Genomic Hatchery Introgression in Brown Trout (Salmo trutta L.): Development of a Diagnostic SNP Panel for Monitoring the Impacted Mediterranean Rivers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Library Preparation and Reads Processing
2.3. SNP Calling and Genotyping: Defining a SNP Panel for All Locations Studied
2.4. Detection of SNPs Variation between Hatchery and Native Fish
2.5. Multiplex MassARRAY Design and Implementation
3. Results
3.1. Identification of SNPs to Assess Hatchery Ancestry in Brown Trout from Iberian Peninsula
3.2. Design and Validation of a Cost-Effective Tool Using Massarray Genotyping
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland, 2007; pp. 408–413. [Google Scholar]
- Sanz, N. Phylogeographic History of Brown Trout: A Review. In Brown Trout: Biology, Ecology and Management; Lobón-Cerviá, J., Sanz, N., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 17–63. [Google Scholar]
- Ferguson, A. Genetic differences among brown trout, Salmo trutta, stocks and their importance for the conservation and management of the species. Freshw. Biol. 1989, 21, 35–46. [Google Scholar] [CrossRef]
- García-Marín, J.L.; Sanz, N.; Pla, G. Erosion of the native genetic resources of brown trout in Spain. Ecol. Freshw. Fish 1999, 8, 151–158. [Google Scholar] [CrossRef]
- Huusko, A.; Vainikka, A.; Syrjänen, J.T.; Orell, P.; Louhi, P.; Vehanen, T. Life-History of the Adfluvial Brown Trout (Salmo trutta L.) in Eastern Fennoscandia. In Brown Trout: Biology, Ecology and Management; Lobón-Cerviá, J., Sanz, N., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 267–295. [Google Scholar]
- Lobón-Cerviá, J. Introduction. Princess of the Streams: The Brown Trout Salmo trutta L. as Aquatic Royalty. In Brown Trout: Biology, Ecology and Management; Lobón-Cerviá, J., Sanz, N., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018; pp. 1–13. [Google Scholar]
- Denic, M.; Geist, J. Habitat suitability analysis for lacustrine brown trout (Salmo trutta) in Lake Walchensee, Germany: Implications for the conservation of an endangered flagship species. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 9–17. [Google Scholar] [CrossRef]
- Freyhof, J. Salmo trutta, The IUCN Red List of Threatened Species 2013. IUCN Red List. Threat. Species 2013, 10, 312. [Google Scholar] [CrossRef]
- Doadrio, I. Atlas y Libro Rojo de los Peces Continentales de España; Ministerio de Medio Ambiente: Madrid, Spain, 2001; pp. 129–131. [Google Scholar]
- Birnie-Gauvin, K.; Candee, M.M.; Baktoft, H.; Larsen, M.H.; Koed, A.; Aarestrup, K. River connectivity reestablished: Effects and implications of six weir removals on brown trout smolt migration. River Res. Appl. 2018, 34, 548–554. [Google Scholar] [CrossRef]
- Dodd, J.R.; Cowx, I.G.; Bolland, J.D. Efficiency of a nature-like bypass channel for restoring longitudinal connectivity for a river-resident population of brown trout. J. Environ. Manag. 2017, 204, 318–326. [Google Scholar] [CrossRef]
- Barlaup, B.T.; Gabrielsen, S.E.; Skoglund, H.; Wiers, T. Addition of spawning gravel—a means to restore spawning habitat of atlantic salmon (Salmo salar L.), and anadromous and resident brown trout (Salmo trutta L.) in regulated rivers. River Res. Appl. 2014, 30, 132–133. [Google Scholar] [CrossRef]
- Durrant, C.J.; Stevens, J.R.; Hogstrand, C.; Bury, N.R. The effect of metal pollution on the population genetic structure of brown trout (Salmo trutta L.) residing in the River Hayle, Cornwall, UK. Environ. Pollut. 2011, 159, 3595–3603. [Google Scholar] [CrossRef]
