MiR-218-5p Affects Subcutaneous Adipogenesis by Targeting ACSL1, a Novel Candidate for Pig Fat Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation, Primary Culture, and Differentiation of Porcine Preadipocytes
2.3. Quantitative Real-Time RT-PCR (qRT-PCR)
2.4. Transfection of miRNA, siRNA, and Plasmid
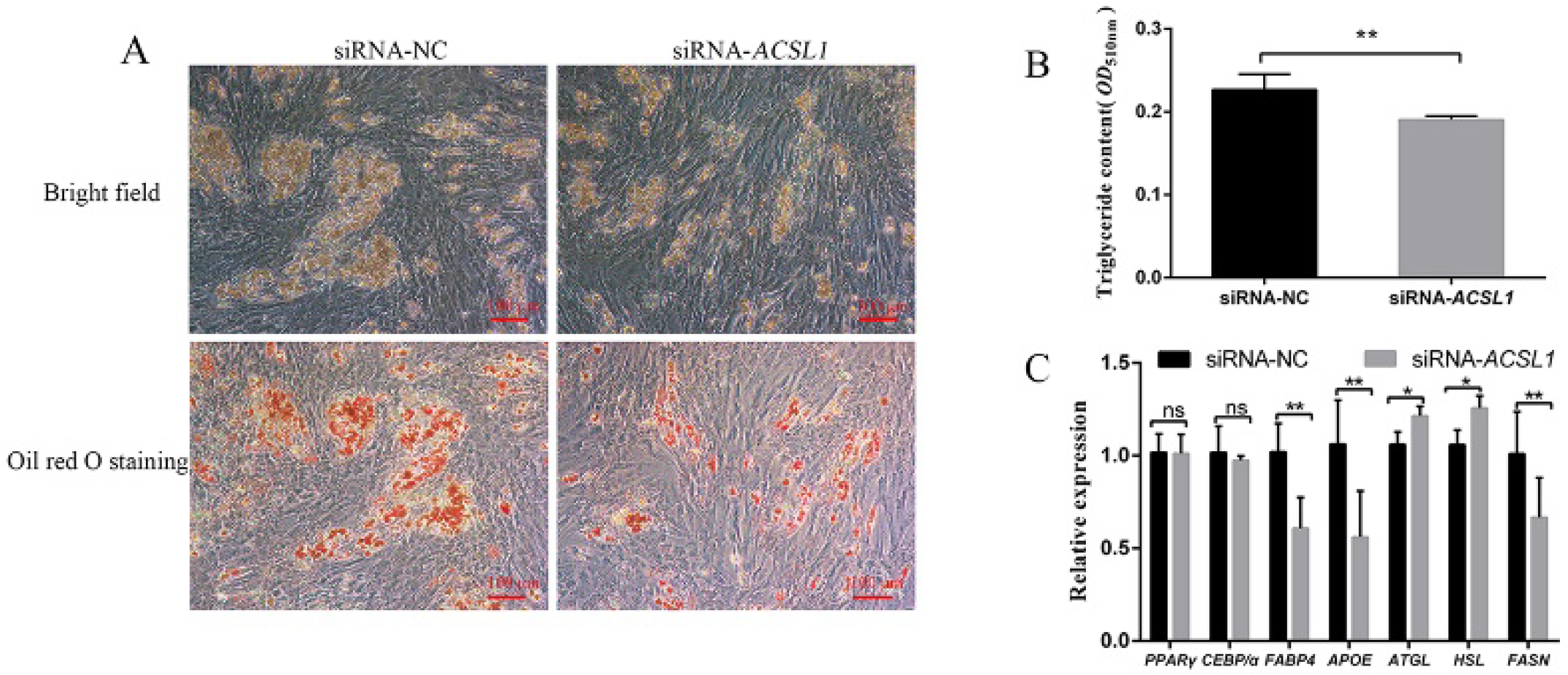
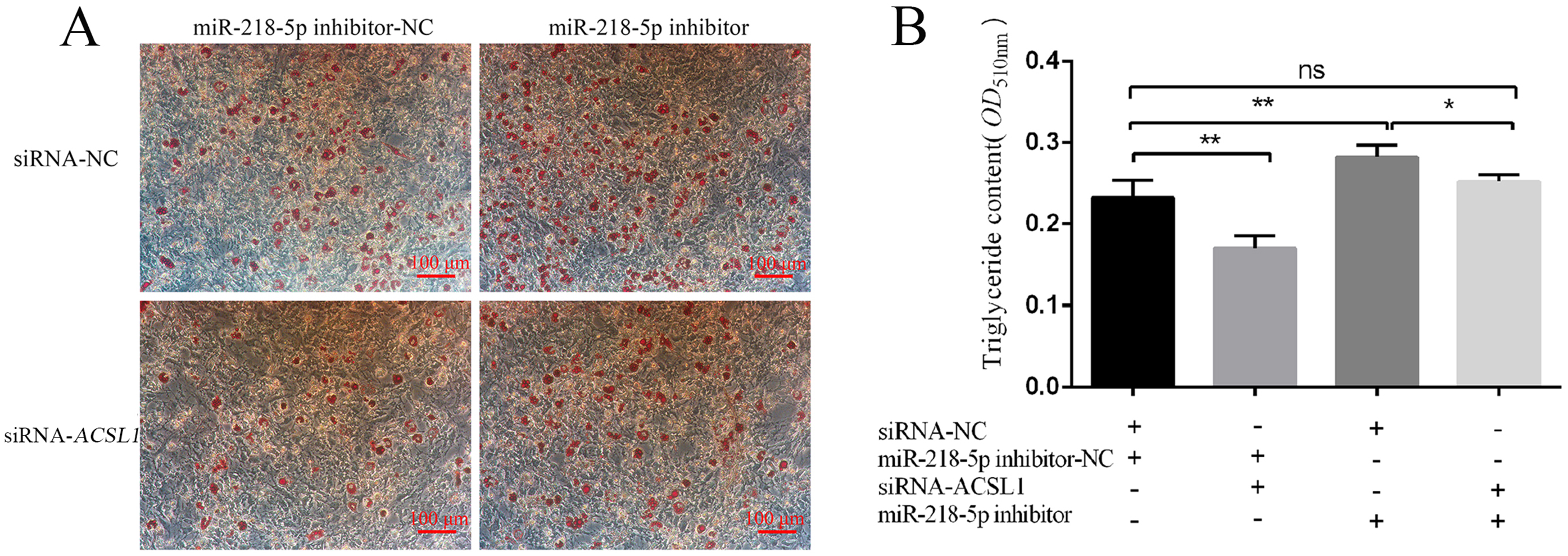
2.5. Determination of Oil Red O Staining and Triglyceride Contents of Adipocytes
2.6. Western Blotting Analysis
2.7. Construction of ACSL1 Overexpression Plasmid
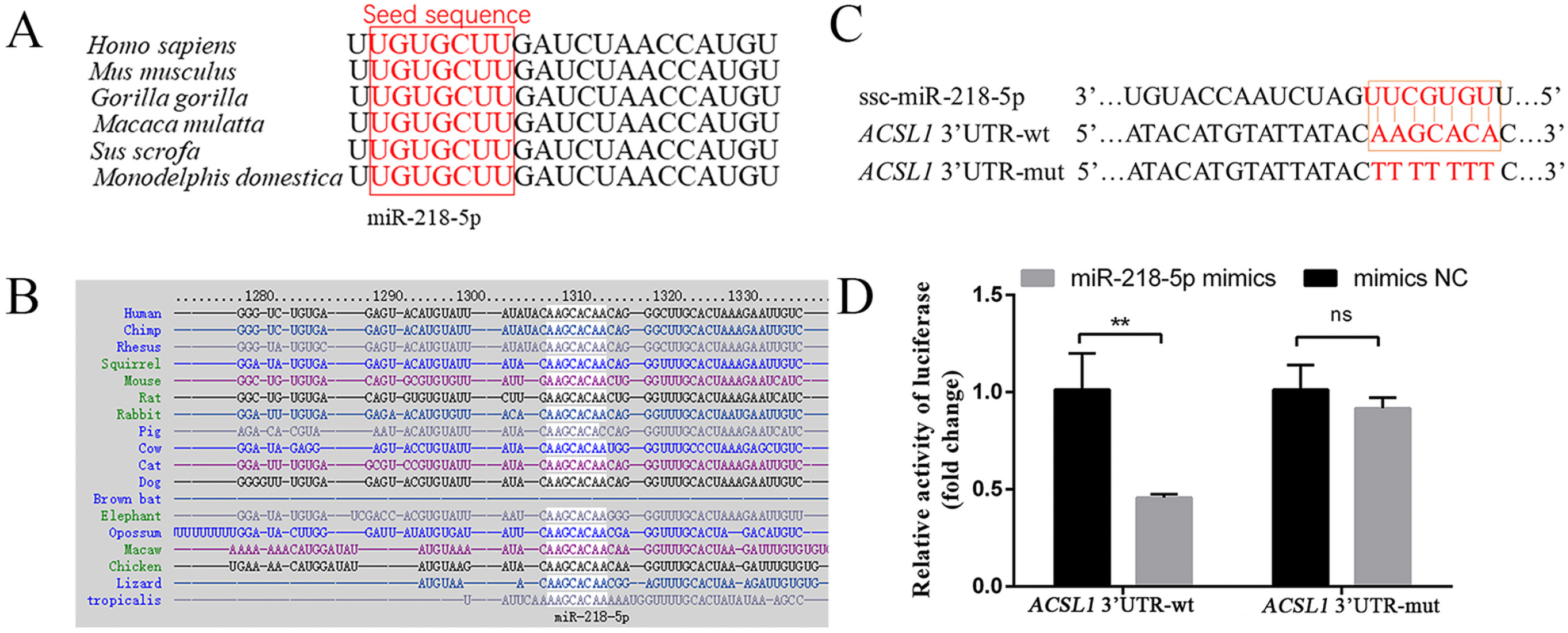
2.8. Construction of Luciferase Reporter Vector
2.9. Dual-Luciferase Activity Assay
2.10. Statistical Analysis
3. Results
3.1. Changes of Preadipocyte Differentiation and the Expression of ACSL1 in Differentiated Preadipocytes
3.2. Effect of siRNA Interference of ACSL1 on Differentiation of Preadipocytes
3.3. Effect of ACSL1 Overexpression on Differentiation of Preadipocytes
3.4. MiR-218-5p Targeting the 3′UTR of ACSL1
3.5. MiR-218-5p Inhibits Preadipocyte Differentiation and ACSL1 Expression
3.6. MiR-218-5p Regulates Preadipocyte Differentiation by Targeting ACSL1
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ros-Freixedes, R.; Reixach, J.; Bosch, L.; Tor, M.; Estany, J. Genetic correlations of intramuscular fat content and fatty acid composition among muscles and with subcutaneous fat in Duroc pigs. J. Anim. Sci. 2014, 92, 5417–5425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.M.; Elabd, C.; Delteil, F.; Astier, J.; Vernochet, C.; Saint-Marc, P.; Guesnet, J.; Guezennec, A.; Amri, E.Z.; Dani, C.; et al. Adipocyte differentiation of multipotent cells established from human adipose tissue. Biochem. Biophys. Res. Commun. 