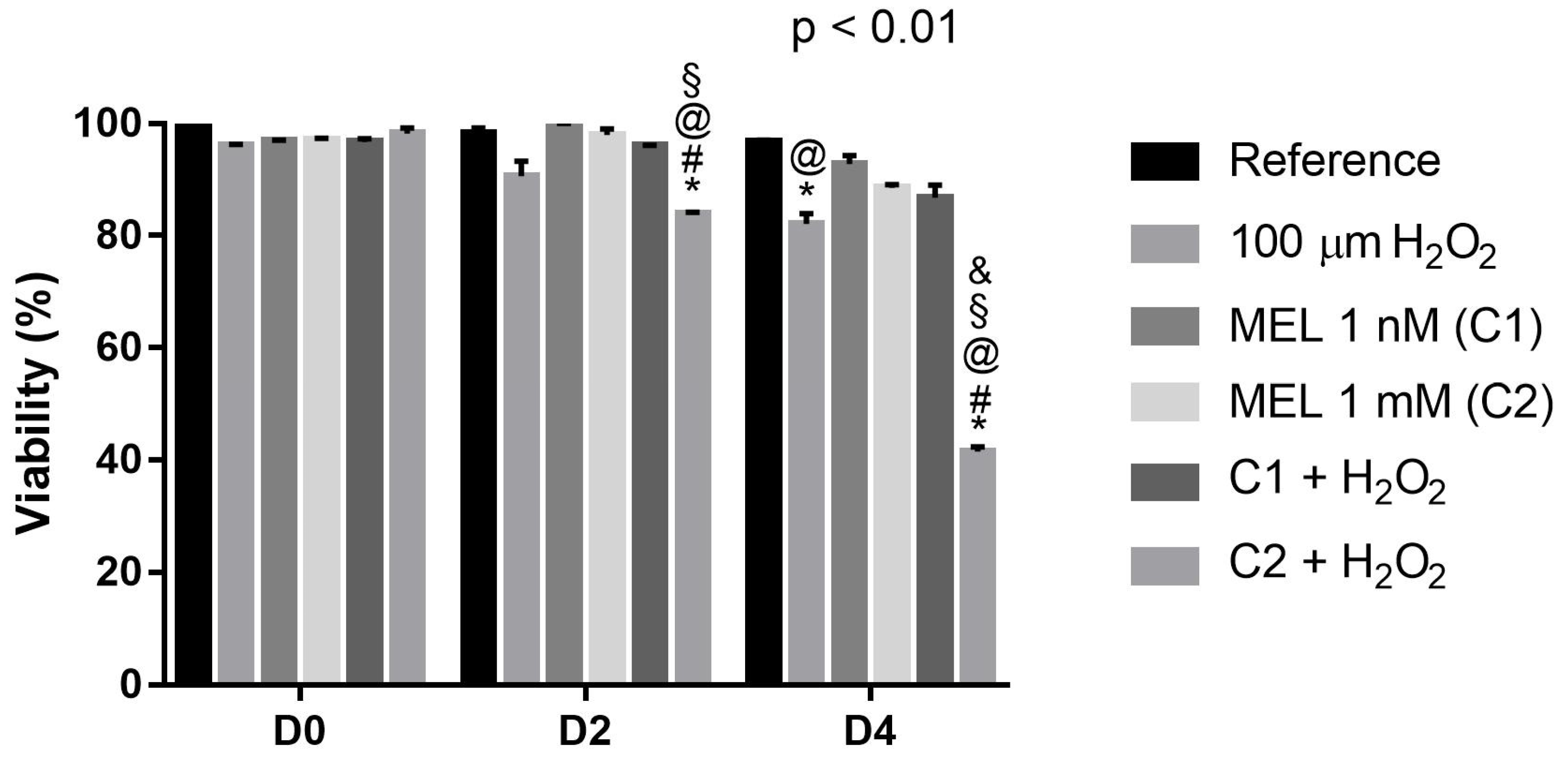
3.1. Protective Role of Melatonin on Cell Viability
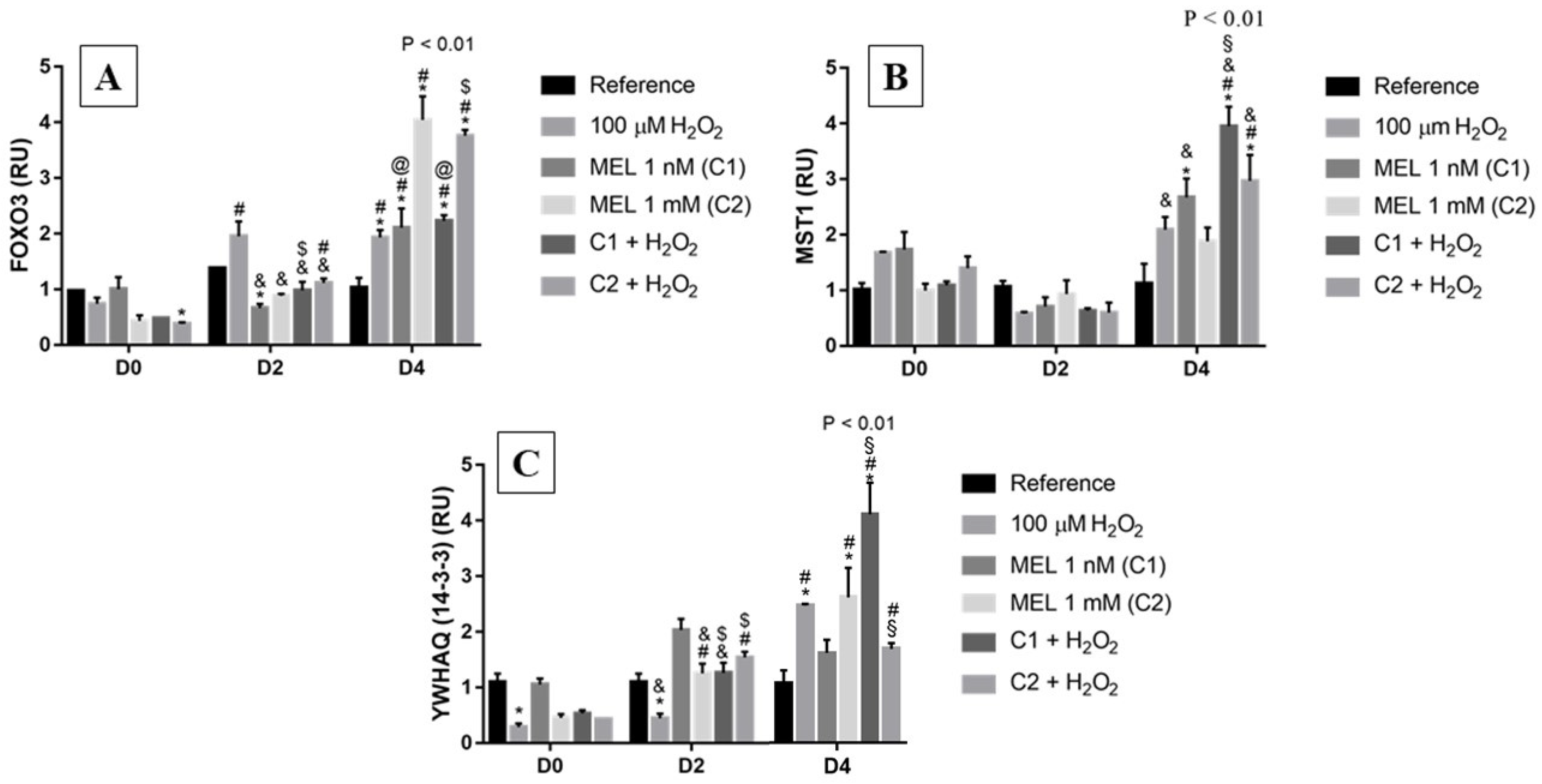
3.2. FOXO3 and Regulators of Its Subcellular Localization
3.3. Keap1/Nrf2/ARE Signaling Pathway
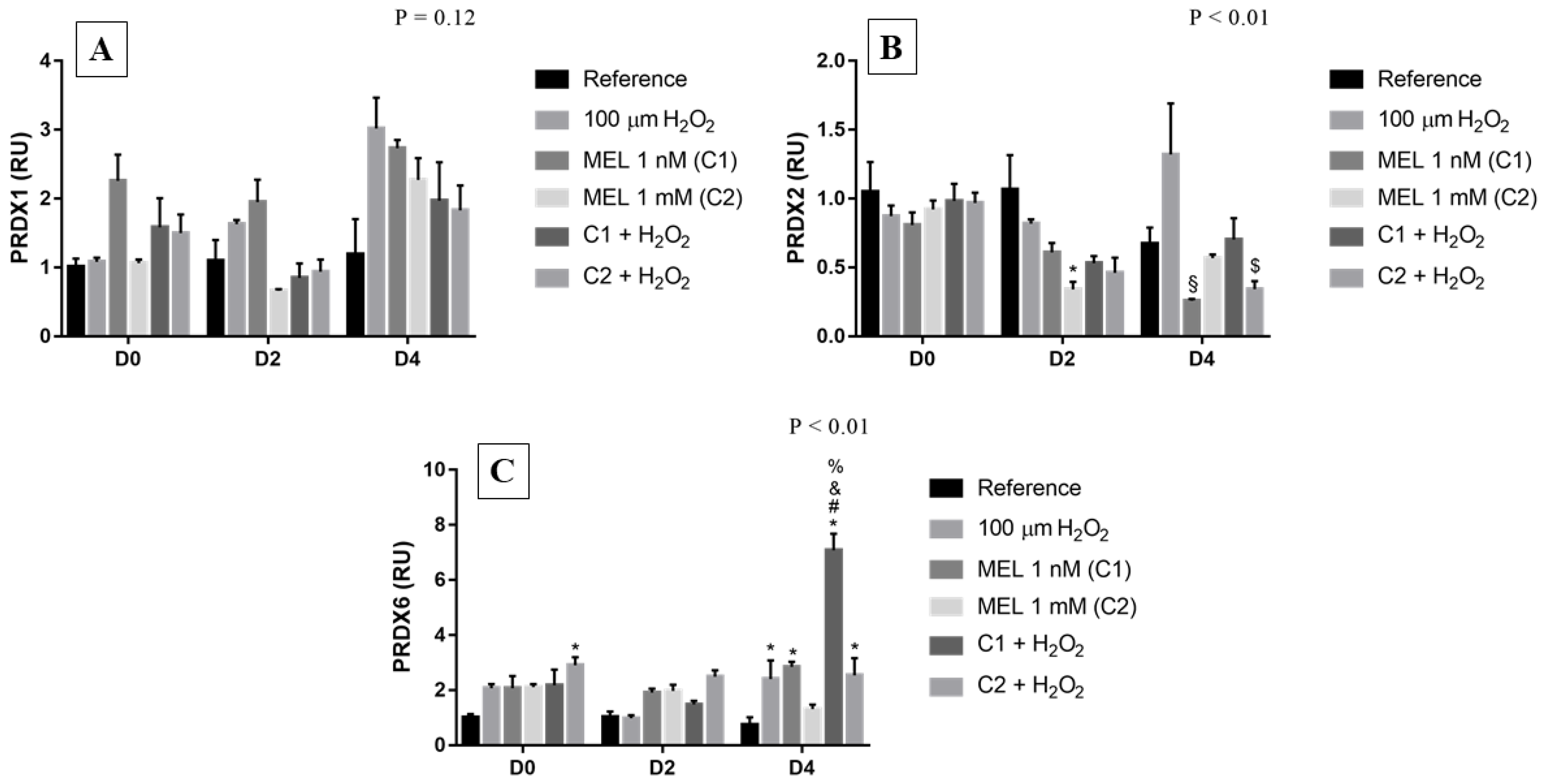
3.4. Peroxiredoxins (PRDX)
3.5. CAT, SOD1 and GPX1
3.6. Proteasome Subunits: PSMB5 and PSMB6
3.7. BIM
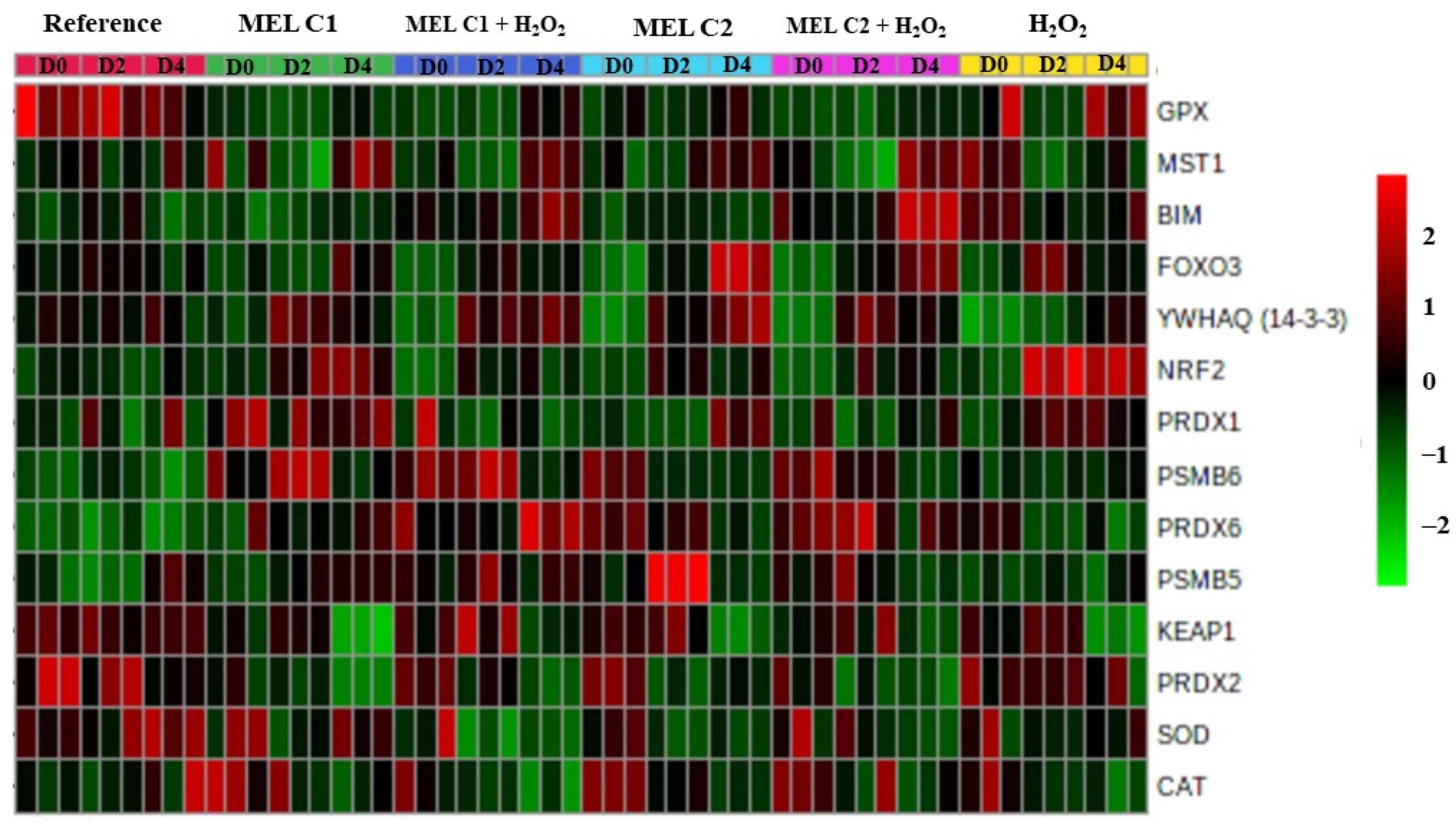
3.8. Overview of the Expression Pattern of the Genes Involved in the Redox Adaptation Mechanisms in K562 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the Balance between ROS and Antioxidants: When to Use the Synthetic Antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Sánchez-ramos, C.; Prieto-arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox Regulation of FoxO Transcription Factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Calnan, D.R. Brunet, a the FoxO Code. Oncogene 2008, 27, 2276–2288. [Google Scholar] [CrossRef] [PubMed]
- Brunet, A.; Bonni, A.; Zigmond, M.J.; Lin, M.Z.; Juo, P.; Hu, L.S.; Anderson, M.J.; Arden, K.C.; Blenis, J.; Greenberg, M.E. Akt Promotes Cell Survival by Phosphorylating and Inhibiting a Forkhead Transcription Factor. Cell 1999, 96, 857–868. [Google Scholar] [CrossRef]
- Boccitto, M.; Kalb, R.G. Regulation of Foxo-Dependent Transcription by Post-Translational Modifications. Curr. Drug Targets 2011, 12, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Tzivion, G.; Dobson, M.; Ramakrishnan, G. FoxO Transcription Factors; Regulation by AKT and 14-3-3 Proteins. BBA-Mol. Cell Res. 2011, 1813, 1938–1945. [Google Scholar] [CrossRef]
- Wang, X.; Hu, S.; Liu, L. Phosphorylation and Acetylation Modifications of FOXO3a: Independently or Synergistically? (Review). Oncol. Lett. 2017, 13, 2867–2872. [Google Scholar] [CrossRef]