- Linde, A.R.; Sánchez-Galán, S.; García-Vázquez, E. Heavy metal contamination of European eel (Anguilla anguilla) and brown trout (Salmo trutta) caught in wild ecosystems in Spain. J. Food Prot. 2004, 67, 2332–2336. [Google Scholar] [CrossRef]
- Nusbaumer, D.; Marques da Cunha, L.; Wedekind, C. Testing for population differences in evolutionary responses to pesticide pollution in brown trout (Salmo trutta). Evol. Appl. 2021, 14, 462–475. [Google Scholar] [CrossRef]
- O’Connor, J.D.; Murphy, S.; Lally, H.T.; O’Connor, I.; Nash, R.; O’Sullivan, J.; Bruen, M.; Heerey, L.; Koelmans, A.A.; Cullagh, A.; et al. Microplastics in brown trout (Salmo trutta Linnaeus, 1758) from an Irish riverine system. Environ. Pollut. 2020, 267, 115572. [Google Scholar] [CrossRef] [PubMed]
- Almodóvar, A.; Nicola, G.G.; Ayllón, D.; Elvira, B. Global warming threatens the persistence of Mediterranean brown trout. Glob. Chang. Biol. 2012, 18, 1549–1560. [Google Scholar] [CrossRef] [Green Version]
- Almodóvar, A.; Nicola, G.G. Angling impact on conservation of Spanish stream-dwelling brown trout Salmo trutta. Fish. Manag. Ecol. 2004, 11, 173–182. [Google Scholar] [CrossRef]
- Almodóvar, A.; Nicola, G.G.; Elvira, B.; García-Marín, J.L. Introgression variability among Iberian brown trout Evolutionary Significant Units: The influence of local management and environmental features. Freshw. Biol. 2006, 51, 1175–1187. [Google Scholar] [CrossRef]
- Apostolidis, A.P.; Madeira, M.J.; Hansen, M.M.; Machordom, A. Genetic structure and demographic history of brown trout (Salmo trutta) populations from the southern Balkans. Freshw. Biol. 2008, 53, 1555–1566. [Google Scholar] [CrossRef]
- Berrebi, P.; Poteaux, C.; Fissier, M.; Cattaneo-Berrebi, G. Stocking impact and allozyme diversity in brown trout from Mediterranean southern France. J. Fish Biol. 2000, 56, 949–960. [Google Scholar] [CrossRef]
- Jug, T.; Berrebi, P.; Snoj, A. Distribution of non-native trout in Slovenia and their introgression with native trout populations as observed through microsatellite DNA analysis. Biol. Conserv. 2005, 123, 381–388. [Google Scholar] [CrossRef]
- Splendiani, A.; Ruggeri, P.; Giovannotti, M.; Pesaresi, S.; Occhipinti, G.; Fioravanti, T.; Lorenzoni, M.; Nisi Cerioni, P.; Caputo Barucchi, V. Alien brown trout invasion of the Italian peninsula: The role of geological, climate and anthropogenic factors. Biol. Invasions 2016, 18, 2029–2044. [Google Scholar] [CrossRef]
- Berrebi, P.; Marić, S.; Snoj, A.; Hasegawa, K. Brown trout in Japan—Introduction history, distribution and genetic structure. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 18. [Google Scholar] [CrossRef]
- Cambray, J.A. The global impact of alien trout species—a review; with reference to their impact in south africa. Afr. J. Aquat. Sci. 2003, 28, 61–67. [Google Scholar] [CrossRef]
- MacCrimmon, H.R.; Marshall, T.L.; Gots, B.L. World Distribution of Brown Trout, Salmo trutta: Further Observations. J. Fish. Res. Board Can. 1970, 27, 811–818. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Seddon, P.J. Directions in reintroduction biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Bernaś, R.; Poćwierz-Kotus, A.; Árnyasi, M.; Kent, M.P.; Lien, S.; Wenne, R. Genetic differentiation in hatchery and stocked populations of sea trout in the Southern Baltic: Selection evidence at SNP loci. Genes 2020, 11, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Marín, J.L.; Jorde, P.E.; Ryman, N.; Utter, F.; Pla, C. Management implications of genetic differentiation between native and hatchery populations of brown trout (Salmo trutta) in Spain. Aquaculture 1991, 95, 235–249. [Google Scholar] [CrossRef]