2004, 315, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, X.; Weijun, P.; Ning, W.; Yu, W.; Wenkai, R.; Gongshe, Y. Knockdown of both FoxO1 and C/EBPbeta promotes adipogenesis in porcine preadipocytes through feedback regulation. Cell Biol. Int. 2013, 37, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Xu, G.; Jiang, C.; Hou, L.; Wu, Z.; Wang, C. PRDM16 represses the pig white lipogenesis through promoting lipolysis activity. Biomed. Res. Int. 2019, 2019, 1969413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Wang, Y.; Liang, X.; Wu, X.; Liu, J.; Yang, S.; Tao, C.; Zhang, J.; Tian, J.; Zhao, J.; et al. Stearoyl-CoA desaturase is essential for porcine adipocyte differentiation. Int. J. Mol. Sci. 2020, 21, 2446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Zhou, J.; Hooi, S.C.; Jiang, Y.M.; Lu, G.D. Fatty acid activation in carcinogenesis and cancer development: Essential roles of long-chain acyl-CoA synthetases. Oncol. Lett. 2018, 16, 1390–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brasaemle, D.L.; Dolios, G.; Shapiro, L.; Wang, R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3-L1 adipocytes. J. Biol. Chem. 2004, 279, 46835–46842. [Google Scholar] [CrossRef] [Green Version]
- Parkes, H.A.; Preston, E.; Wilks, D.; Ballesteros, M.; Carpenter, L.; Wood, L.; Kraegen, E.W.; Furler, S.M.; Cooney, G.J. Overexpression of acyl-CoA synthetase-1 increases lipid deposition in hepatic (HepG2) cells and rodent liver in vivo. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E737–E744. [Google Scholar] [CrossRef]
- Li, L.O.; Ellis, J.M.; Paich, H.A.; Wang, S.; Gong, N.; Altshuller, G.; Thresher, R.J.; Koves, T.R.; Watkins, S.M.; Muoio, D.M.; et al. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J. Biol. Chem. 2009, 284, 27816–27826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, R.; Poschmann, J.; Sukarieh, R.; Too, P.G.; Julien, S.G.; Xu, F.; Teh, A.L.; Holbrook, J.D.; Ng, K.L.; Chong, Y.S.; et al. ACSL1 is associated with fetal programming of insulin sensitivity and cellular lipid content. Mol. Endocrinol. 