- Lim, J.H.; Kim, K.; Kim, S.W.; Hwang, O.; Choi, H.J. Bromocriptine Activates NQO1 via Nrf2-PI3K/Akt Signaling: Novel Cytoprotective Mechanism against Oxidative Damage. Pharmacol. Res. 2008, 57, 325–331. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, Y.; Shao, Q.; Wang, Z.; Zhu, C.; Shi, J.; Ma, K.; Yan, X.; Chen, N. DJ-1 Regulating PI3K-Nrf2 Signaling Plays a Significant Role in Bibenzyl Compound 20C-Mediated Neuroprotection against Rotenone-induced Oxidative Insult. Toxicol. Lett. 2017, 271, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Mao, X.; Liu, S.; Chen, W.; Huang, D.; Zhang, C.; Chen, B.; Shen, X.; Yu, Z. Ginsenoside Rb1 Improves Postoperative Fatigue Syndrome by Reducing Skeletal Muscle Oxidative Stress through Activation of the PI3K / Akt / Nrf2 Pathway in Aged Rats. Eur. J. Pharmacol. 2014, 740, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.; Reiter, R.J. The Keap1-Nrf2-Antioxidant Response Element Pathway: A Review of Its Regulation by Melatonin and the Proteasome. Mol. Cell. Endocrinol. 2015, 401, 213–220. [Google Scholar] [CrossRef]
- Robledinos-antón, N.; Fernández-ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Wakabayashi, N.; Greenlaw, J.L.; Yamamoto, M.; Kensler, T.W. Antioxidants Enhance Mammalian Proteasome Expression through the Keap1-Nrf2 Signaling Pathway. Mol. Cell Biol. 2003, 23, 8786–8794. [Google Scholar] [CrossRef]
- Lee, S.; Hur, E.; Ryoo, I.; Jung, K.; Kwak, J.; Kwak, M. Involvement of the Nrf2-Proteasome Pathway in the Endoplasmic Reticulum Stress Response in Pancreatic β -Cells. Toxicol. Appl. Pharmacol. 2012, 264, 431–438. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a Natural Ally against Oxidative Stress: A Physicochemical Examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and Its Metabolites vs Oxidative Stress: From Individual Actions to Collective Protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef]