- Kohout, J.; Jašková, I.; Papoušek, I.; Šedivá, A.; Šlechta, V. Effects of stocking on the genetic structure of brown trout, Salmo trutta, in Central Europe inferred from mitochondrial and nuclear DNA markers. Fish. Manag. Ecol. 2012, 19, 252–263. [Google Scholar] [CrossRef]
- Laikre, L.; Palmé, A.; Josefsson, M.; Utter, F.; Ryman, N. Release of alien populations in Sweden. Ambio 2006, 35, 255–261. [Google Scholar] [CrossRef]
- Pinter, K.; Epifanio, J.; Unfer, G. Release of hatchery-reared brown trout (Salmo trutta) as a threat to wild populations? A case study from Austria. Fish. Res. 2019, 219, 105296. [Google Scholar] [CrossRef]
- Arias, J.; Sánchez, L.; Martínez, P. Low stocking incidence in brown trout populations from northwestern Spain monitored by LDH-5* diagnostic marker. J. Fish Biol. 1995, 47, 170–176. [Google Scholar] [CrossRef]
- Saint-Pé, K.; Leitwein, M.; Tissot, L.; Poulet, N.; Guinand, B.; Berrebi, P.; Marselli, G.; Lascaux, J.M.; Gagnaire, P.A.; Blanchet, S. Development of a large SNPs resource and a low-density SNP array for brown trout (Salmo trutta) population genetics. BMC Genom. 2019, 20, 582. [Google Scholar] [CrossRef] [Green Version]
- Champagnon, J.; Elmberg, J.; Guillemain, M.; Gauthier-Clerc, M.; Lebreton, J.D. Conspecifics can be aliens too: A review of effects of restocking practices in vertebrates. J. Nat. Conserv. 2012, 20, 231–241. [Google Scholar] [CrossRef]
- Berrebi, P.; Horvath, Á.; Splendiani, A.; Palm, S.; Bernaś, R. Genetic diversity of domestic brown trout stocks in Europe. Aquaculture 2021, 544, 737043. [Google Scholar] [CrossRef]
- Snoj, A.; Marić, S.; Bajec, S.S.; Berrebi, P.; Janjani, S.; Schöffmann, J. Phylogeographic structure and demographic patterns of brown trout in North-West Africa. Mol. Phylogenet. Evol. 2011, 61, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Arias, J.; Castro, J.; Sánchez, L. Differential stocking incidence in brown trout (Salmo trutta) populations from Northwestern Spain. Aquaculture 1993, 114, 203–216. [Google Scholar] [CrossRef]
- Vera, M.; Martínez, P.; Bouza, C. Stocking impact, population structure and conservation of wild brown trout populations in inner Galicia (NW Spain), an unstable hydrologic region. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 435–443. [Google Scholar] [CrossRef]
- Talarico, L.; Marta, S.; Rossi, A.R.; Crescenzo, S.; Petrosino, G.; Martinoli, M.; Tancioni, L. Balancing selection, genetic drift, and human-mediated introgression interplay to shape MHC (functional) diversity in Mediterranean brown trout. Ecol. Evol. 2021, 11, 10026–10041. [Google Scholar] [CrossRef] [PubMed]
- Morán, P.; Pendás, A.M.; García-Vázquez, E.; Izquierdo, J. Failure of a stocking policy, of hatchery reared brown trout, Salmo trutta L., in Asturias, Spain, detected using LDH-5* as a genetic marker. J. Fish Biol. 1991, 39, 117–121. [Google Scholar] [CrossRef]
- Hamilton, K.E.; Ferguson, A.; Taggart, J.B.; Tómasson, T.; Walker, A.; Fahy, E. Post-glacial colonization of brown trout, Salmo trutta L.: Ldh-5 as a phylogeographic marker locus. J. Fish Biol. 1989, 35, 651–664. [Google Scholar] [CrossRef]
- McMeel, O.M.; Hoey, E.M.; Ferguson, A. Partial nucleotide sequences, and routine typing by polymerase chain reaction-restriction fragment length polymorphism, of the brown trout (Salmo trutta) lactate dehydrogenase, LDH-C1*90 and *100 alleles. Mol. Ecol. 2001, 10, 29–34. [Google Scholar] [CrossRef]
- Vera, M.; García-Marín, J.L.; Martínez, P.; Araguas, R.M.; Bouza, C. Identification and conservation of remnant genetic resources of brown trout in relict populations from Western Mediterranean streams. Hydrobiologia 2013, 707, 29–45. [Google Scholar] [CrossRef] [Green Version]