2015, 29, 909–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, K.; Shan, B.; Wei, S.; Li, D.; Han, H.; Wei, W.; Chen, J.; Liu, H.; Zhang, L. A genome-wide landscape of mRNAs, lncRNAs, and circRNAs during subcutaneous adipogenesis in pigs. J. Anim. Sci. Biotechnol. 2018, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Bagga, S.; Bracht, J.; Hunter, S.; Massirer, K.; Holtz, J.; Eachus, R.; Pasquinelli, A.E. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 2005, 122, 553–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheideler, M. MicroRNAs in adipocyte formation and obesity. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, M.; Wang, Y.; Sun, B.; Xiao, Z.; Ye, L.; Zhang, X. MiR-205 modulates abnormal lipid metabolism of hepatoma cells via targeting acyl-CoA synthetase long-chain family member 1 (ACSL1) mRNA. Biochem. Biophys. Res. Commun. 2014, 444, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Lian, S.; Guo, J.R.; Nan, X.M.; Ma, L.; Loor, J.J.; Bu, D.P. MicroRNA Bta-miR-181a regulates the biosynthesis of bovine milk fat by targeting ACSL1. J. Dairy Sci. 2016, 99, 3916–3924. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.H.; Wang, Z.; Yue, Y.X.; Li, H.; Li, Z.J.; Han, R.L.; Tian, Y.D.; Kang, X.T.; Liu, X.J. MiR-34a-5p increases hepatic triglycerides and total cholesterol levels by regulating ACSL1 protein expression in laying hens. Int. J. Mol. Sci. 2019, 20, 4420. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, Q.; Tao, Z.; Shi, L.; Ban, D.; Zhang, B.; Yang, Y.; Zhang, H.; Wu, C. Expression and genome polymorphism of ACSL1 gene in different pig breeds. Mol. Biol. Rep. 2012, 39, 8787–8792. [Google Scholar] [CrossRef]