- Hardeland, R.; Pandi-Perumal, S.R. Melatonin, a Potent Agent in Antioxidative Defense: Actions as a Natural Food Constituent, Gastrointestinal Factor, Drug and Prodrug. Nutr. Metab. 2005, 2, 22. [Google Scholar] [CrossRef]
- Reiter, R.J. Melatonin: Lowering the High Price of Free Radicals. News Physiol. Sci. 2000, 15, 246–250. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Rosales-corral, S.; Galano, A.; Jou, M.; Id, D.A. Melatonin Mitigates Mitochondrial Meltdown: Interactions with SIRT3. Int. J. Mol. Sci. 2018, 19, 2439. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Currò, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Hong, S.-W.; Zheng, H.-M.; Lee, D.-H.; Hong, S.-S. Melatonin Downregulates Nuclear Erythroid 2-Related Factor 2 and Nuclear Factor-kappaB during Prevention of Oxidative Liver Injury in a Dimethylnitrosamine Model. J. Pineal Res. 2009, 47, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Kilic, U.; Kilic, E.; Tuzcu, Z.; Tuzcu, M.; Ozercan, I.H.; Yilmaz, O.; Sahin, F.; Sahin, K. Melatonin Suppresses Cisplatin-Induced Nephrotoxicity via Activation of Nrf-2 / HO-1 Pathway. Nutr. Metab. 2013, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Santofimia-castaño, P.; Ruy, D.C.; Garcia-sanchez, L.; Jimenez-blasco, D.; Fernandez-bermejo, M.; Bolaños, J.P.; Salido, G.M.; Gonzalez, A. Melatonin Induces the Expression of Nrf2-Regulated Antioxidant Enzymes via PKC and Ca2+ Influx Activation in Mouse Pancreatic Acinar Cells. Free Radic. Biol. Med. 2015, 87, 226–236. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, J.; Ji, Q.; Yu, K.; Wang, P.; Song, M.; Cao, Z.; Zhang, X.; Li, Y. Melatonin Alleviates Aluminium Chloride-Induced Immunotoxicity by Inhibiting Oxidative Stress and Apoptosis Associated with the Activation of Nrf2 Signaling Pathway. Ecotoxicol. Environ. Saf. 2019, 173, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, J.; Mi, C.; Xu, B.; Jiao, C.; Li, Y.; Xu, D.; Liu, W.; Xu, Z. Melatonin Antagonizes Mn-Induced Oxidative Injury Through the Activation of Keap1–Nrf2–ARE Signaling Pathway in the Striatum of Mice. Neurotox. Res. 2014, 27, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.; Su, X.; Hu, X.; Zhou, Y.; Huang, L.; Fan, Y.; Li, J.; Lu, L. Melatonin Improves Hypoxic-Ischemic Brain Damage through the Akt/Nrf2/Gpx4 Signaling Pathway. Brain Res. Bull. 2020, 163, 40–48. [Google Scholar] [CrossRef]
- Carbajo-Pescador, S.; Steinmetz, C.; Kashyap, A.; Lorenz, S.; Mauriz, J.L.; Heise, M.; Galle, P.R. Melatonin Induces Transcriptional Regulation of Bim by FoxO3a in HepG2 Cells. Br. J. Cancer 2013, 108, 442–449. [Google Scholar] [CrossRef]
- Jang, H.; Lee, O.H.; Lee, Y.; Yoon, H.; Chang, E.M.; Park, M.; Lee, J.W.; Hong, K.; Kim, J.O.; Kim, N.K.; et al. Melatonin Prevents Cisplatin-Induced Primordial Follicle Loss via Suppression of PTEN/AKT/FOXO3a Pathway Activation in the Mouse Ovary. J. Pineal Res. 2016, 60, 336–347. [Google Scholar] [CrossRef]
- Paula, C.P. De Efeito Da Melatonina Na Proteção Contra Estresse Oxidativo Em Células Eritrocitárias K562-Effect of Melatonin on Protection Against Oxidative Stress In K562 Erythrocyte Cells. Ph.D. Thesis, Universidade Federal de São Carlos, São Carlos, Brazil, 2020; pp. 1–131. [Google Scholar]
