Dkk3/REIC Deficiency Impairs Spermiation, Sperm Fibrous Sheath Integrity and the Sperm Motility of Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Generation of Knock-Out Mouse Model and Genotype Identification
2.2. Periodic Acid Schiff (PAS) Staining
2.3. Immunofluorescence Staining
2.4. Assessment of Sperm Count, Vitality and Motility
2.5. Transmission Electron Microscope (TEM) Examination
2.6. Testicular RNA-Seq and GO Enrichment Analysis
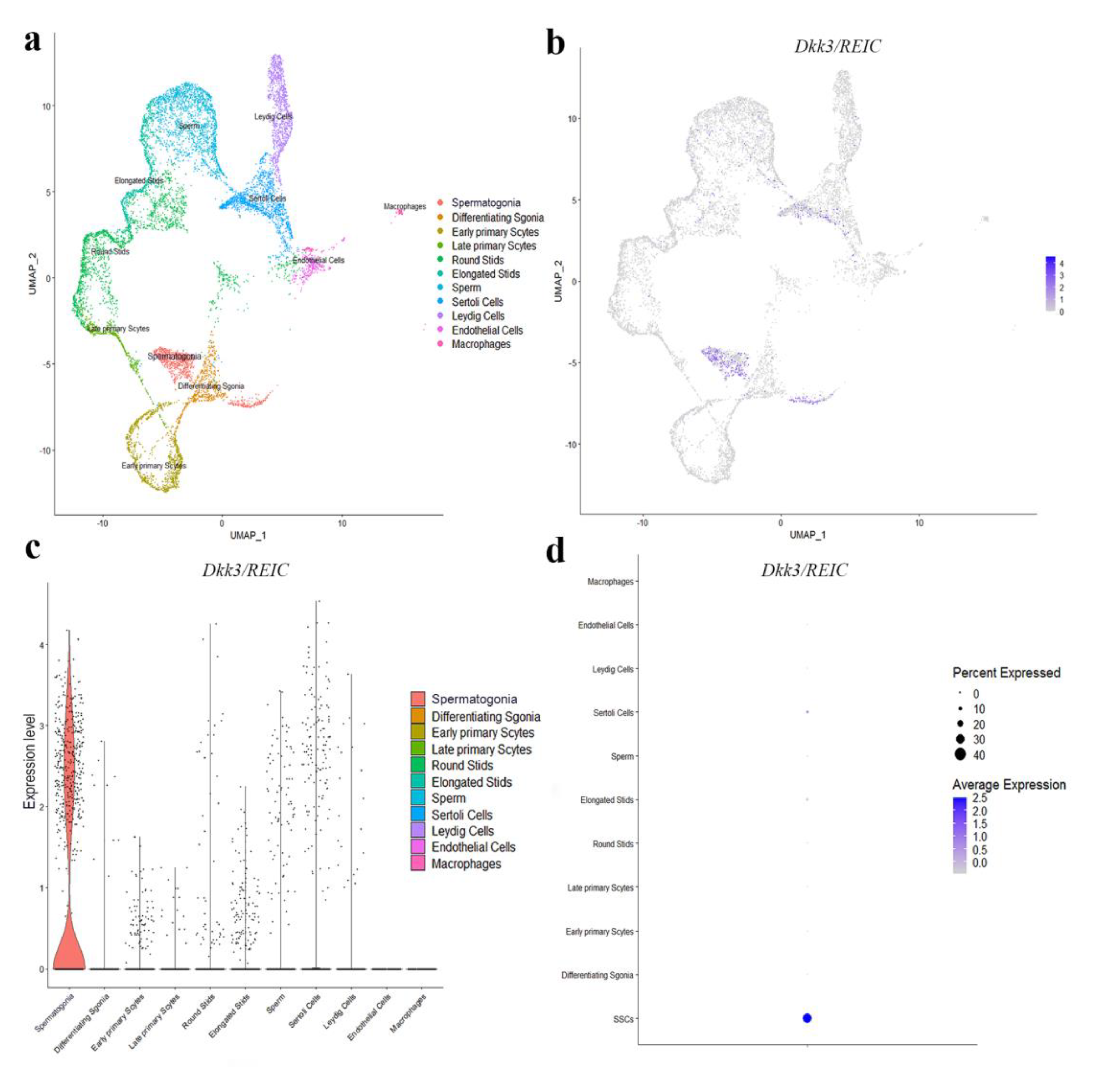
2.7. Analysis of Public Single Cell RNA-Seq Data
2.8. Statistical Analysis
3. Results
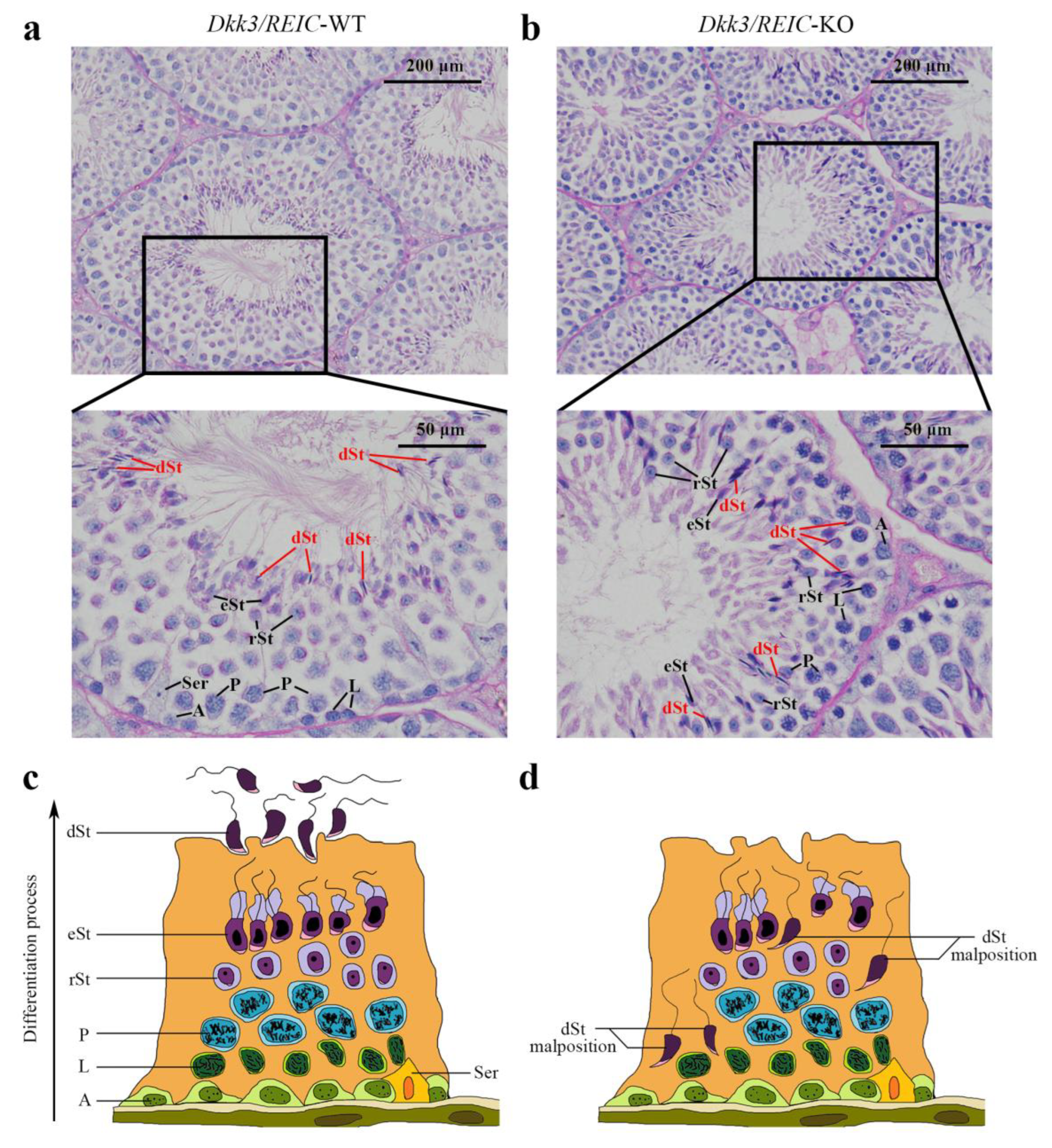
3.1. Spermiation Failuare and Malposition of Differentiated Spermatids Exist in Testicular Sections of Dkk3/REIC-KO Mice
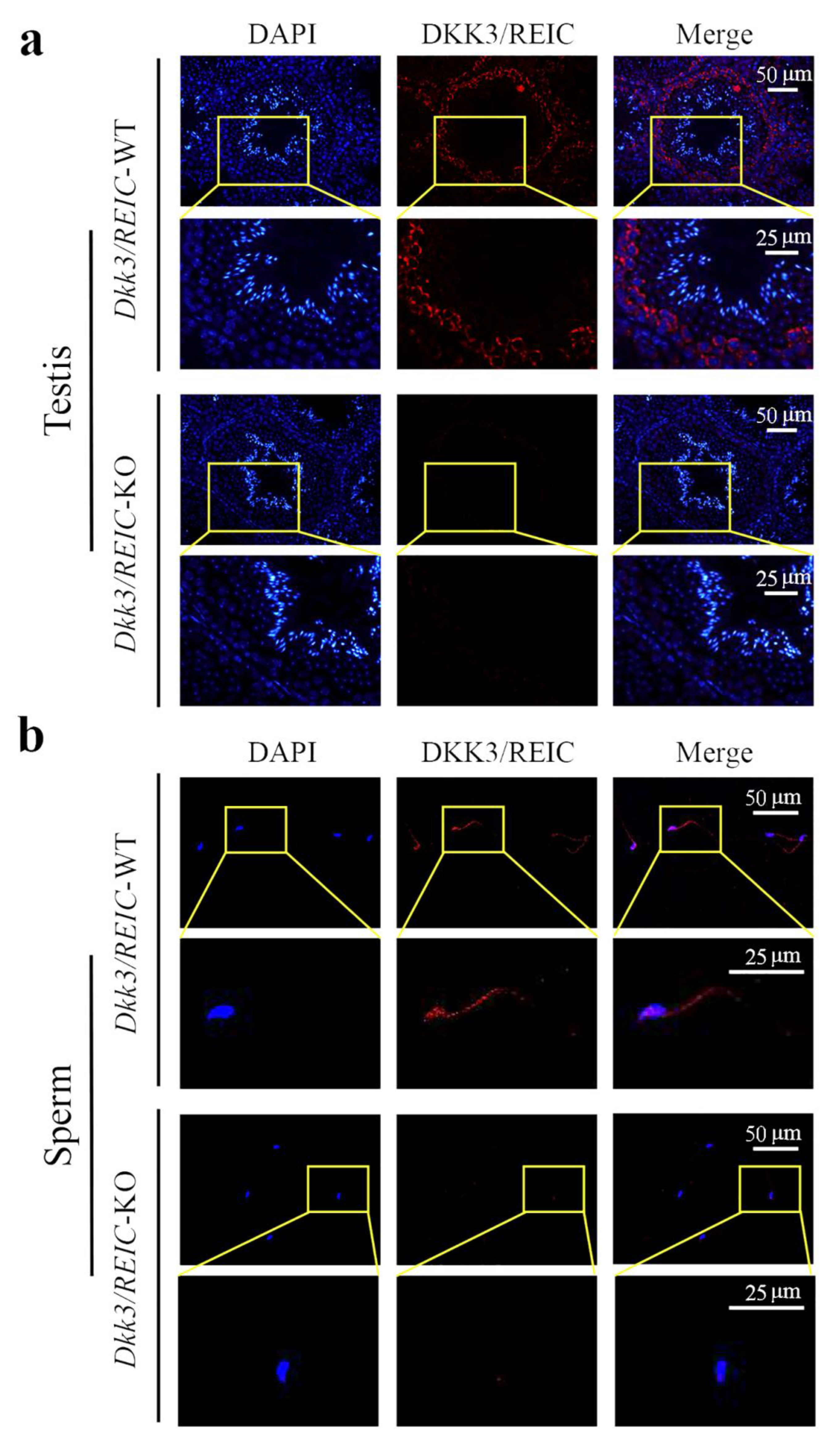
3.2. Expression and Distribution of Dkk3/REIC in Testes and Sperm of Male Mouse
3.3. Sperm Motility Is Impaired in Dkk3/REIC-KO Male Mice While the Sperm Total Count and Vitality Rate Show No Significant Difference
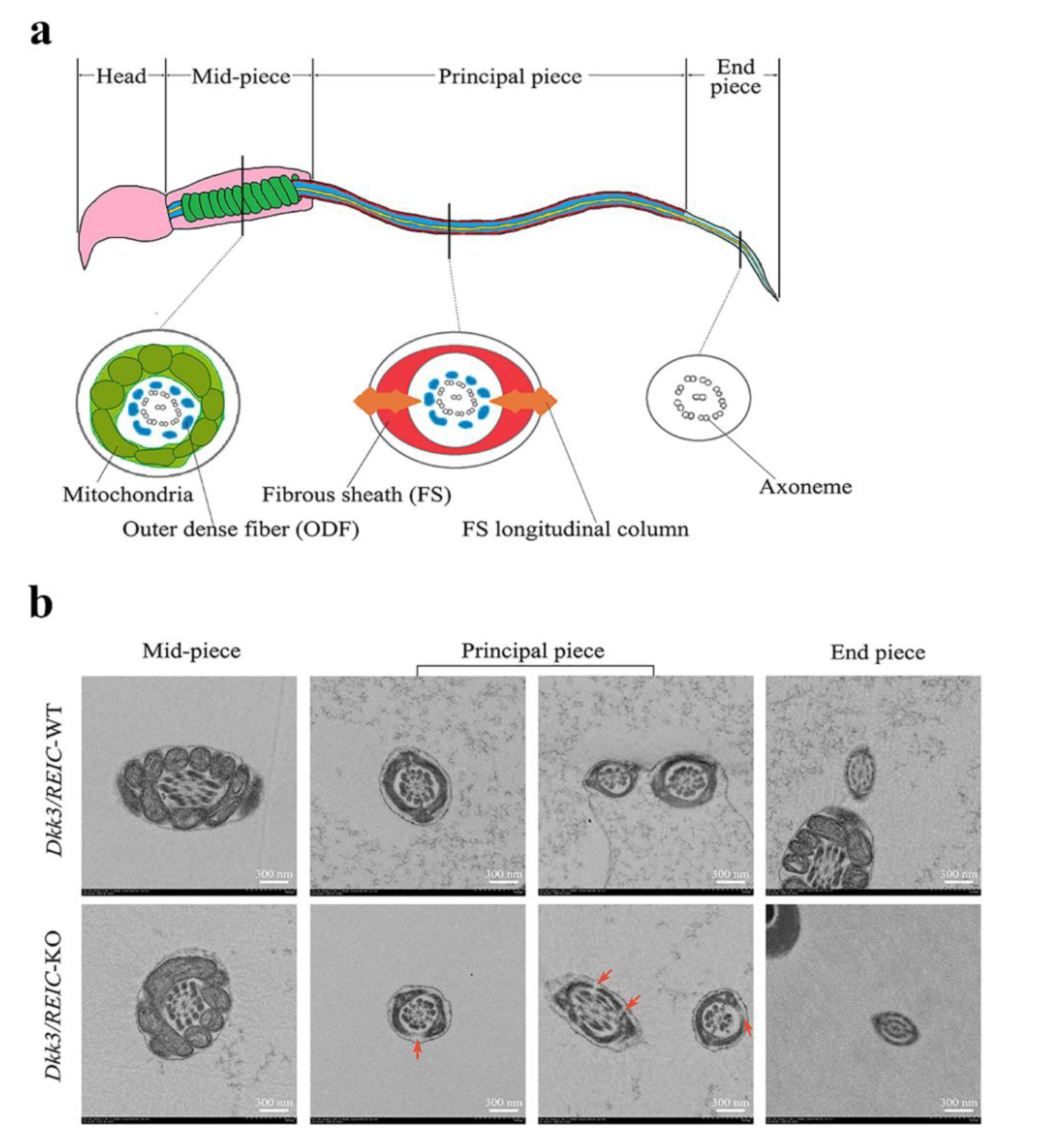
3.4. Ultrastructural Examination Reveals Defects in Flagellum Fibrous Sheath of Dkk3/REIC-KO Mouse Sperm