- Araguas, R.M.; Vera, M.; Aparicio, E.; Sanz, N.; Fernández-Cebrián, R.; Marchante, C.; García-Marín, J.L. Current status of the brown trout (Salmo trutta) populations within eastern Pyrenees genetic refuges. Ecol. Freshw. Fish 2017, 26, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Dijk, E.L.; Auger, H.; Jaszczyszyn, Y.; Thermes, C. Ten years of next-generation sequencing technology. Trends Genet. 2014, 30, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, F.W.; Hohenlohe, P.A.; Luikart, G. Genomics and the future of conservation genetics. Nat. Rev. Genet. 2010, 11, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Ouborg, N.J.; Pertoldi, C.; Loeschcke, V.; Bijlsma, R.K.; Hedrick, P.W. Conservation genetics in transition to conservation genomics. Trends Genet. 2010, 26, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Baird, N.A.; Etter, P.D.; Atwood, T.S.; Currey, M.C.; Shiver, A.L.; Lewis, Z.A.; Selker, E.U.; Cresko, W.A.; Johnson, E.A. Rapid SNP discovery and genetic mapping using sequenced RAD markers. PLoS ONE 2008, 3, e3376. [Google Scholar] [CrossRef]
- Narum, S.R.; Buerkle, C.A.; Davey, J.W.; Miller, M.R.; Hohenlohe, P.A. Genotyping-by-sequencing in ecological and conservation genomics. Mol. Ecol. 2013, 22, 2841–2847. [Google Scholar] [CrossRef]
- Andrews, K.R.; Good, J.M.; Miller, M.R.; Luikart, G.; Hohenlohe, P.A. Harnessing the power of RADseq for ecological and evolutionary genomics. Nat. Rev. Genet. 2016, 17, 81–92. [Google Scholar] [CrossRef] [Green Version]
- Paris, J.R.; Stevens, J.R.; Catchen, J.M. Lost in parameter space: A road map for STACKS. Methods Ecol. Evol. 2017, 8, 1360–1373. [Google Scholar] [CrossRef]
- Tan, M.P.; Wong, L.L.; Razali, S.A.; Afiqah-Aleng, N.; Nor, S.A.M.; Sung, Y.Y.; Van de Peer, Y.; Sorgeloos, P.; Danish-Daniel, M. Applications of Next-Generation Sequencing Technologies and Computational Tools in Molecular Evolution and Aquatic Animals Conservation Studies: A Short Review. Evol. Bioinforma 2019, 15, 1176934319892284. [Google Scholar] [CrossRef] [Green Version]
- Waples, R.S.; Naish, K.A.; Primmer, C.R. Conservation and Management of Salmon in the Age of Genomics. Annu. Rev. Anim. Biosci. 2020, 8, 117–143. [Google Scholar] [CrossRef]
- Hansen, T.; Fjelldal, P.G.; Lien, S.; Smith, M.; Corton, C.; Oliver, K.; Skelton, J.; Betteridge, E.; Doulcan, J.; Fedrigo, O.; et al. The genome sequence of the brown trout, Salmo trutta Linnaeus 1758 [version 1; peer review: 3 approved]. Wellcome Open Res. 2021, 6, 108. [Google Scholar] [CrossRef]
- Wang, S.; Meyer, E.; Mckay, J.K.; Matz, M.V. 2b-RAD: A simple and flexible method for genome-wide genotyping. Nat. Methods 2012, 9, 808–810. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.; Ziaugra, L.; Tabbaa, D. SNP genotyping using the sequenom massARRAY iPLEX Platform. Curr. Protoc. Hum. Genet. 2009, 60, 2121–2122. [Google Scholar] [CrossRef] [PubMed]
- Bouza, C.; Castro, J.; Sánchez, L.; Martínez, P. Allozymic evidence of parapatric differentiation of brown trout (Salmo trutta L.) within an Atlantic river basin of the Iberian Peninsula. Mol. Ecol. 2001, 10, 1455–1469. [Google Scholar] [CrossRef] [PubMed]
- Martínez, P.; Bouza, C.; Castro, J.; Hermida, M.; Pardo, B.G.; Sánchez, L. Analysis of a secondary contact between divergent lineages of brown trout Salmo trutta L. from Duero basin using microsatellites and mtDNA RFLPs. J. Fish Biol. 2007, 71, 195–213. [Google Scholar] [CrossRef]
- Vilas, R.; Bouza, C.; Castro, J.; López, A.; Martínez, P. Management units of brown trout from Galicia (NW: Spain) based on spatial genetic structure analysis. Conserv. Genet. 2010, 11, 897–906. [Google Scholar] [CrossRef]