- Rowley, P.T.; Ohlsson-Wilhelm, B.M.; Farley, B.A.; LaBella, S. Inducers of Erythroid Differentiation in K562 Human Leukemia Cells. Exp. Hematol. 1981, 9, 32–37. [Google Scholar] [PubMed]
- Tennanth, J. Evaluation of the trypan blue technique for determination of cell viability. Transplantation 1964, 2, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real- Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Quinn, G.P.; Keough, M.J. Experimental Design and Data Analysis for Biologists; Cambridge University Press: New York, NY, USA, 2002; Volumr 4, ISBN 9780521811286. [Google Scholar]
- Xia, J.; Wishart, D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinf. 2016, 55, 1–91. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J. Knockout Mouse Models for Peroxiredoxins. Antioxidants 2020, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of Antioxidant Enzymes: A Significant Role for Melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Zapico, C.; Coto-Montes, A. Melatonin as Antioxidant Under Pathological Processes. Recent Pat. Endocr. Metab. Immune Drug Discov. 2007, 1, 63–82. [Google Scholar] [CrossRef]
- Emamgholipour, S.; Hossein-nezhad, A.; Ansari, M. Can Melatonin Act as an Antioxidant in Hydrogen Peroxide-Induced Oxidative Stress Model in Human Peripheral Blood Mononuclear Cells ? Biochem. Res. Int. 2016, 2016, 5857940. [Google Scholar] [CrossRef]
- Silva, D.; Chaves, N.A.; Miyamoto, S.; Almeida, E.A. Prolonged Erythrocyte Auto-Incubation as an Alternative Model for Oxidant Generation System. Toxicol. Vitr. 2019, 56, 62–74. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. On the Free Radical Scavenging Activities of Melatonin’s Metabolites, AFMK and AMK. J. Pineal Res. 2013, 54, 245–257. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Reiter, R.J.; Plummer, B.F.; Limson, J.; Weintraub, S.T.; Qi, W. Melatonin directly scavenges hydrogen peroxide: A potentially new metabolic pathway of melatonin. Free Radic. Biol. Med. 2000, 29, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Wang, H.; Xu, J.; Li, T.; Zhang, L.; Ding, Y.; Zhu, L.; He, J.; Zhou, M. Melatonin Stimulates Antioxidant Enzymes and Reduces Oxidative Stress in Experimental Traumatic Brain Injury : The Nrf2–ARE Signaling Pathway as a Potential Mechanism. Free Radic. Biol. Med. 2014, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Ishii, T.; Wakabayashi, N.; Yamamoto, M. Regulatory Mechanisms of Cellular Response to Oxidative Stress. Free Radic. Res. 1999, 31, 319–324. [Google Scholar] [CrossRef] [PubMed]
- VENUGOPAL, R.; JAISWAL, A.K. Nrf1 and Nrf2 Positively and c-Fos and Fra1 Negatively Regulate the Human Antioxidant Response Element-Mediated Expression of NAD(P)H: Quinone Oxidoreductase 1 Gene. Proc. Natl. Acad. Sci. USA 1996, 93, 14960–14965. [Google Scholar] [CrossRef]
- Sahni, S.K.; Rydkina, E.; Sahni, A. The Proteasome Inhibitor MG132 Induces Nuclear Translocation of Erythroid Transcription Factor Nrf2 and Cyclooxygenase-2 Expression in Human Vascular Endothelial Cells. Trombos. Res. 2008, 122, 820–825. [Google Scholar] [CrossRef]
- Dreger, H.; Westphal, K.; Wilck, N.; Baumann, G.; Stangl, V.; Stangl, K.; Meiners, S. Protection of Vascular Cells from Oxidative Stress by Proteasome Inhibition Depends on Nrf2. Cardiovasc. Res. 2010, 85, 395–403. [Google Scholar] [CrossRef]
- Vriend, J.; Reiter, R.J. Melatonin as a Proteasome Inhibitor. Is There Any Clinical Evidence? Life Sci. 2014, 115, 8–14. [Google Scholar] [CrossRef]