- Shan, T.; Liu, W.; Kuang, S. Fatty acid binding protein 4 expression marks a population of adipocyte progenitors in white and brown adipose tissues. FASEB J. 2013, 27, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.H.; Reardon, C.A.; Mazzone, T. Endogenous ApoE Expression Modulates Adipocyte Triglyceride Content and Turnover. Diabetes 2006, 55, 3394–3402. [Google Scholar] [CrossRef] [Green Version]
- Schmid, B.; Rippmann, J.F.; Tadayyon, M.; Hamilton, B.S. Inhibition of fatty acid synthase prevents preadipocyte differentiation. Biochem. Biophys. Res. Commun. 2005, 328, 1073–1082. [Google Scholar] [CrossRef]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat Mobilization in Adipose Tissue Is Promoted by Adipose Triglyceride Lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef] [Green Version]
- Holm, C. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 2003, 31, 1120–1124. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, S.; Liu, S.; Wang, Y.; Jin, H.; Ma, H.; Luo, X.; Lian, Z.; Cao, Y. Effects of Long-Chain Fatty Acyl-CoA Synthetase 1 on Diglyceride Synthesis and Arachidonic Acid Metabolism in Sheep Adipocytes. Int. J. Mol. Sci. 2020, 21, 2044. [Google Scholar] [CrossRef] [Green Version]
- Lobo, S.; Wiczer, B.M.; Bernlohr, D.A. Functional Analysis of Long-chain Acyl-CoA Synthetase 1 in 3T3-L1 Adipocytes. J. Biol. Chem. 2009, 284, 18347–18356. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Li, X.; Meng, H.; Chen, L.; Meng, F. ACSL1 affects Triglyceride Levels through the PPARγ Pathway. Int. J. Med. Sci. 2020, 17, 720–727. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Raza, S.H.A.; Tian, H.; Shi, B.; Luo, Y.; Wang, J.; Liu, X.; Li, S.; Bai, Y.; Hu, J. Effects of overexpression of ACSL1 gene on the synthesis of unsaturated fatty acids in adipocytes of bovine. Arch. Biochem. Biophys. 2020, 695, 108648. [Google Scholar] [CrossRef]