- Bouza, C.; Vilas, R.; Castro, J.; Martínez, P. Mitochondrial haplotype variability of brown trout populations from Northwestern Iberian Peninsula, a secondary contact area between lineages. Conserv. Genet. 2008, 9, 917–920. [Google Scholar] [CrossRef]
- Cortey, M.; Pla, C.; García-Marín, J.L. Historical biogeography of Mediterranean trout. Mol. Phylogenet. Evol. 2004, 33, 831–844. [Google Scholar] [CrossRef]
- Vera, M.; Bouza, C.; Casanova, A.; Heras, S.; Martínez, P.; García-Marín, J.L. Identification of an endemic Mediterranean brown trout mtDNA group within a highly perturbed aquatic system, the Llobregat River (NE Spain). Hydrobiologia 2019, 827, 277–291. [Google Scholar] [CrossRef]
- Catchen, J.M.; Amores, A.; Hohenlohe, P.; Cresko, W.; Postlethwait, J.H. Stacks: Building and genotyping loci de novo from short-read sequences. G3 Genes Genomes Genet. 2011, 1, 171–182. [Google Scholar] [CrossRef] [Green Version]
- Catchen, J.; Hohenlohe, P.A.; Bassham, S.; Amores, A.; Cresko, W.A. Stacks: An analysis tool set for population genomics. Mol. Ecol. 2013, 22, 3124–3140. [Google Scholar] [CrossRef] [Green Version]
- Rochette, N.C.; Rivera-Colón, A.G.; Catchen, J.M. Stacks 2: Analytical methods for paired-end sequencing improve RADseq-based population genomics. Mol. Ecol. 2019, 28, 4737–4754. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, K.A.; Davidson, W.S. Autopolyploidy genome duplication preserves other ancient genome duplications in Atlantic salmon (Salmo salar). PLoS ONE 2017, 12, e0173053. [Google Scholar] [CrossRef] [PubMed]
- Leitwein, M.; Guinand, B.; Pouzadoux, J.; Desmarais, E.; Berrebi, P.; Gagnaire, P.A. A dense brown trout (Salmo trutta) linkage map reveals recent chromosomal rearrangements in the Salmo genus and the impact of selection on linked neutral diversity. G3 Genes Genomes Genet. 2017, 7, 1365–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, M.A.; Buser, T.J.; Alfaro, M.E.; López, J.A. Addressing incomplete lineage sorting and paralogy in the inference of uncertain salmonid phylogenetic relationships. PeerJ 2020, 8, e9389. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [Green Version]
- Stanley, R.R.E.; Jeffery, N.W.; Wringe, B.F.; DiBacco, C.; Bradbury, I.R. GENEPOPEDIT: A simple and flexible tool for manipulating multilocus molecular data in R. Mol. Ecol. Resour. 2017, 17, 12–18. [Google Scholar] [CrossRef]
- Lischer, H.E.L.; Excoffier, L. PGDSpider: An automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics 2012, 28, 298–299. [Google Scholar] [CrossRef] [Green Version]
- Puritz, J.B.; Hollenbeck, C.M.; Gold, J.R. dDocent: A RADseq, variant-calling pipeline designed for population genomics of non-model organisms. PeerJ 2014, 2, e431. [Google Scholar] [CrossRef] [Green Version]
- Fisher, R.A. Statistical Methods for Research Workers, 1st ed.; Oliver and Boyd: Edinburgh, UK, 1932. [Google Scholar]
- Martínez, P.; Viñas, A.; Bouza, C.; Arias, J.; Amaro, R.; Sánchez, L. Cytogenetical characterization of hatchery stocks and natural populations of Sea and Brown Trout from northwestern Spain. Heredity 1991, 66, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of Population Structure Using Multilocus Genotype Data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Besnier, F.; Glover, K.A. ParallelStructure: A R Package to Distribute Parallel Runs of the Population Genetics Program STRUCTURE on Multi-Core Computers. PLoS ONE 2013, 8, e70651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanz, N.; Araguas, R.M.; Fernández, R.; Vera, M.; García-Marín, J.L. Efficiency of markers and methods for detecting hybrids and introgression in stocked populations. Conserv. Genet. 2009, 10, 225–236. [Google Scholar] [CrossRef]