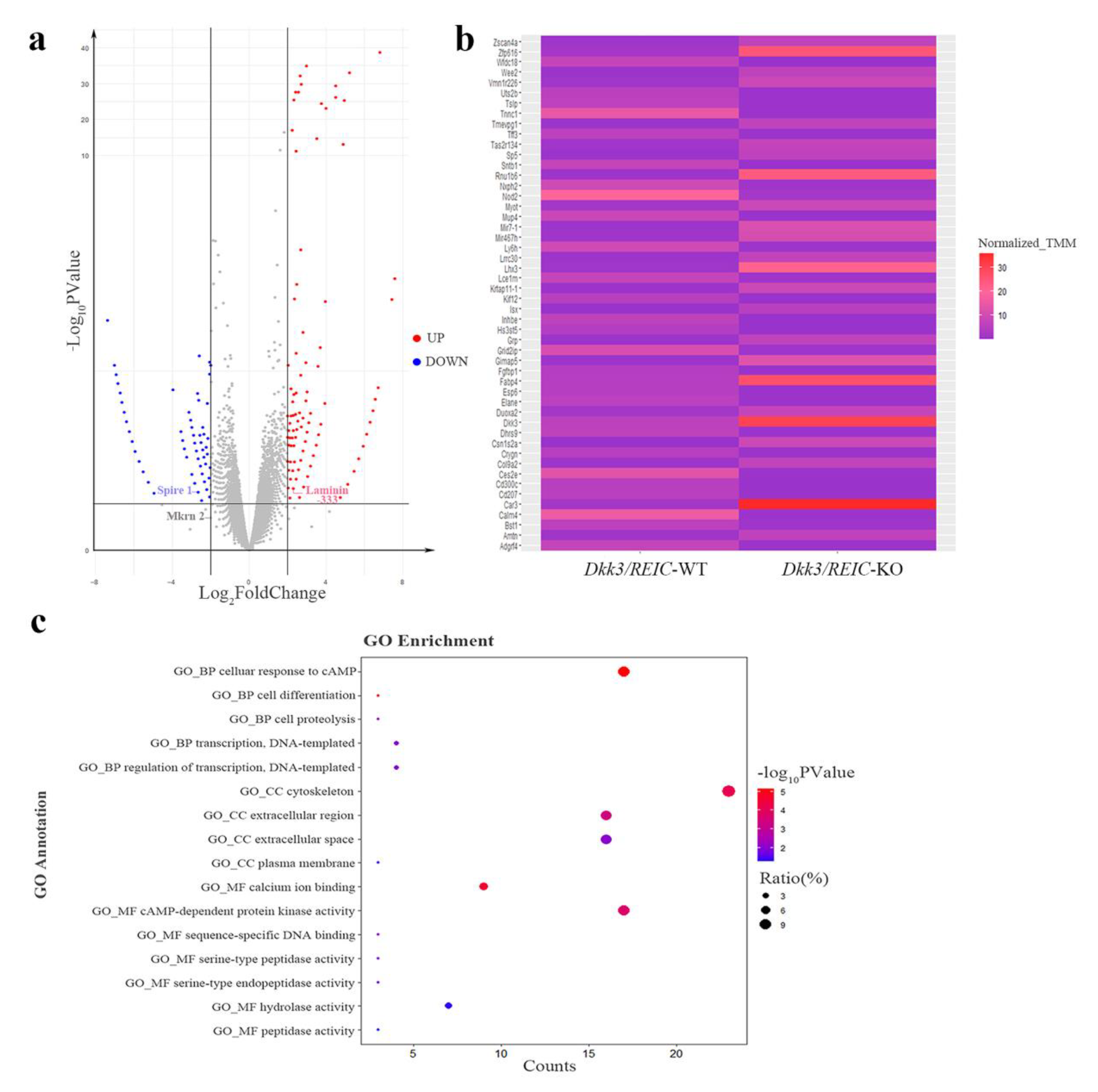
3.5. RNA Sequencing of Testicular Transcriptome from Dkk3/REIC-WT and Dkk3/REIC-KO Mice and GO Enrichment Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pathak, U.I.; Gabrielsen, J.S.; Lipshultz, L.I. Cutting-Edge Evaluation of Male Infertility. Urol. Clin. N. Am. 2020, 47, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Krausz, C. Male infertility: Pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Usui, K.; Sanjo, H.; Takeshima, T.; Kawahara, T.; Uemura, H.; Yumura, Y. Genetic disorders and male infertility. Reprod. Med. Biol. 2020, 19, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Lin, W.; Sun, J.; Watanabe, M.; Xu, A.; Araki, M.; Nasu, Y.; Tang, Z.; Huang, P. The role of Wnt signaling in male reproductive physiology and pathology. Mol. Hum. Reprod. 2021, 27, gaaa085. [Google Scholar] [CrossRef]
- Cheng, J.M.; Tang, J.X.; Li, J.; Wang, Y.Q.; Wang, X.X.; Zhang, Y.; Chen, S.R.; Liu, Y.X. Role of WNT signaling in epididymal sperm maturation. J. Assist. Reprod. Genet. 2018, 35, 229–236. [Google Scholar] [CrossRef]