- Pickering, A.M.; Linder, R.A.; Zhang, H.; Forman, H.J.; Davies, K.J.A. Nrf2-Dependent Induction of Proteasome and Pa28αβ Regulator Are Required for Adaptation to Oxidative Stress. J. Biol. Chem. 2012, 287, 10021–10031. [Google Scholar] [CrossRef]
- Menon, V.; Ghaffari, S. Transcription Factors FOXO in the Regulation of Homeostatic Hematopoiesis. Curr. Opin. Hematol. 2018, 25, 290–298. [Google Scholar] [CrossRef]
- Costas, M.A. Vida Y Muerte de La Célula: Las Señales Intracelulares. Medicina 2006, 66, 281–284. [Google Scholar]
- Fu, H.; Subramanian, R.R.; Masters, S.C. 14-3-3 P ROTEINS: Structure, Function, and Regulation. Annu. Rev. Pharmacol. Toxicol 2000, 40, 617–647. [Google Scholar] [CrossRef] [PubMed]
- Kleppe, R.; Martinez, A.; Ove, S.; Haavik, J. The 14-3-3 Proteins in Regulation of Cellular Metabolism. Semin. Cell Dev. Biol. 2011, 22, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Tian, J.; Zhou, D.; Chen, L. Mst1 and Mst2 Kinases: Regulations and Diseases. Cell Biosci. 2013, 3, 31. [Google Scholar] [CrossRef] [PubMed]




| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| ACTB | 5′-CAAGCAGGAGTATGACGAGTC-3′ | 5′-GCCATGCCAATCTCATCTTG-3′ |
| PRDX1 | 5′-TGTAAATGACCTCCCTGTTGG-3′ | 5′-TATCACTGCCAGGTTTCCAG-3′ |
| PRDX2 | 5′-CTGTTAATGATTTGCCTGTGGG-3′ | 5′-TGGGCTTAATCGTGTCACTG-3′ |
| PRDX6 | 5′-CACGACTTTCTGGGAGACT-3′ | 5′-GGGCAATCAACTTAACATTCCTC-3′ |
| CAT | 5′-TGAATGAGGAACAGAGGAAACG-3′ | 5′-GTACTTGTCCAGAAGAGCCTG-3′ |
| SOD1 | 5′-GGGCAAAGGTGGAAATGAAG-3′ | 5′-CAGCTAGCAGGATAACAGATGAG-3′ |
| GPX1 | 5′-TTCCAGACCATTGACATCGAG-3′ | 5’-CACCCTCATAGATGAAAACCCC-3′ |
| FOXO3 | 5′-GCGTGCCCTACTTCAAGGATAAG-3′ | 5′-GACCCGCATGAATCGACTATG-3′ |
| MST1 | 5′-CCTCCCACATTCCGAAAACCA-3′ | 5′-GCACTCCTGACAAATGGGTG-3′ |