- Gilbert, K.J.; Andrew, R.L.; Bock, D.; Franklin, M.T.; Moore, B.; Kane, N.C.; Rennison, D.J.; Veen, T.; Vines, T.H. Recommendations for utilizing and reporting population genetic analyses: The reproducibility of genetic clustering using the program structure. Mol. Ecol. 2012, 21, 4925–4930. [Google Scholar] [CrossRef]
- Linck, E.; Battey, C.J. Minor allele frequency thresholds strongly affect population structure inference with genomic data sets. Mol. Ecol. Resour. 2019, 19, 639–647. [Google Scholar] [CrossRef]
- Rousset, F. GENEPOP’007: A complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-statistics for the analysis of population structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.S.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.A.; Ong, B. The MassARRAY® System for Targeted SNP Genotyping. In Genotyping Methods and Protocols; White, S., Cantsilieris, S., Eds.; Springer Nature: New York, NY, USA, 2017; pp. 77–94. [Google Scholar]
- Madden, T. The BLAST sequence analysis tool. In The NCBI Handbook, 2nd ed.; McEntyre, J., Ostell, J., Eds.; National Center for Biotechnology Information: Bethesda, MD, USA, 2013; pp. 361–370. [Google Scholar]
- Bernatchez, L.; Guyomard, R.; Bonhomme, F. DNA sequence variation of the mitochondrial control region among geographically and morphologically remote European brown trout Saltno trutta populations. Mol. Ecol. 1992, 1, 161–173. [Google Scholar] [CrossRef]
- Vera, M.; Cortey, M.; Sanz, N.; García-Marín, J.L. Maintenance of an endemic lineage of brown trout (Salmo trutta) within the Duero river basin. J. Zool. Syst. Evol. Res. 2010, 48, 181–187. [Google Scholar] [CrossRef]
- Machordom, A.; Suárez, J.; Almodóvar, A.; Bautista, J.M. Mitochondrial haplotype variation and phylogeography of Iberian brown trout populations. Mol. Ecol. 2000, 9, 1324–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortey, M.; Vera, M.; Pla, C.; García-Marín, J.L. Northern and Southern expansions of Atlantic brown trout (Salmo trutta) populations during the Pleistocene. Biol. J. Linn. Soc. 2009, 97, 904–917. [Google Scholar] [CrossRef] [Green Version]
- Vera, M.; García-Marín, J.L.; Martínez, P.; Bouza, C. Phylogenetic diversity within the endemic brown trout Duero lineage: Implications for conservation and management. Mar. Freshw. Res. 2015, 66, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Bernatchez, L. The evolutionary history of brown trout (Salmo trutta L.) inferred from phylogeographic, nested clade, and mismatch analyses of mitochondrial DNA variation. Evolution 2001, 55, 351–379. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Cebrián, R.; Araguas, R.M.; Sanz, N.; García-Marín, J.L. Genetic risks of supplementing trout populations with native stocks: A simulation case study from current Pyrenean populations. Can. J. Fish. Aquat. Sci. 2014, 71, 1243–1255. [Google Scholar] [CrossRef] [Green Version]
- Bekkevold, D.; Höjesjö, J.; Nielsen, E.E.; Aldvén, D.; Als, T.D.; Sodeland, M.; Kent, M.P.; Lien, S.; Hansen, M.M. Northern European Salmo trutta (L.) populations are genetically divergent across geographical regions and environmental gradients. Evol. Appl. 2020, 13, 400–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linløkken, A.N.; Haugen, T.O.; Kent, M.P.; Lien, S. Genetic differences between wild and hatchery-bred brown trout (Salmo trutta L.) in single nucleotide polymorphisms linked to selective traits. Ecol. Evol. 2017, 7, 4963–4972. [Google Scholar] [CrossRef] [Green Version]