- Baetta, R.; Banfi, C. Dkk (Dickkopf) Proteins. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1330–1342. [Google Scholar] [CrossRef]
- Tsuji, T.; Miyazaki, M.; Sakaguchi, M.; Inoue, Y.; Namba, M. A REIC gene shows down-regulation in human immortalized cells and human tumor-derived cell lines. Biochem. Biophys. Res. Commun. 2000, 268, 20–24. [Google Scholar] [CrossRef]
- Kohn, M.J.; Kaneko, K.J.; DePamphilis, M.L. DkkL1 (Soggy), a Dickkopf family member, localizes to the acrosome during mammalian spermatogenesis. Mol. Reprod. Dev. 2005, 71, 516–522. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.J.; Hou, X.J.; Liao, S.Y.; Wang, X.X.; Cooke, H.J.; Zhang, M.; Han, C. SLXL1, a novel acrosomal protein, interacts with DKKL1 and is involved in fertilization in mice. PLoS ONE 2011, 6, e20866. [Google Scholar] [CrossRef] [Green Version]
- Fujita, H.; Bando, T.; Oyadomari, S.; Ochiai, K.; Watanabe, M.; Kumon, H.; Ohuchi, H. Dkk3/REIC, an N-glycosylated Protein, Is a Physiological Endoplasmic Reticulum Stress Inducer in the Mouse Adrenal Gland. Acta Med. Okayama 2020, 74, 199–208. [Google Scholar] [CrossRef]