| YWHAQ (14-3-3) | 5′-GGGTTGCATCTCTTTCTTGC-3′ | 5′-GCACTCCTGACAAATGGGTG-3′ |
| NRF2 | 5′-GCTACGTGATGAAGATGGAAAAC-3′ | 5′-AGCTCAGAAAAGGTCAAATCCTC-3′ |
| KEAP1 | 5′-AACAGAGACGTGGACTTTCG-3′ | 5′-GTGTCTGTATCTGGGTCGTAAC-3′ |
| BIM | 5′-AACCACTATCTCAGTGCAAT-3′ | 5′-GGTCTTCGGCTGCTTGGTAA-3′ |
| PSMB5 | 5′-CCATACCTGCTAGGCACCAT-3′ | 5′-GCACCTCCTGAGTAGGCATC-3′ |
| PSMB6 | 5′-CCTATTCACGACCGCATTTT-3′ | 5′-TCCCGGTAGGTAGCATCAAC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, F.F.; Bernardo, V.S.; de Paula, C.P.; da Silva, J.P.M.d.O.; de Almeida, E.A.; da Cunha, A.F.; da Silva, D.G.H. Influence of Melatonin Treatment on Cellular Mechanisms of Redox Adaptation in K562 Erythroleukemic Cells. Genes 2022, 13, 2337. https://doi.org/10.3390/genes13122337
Torres FF, Bernardo VS, de Paula CP, da Silva JPMdO, de Almeida EA, da Cunha AF, da Silva DGH. Influence of Melatonin Treatment on Cellular Mechanisms of Redox Adaptation in K562 Erythroleukemic Cells. Genes. 2022; 13(12):2337. https://doi.org/10.3390/genes13122337
Chicago/Turabian StyleTorres, Flaviene Felix, Victoria Simões Bernardo, Carla Peres de Paula, João Pedro Maia de Oliveira da Silva, Eduardo Alves de Almeida, Anderson Ferreira da Cunha, and Danilo Grünig Humberto da Silva. 2022. "Influence of Melatonin Treatment on Cellular Mechanisms of Redox Adaptation in K562 Erythroleukemic Cells" Genes 13, no. 12: 2337. https://doi.org/10.3390/genes13122337
APA StyleTorres, F. F., Bernardo, V. S., de Paula, C. P., da Silva, J. P. M. d. O., de Almeida, E. A., da Cunha, A. F., & da Silva, D. G. H. (2022). Influence of Melatonin Treatment on Cellular Mechanisms of Redox Adaptation in K562 Erythroleukemic Cells. Genes, 13(12), 2337. https://doi.org/10.3390/genes13122337