- Leitwein, M.; Gagnaire, P.A.; Desmarais, E.; Guendouz, S.; Rohmer, M.; Berrebi, P.; Guinand, B. Genome-wide nucleotide diversity of hatchery-reared Atlantic and Mediterranean strains of brown trout Salmo trutta compared to wild Mediterranean populations. J. Fish Biol. 2016, 89, 2717–2734. [Google Scholar] [CrossRef]
- Casanova, A.; Maroso, F.; Blanco, A.; Hermida, M.; Ríos, N.; García, G.; Manuzzi, A.; Zane, L.; Verissimo, A.; García-Marín, J.L.; et al. Low impact of different SNP panels from two building-loci pipelines on RAD-Seq population genomic metrics: Case study on five diverse aquatic species. BMC Genom. 2021, 22, 150. [Google Scholar] [CrossRef]
- Leitwein, M.; Gagnaire, P.A.; Desmarais, E.; Berrebi, P.; Guinand, B. Genomic consequences of a recent three-way admixture in supplemented wild brown trout populations revealed by local ancestry tracts. Mol. Ecol. 2018, 27, 3466–3483. [Google Scholar] [CrossRef]
- Splendiani, A.; Giovannotti, M.; Righi, T.; Fioravanti, T.; Cerioni, P.N.; Lorenzoni, M.; Carosi, A.; La Porta, G.; Barucchi, V.C. Introgression despite protection: The case of native brown trout in Natura 2000 network in Italy. Conserv. Genet. 2019, 20, 343–356. [Google Scholar] [CrossRef]
- Hansen, M.M.; Nielsen, E.E.; Bekkevold, D.; Mensberg, K.-L.D. Admixture analysis and stocking impact assessment in brown trout (Salmo trutta), estimated with incomplete baseline data. Can. J. Fish. Aquat. Sci. 2001, 58, 1853–1860. [Google Scholar] [CrossRef]
- Prado, F.D.; Vera, M.; Hermida, M.; Blanco, A.; Bouza, C.; Maes, G.E.; Volckaert, F.A.M.; AquaTrace Consortium; Martínez, P. Tracing the genetic impact of farmed turbot Scophthalmus maximus on wild populations. Aquac. Environ. Interact. 2018, 10, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Walsh, P.S.; Metzger, D.; Higuchi, R. Chelex 100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 1991, 10, 506–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roques, S.; Chancerel, E.; Boury, C.; Pierre, M.; Acolas, M.L. From microsatellites to single nucleotide polymorphisms for the genetic monitoring of a critically endangered sturgeon. Ecol. Evol. 2019, 9, 7017–7029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Gowan, S.; Anil, A.; Beck, B.H.; Thongda, W.; Kucuktas, H.; Kaltenboeck, L.; Peatman, E. Discovery and validation of gene-linked diagnostic SNP markers for assessing hybridization between Largemouth bass (Micropterus salmoides) and Florida bass (M. floridanus). Mol. Ecol. Resour. 2015, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Maroso, F.; Casanova, A.; do Prado, F.D.; Bouza, C.; Pardo, B.G.; Blanco, A.; Hermida, M.; Fernández, C.; Vera, M.; Martínez, P. Species identification of two closely exploited flatfish, turbot (Scophthalmus maximus) and brill (Scophthalmus rhombus), using a ddRADseq genomic approach. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1253–1260. [Google Scholar] [CrossRef]
- Maroso, F.; De Gracia, C.P.; Iglesias, D.; Cao, A.; Díaz, S.; Villalba, A.; Vera, M.; Martínez, P. A useful SNP panel to distinguish two cockle species, Cerastoderma edule and C. glaucum, co-occurring in some European beds, and their putative hybrids. Genes 2019, 10, 760. [Google Scholar] [CrossRef] [Green Version]
- Thongda, W.; Lewis, M.; Zhao, H.; Bowen, B.; Lutz-Carrillo, D.J.; Peoples, B.K.; Peatman, E. Species-diagnostic SNP markers for the black basses (Micropterus spp.): A new tool for black bass conservation and management. Conserv. Genet. Resour. 2020, 12, 319–328. [Google Scholar] [CrossRef]