- Inoue, J.; Fujita, H.; Bando, T.; Kondo, Y.; Kumon, H.; Ohuchi, H. Expression analysis of Dickkopf-related protein 3 (Dkk3) suggests its pleiotropic roles for a secretory glycoprotein in adult mouse. J. Mol. Histol. 2017, 48, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampson, M.J.; Decker, W.K.; Beaudet, A.L.; Ruitenbeek, W.; Armstrong, D.; Hicks, M.J.; Craigen, W.J. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J. Biol. Chem. 2001, 276, 39206–39212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, S.A.; Miyata, H.; Satouh, Y.; Aitken, R.J.; Baker, M.A.; Ikawa, M. CABYR is essential for fibrous sheath integrity and progressive motility in mouse spermatozoa. J. Cell Sci. 2016, 129, 4379–4387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, D.S.; Wadhwa, N.; Kunj, N.; Sarda, K.; Pradhan, B.S.; Majumdar, S.S. Dickkopf homolog 3 (DKK3) plays a crucial role upstream of WNT/β-CATENIN signaling for Sertoli cell mediated regulation of spermatogenesis. PLoS ONE 2013, 8, e63603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, E.A.; de Rooij, D.G. Staging of mouse seminiferous tubule cross-sections. Methods Mol. Biol. 2009, 558, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Toyama, Y.; Maekawa, M.; Yuasa, S. Ectoplasmic specializations in the Sertoli cell: New vistas based on genetic defects and testicular toxicology. Anat. Sci. Int. 2003, 78, 1–16. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis 2014, 4, e979623. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.H.; Mruk, D.D.; Wong, E.W.; Lee, W.M.; Cheng, C.Y. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 8950–8955. [Google Scholar] [CrossRef] [Green Version]
- Wen, Q.; Li, N.; Xiao, X.; Lui, W.Y.; Chu, D.S.; Wong, C.K.C.; Lian, Q.; Ge, R.; Lee, W.M.; Silvestrini, B.; et al. Actin nucleator Spire 1 is a regulator of ectoplasmic specialization in the testis. Cell Death Dis. 2018, 9, 208. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.C.; Xie, Y.X.; Wang, C.S.; Shi, Z.M.; Jiang, C.F.; Tang, Y.Y.; Qian, X.; Wang, L.; Jiang, B.H. Mkrn2 deficiency induces teratozoospermia and male infertility through p53/PERP-mediated apoptosis in testis. Asian J. Androl. 