- Xu, Y.; He, Q.; Lu, Y.; Tao, F.; Zhao, L.; Ou, R. MicroRNA-218-5p inhibits cell growth and metastasis in cervical cancer via LYN/NF-κB signaling pathway. Cancer Cell Int. 2018, 18, 198. [Google Scholar] [CrossRef]
- Peng, P.; Chen, T.; Wang, Q.; Zhang, Y.; Zheng, F.; Huang, S.; Tang, Y.; Yang, C.; Ding, W.; Ren, D.; et al. Decreased miR-218–5p Levels as a Serum Biomarker in Bone Metastasis of Prostate Cancer. Oncol. Res. Treat. 2019, 42, 165–185. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, H.; Ye, F.; Zhuang, Z. The Multifunction Of miR-218-5p-Cx43 Axis in Breast Cancer. OncoTargets Ther. 2019, 12, 8319–8328. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Chen, Y.; Yang, N.; Chen, Q.; Bao, Z.; Liu, M.; Hu, S.; Li, J.; Wu, X. MiR-218-5p regulates skin and hair follicle development through Wnt/β-catenin signaling pathway by targeting SFRP2. J. Cell. Physiol. 2019, 234, 20329–20341. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Lutz, H.; Huang, K.; Su, T.; Cores, J.; Dinh, P.-U.C.; Cheng, K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci. Adv. 2020, 6, eaba1685. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, B.; Yan, M.; Yang, K.; Lin, W.; Yan, J.; Wei, S.; Wei, W.; Chen, J.; Zhang, L. MiR-218-5p Affects Subcutaneous Adipogenesis by Targeting ACSL1, a Novel Candidate for Pig Fat Deposition. Genes 2022, 13, 260. https://doi.org/10.3390/genes13020260
Shan B, Yan M, Yang K, Lin W, Yan J, Wei S, Wei W, Chen J, Zhang L. MiR-218-5p Affects Subcutaneous Adipogenesis by Targeting ACSL1, a Novel Candidate for Pig Fat Deposition. Genes. 2022; 13(2):260. https://doi.org/10.3390/genes13020260
Chicago/Turabian StyleShan, Baosen, Mengting Yan, Kai Yang, Weimin Lin, Jiayu Yan, Shengjuan Wei, Wei Wei, Jie Chen, and Lifan Zhang. 2022. "MiR-218-5p Affects Subcutaneous Adipogenesis by Targeting ACSL1, a Novel Candidate for Pig Fat Deposition" Genes 13, no. 2: 260. https://doi.org/10.3390/genes13020260