- DOGC. Resolució ARP/260/2021, De 5 de Febrer, per la Qual S’ordena la Pesca a les Aigües Continentals de Catalunya Durant la Temporada 2021. Diari Oficial de la Generalitat de Catalunya. 2021. No. 8337. Available online: https://portaldogc.gencat.cat/utilsEADOP/PDF/8337/1835056.pdf (accessed on 15 November 2021).

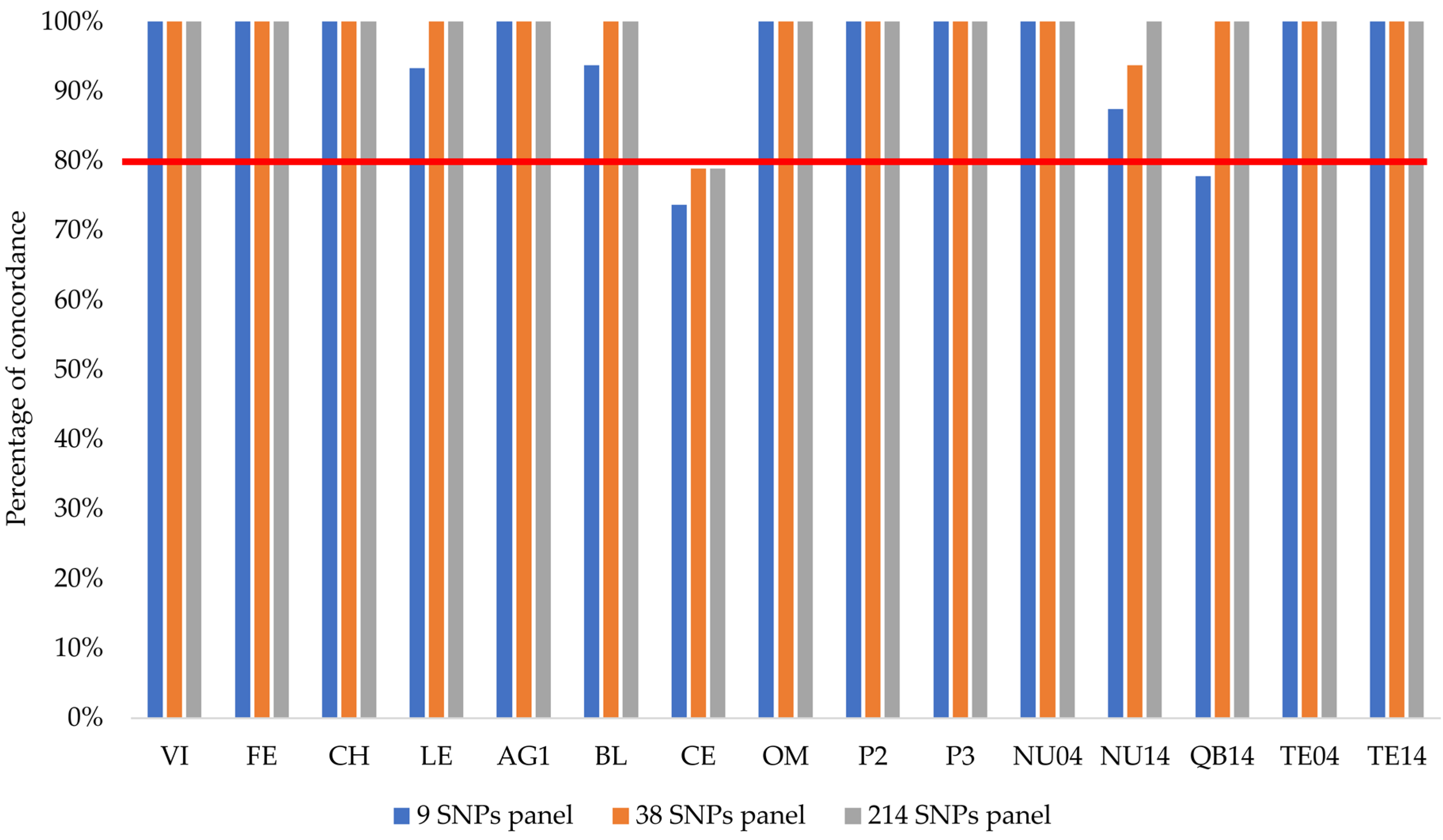
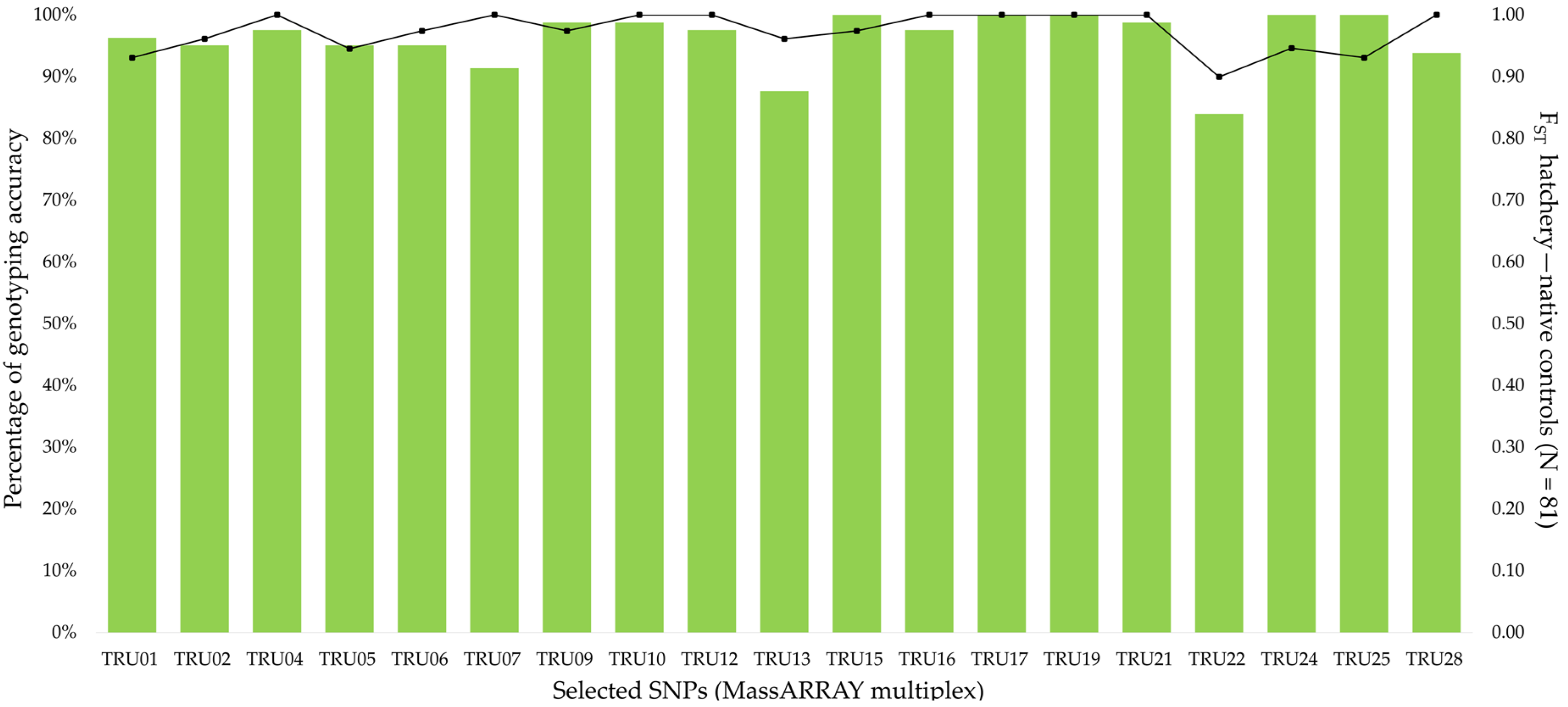
| Locations | Year | Code | N | Native mtDNA Lineage |
|---|---|---|---|---|
| Samples for 2b-RADseq analysis | ||||
| Miño-Sil River basin | 59 (56) | |||
| 1. Viñao River | 2003 | VI | 16 (15) | AT |
| 2. Ferreira River | 2003 | FE | 14 (13) | AT/DU |
| 3. Chamoso River | 2003 | CH | 13 (13) | AT/DU |
| 4. Lea River | 2003 | LE | 16 (15) | DU |
| Duero River basin | 119 (106) | |||
| 5. Águeda River | 2002 | AG1 | 20 (16) | AT |
| 6. Porto do Rei Búbal River | 2002 | BL | 19 (16) | AT |
| 7. Cega River | 2002 | CE | 20 (19) | AT/DU |
| 8. Omaña River | 2002 | OM | 20 (20) | DU |
| 9. Pisuerga River 2 | 2002 | P2 | 20 (18) | DU |
| 10. Pisuerga River 3 | 2002 | P3 | 20 (17) | DU |
| Ter River basin | 82 (82) | |||
| 11. Núria River | 2004 | NU04 | 16 (16) | AD/ME |
| 11. Núria River | 2014 | NU14 | 16 (16) | AD/ME |
| 12. Queralbs, in Freser River | 2014 | QB14 | 18 (18) | AD/ME |
| 13. Ter River | 2004 | TE04 | 14 (14) | AD/ME |
| 13. Ter River | 2014 | TE14 | 18 (18) | AD/ME |
| Hatchery | 39 (39) | |||
| 14. Hatchery release individuals | 2014 | BA14 | 19 (19) | AT |
| 14. Hatchery spawners | 2002 | S | 20 (20) | AT |
| Samples for MassARRAY | ||||
| Ebro River basin | 130 | |||
| 15. Segre, Queixans | 2016 | QU16 | 30 | AD/ME |
| 16. Segre, Meranges | 2016 | ME16 | 24 | ME |
| 17. Segre, Prullans | 2016 | PR16 | 30 | ME |
| 18. Segre, Martinet | 2017 | MA17 | 33 | AD/ME |
| 19. Segre, Els Hostalets de Tost | 2018 | TS18 | 4 | ME |
| 20. Segre, Organyà | 2018 | OR18 | 9 | ME |
| Llobregat River basin | 95 | |||
| 21. Cardener River | 2018 | CA18 | 17 | ME |
| 22. Aiguadora; Bancells Mill) | 2017 | CT17 | 14 | ME |
| 23. Gressolet | 2017 | GRE17 | 30 | ME |
| 24. Riutort | 2017 | RT17 | 34 | ME |
| Ter River basin | 16 | |||
| 25. Querós Creek | 2018 | RQS18 | 16 | NA |
| Location Codes | qH (19 SNPs) | qH (5 Microsatellites) | LDH-C*90 |
|---|---|---|---|
| QU16 | 0.056 | 0.027 | 0.050 |
| ME16 | 0.024 | 0.381 | 0.022 |
| PR16 | 0.241 | 0.074 | 0.217 |
| MA17 | 0.210 | 0.071 | 0.197 |
| TS18 | 0.496 | 0.159 | 0.250 |
| OR18 | 0.439 | 0.293 | 0.556 |
| CA18 | 0.147 | 0.029 | 0.059 |
| CT17 | 0.120 | 0.058 | 0.143 |
| GRE17 | 0.570 | 0.097 | 0.667 |
| RT17 | 0.545 | 0.210 | 0.397 |
| RQS18 | 0.980 | 0.400 | 0.969 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casanova, A.; Heras, S.; Abras, A.; Roldán, M.I.; Bouza, C.; Vera, M.; García-Marín, J.L.; Martínez, P. Genomic Hatchery Introgression in Brown Trout (Salmo trutta L.): Development of a Diagnostic SNP Panel for Monitoring the Impacted Mediterranean Rivers. Genes 2022, 13, 255. https://doi.org/10.3390/genes13020255
Casanova A, Heras S, Abras A, Roldán MI, Bouza C, Vera M, García-Marín JL, Martínez P. Genomic Hatchery Introgression in Brown Trout (Salmo trutta L.): Development of a Diagnostic SNP Panel for Monitoring the Impacted Mediterranean Rivers. Genes. 2022; 13(2):255. https://doi.org/10.3390/genes13020255
Chicago/Turabian StyleCasanova, Adrián, Sandra Heras, Alba Abras, María Inés Roldán, Carmen Bouza, Manuel Vera, José Luis García-Marín, and Paulino Martínez. 2022. "Genomic Hatchery Introgression in Brown Trout (Salmo trutta L.): Development of a Diagnostic SNP Panel for Monitoring the Impacted Mediterranean Rivers" Genes 13, no. 2: 255. https://doi.org/10.3390/genes13020255