2020, 22, 414–421. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Li, W.; Zhang, Z.; Shang, X.; Zhang, D.; Li, Y.; Zhang, S.; Liu, J.; Hess, R.A.; et al. Intraflagellar transporter protein (IFT27), an IFT25 binding partner, is essential for male fertility and spermiogenesis in mice. Dev. Biol. 2017, 432, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Macleod, J.; Mei, X.; Erlichman, J.; Orr, G.A. Association of the regulatory subunit of a type II cAMP-dependent protein kinase and its binding proteins with the fibrous sheath of rat sperm flagellum. Eur. J. Biochem. 1994, 225, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Luconi, M.; Cantini, G.; Baldi, E.; Forti, G. Role of a-kinase anchoring proteins (AKAPs) in reproduction. Front. Biosci. 2011, 16, 1315–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, S.; Brothag, C.; Vijayaraghavan, S. Signaling Enzymes Required for Sperm Maturation and Fertilization in Mammals. Front. Cell Dev. Biol. 2019, 7, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.; Sa, R.; Barros, A.; Sousa, M. Major regulatory mechanisms involved in sperm motility. Asian J. Androl. 2017, 19, 5–14. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, R.; Lin, W.; Fujita, H.; Sun, J.; Kinoshita, R.; Ochiai, K.; Futami, J.; Watanabe, M.; Ohuchi, H.; Sakaguchi, M.; et al. Dkk3/REIC Deficiency Impairs Spermiation, Sperm Fibrous Sheath Integrity and the Sperm Motility of Mice. Genes 2022, 13, 285. https://doi.org/10.3390/genes13020285
Xue R, Lin W, Fujita H, Sun J, Kinoshita R, Ochiai K, Futami J, Watanabe M, Ohuchi H, Sakaguchi M, et al. Dkk3/REIC Deficiency Impairs Spermiation, Sperm Fibrous Sheath Integrity and the Sperm Motility of Mice. Genes. 2022; 13(2):285. https://doi.org/10.3390/genes13020285
Chicago/Turabian StyleXue, Ruizhi, Wenfeng Lin, Hirofumi Fujita, Jingkai Sun, Rie Kinoshita, Kazuhiko Ochiai, Junichiro Futami, Masami Watanabe, Hideyo Ohuchi, Masakiyo Sakaguchi, and et al. 2022. "Dkk3/REIC Deficiency Impairs Spermiation, Sperm Fibrous Sheath Integrity and the Sperm Motility of Mice" Genes 13, no. 2: 285. https://doi.org/10.3390/genes13020285








