Transcriptome Profiling Reveals Role of MicroRNAs and Their Targeted Genes during Adventitious Root Formation in Dark-Pretreated Micro-Shoot Cuttings of Tetraploid Robinia pseudoacacia L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Sample Collection
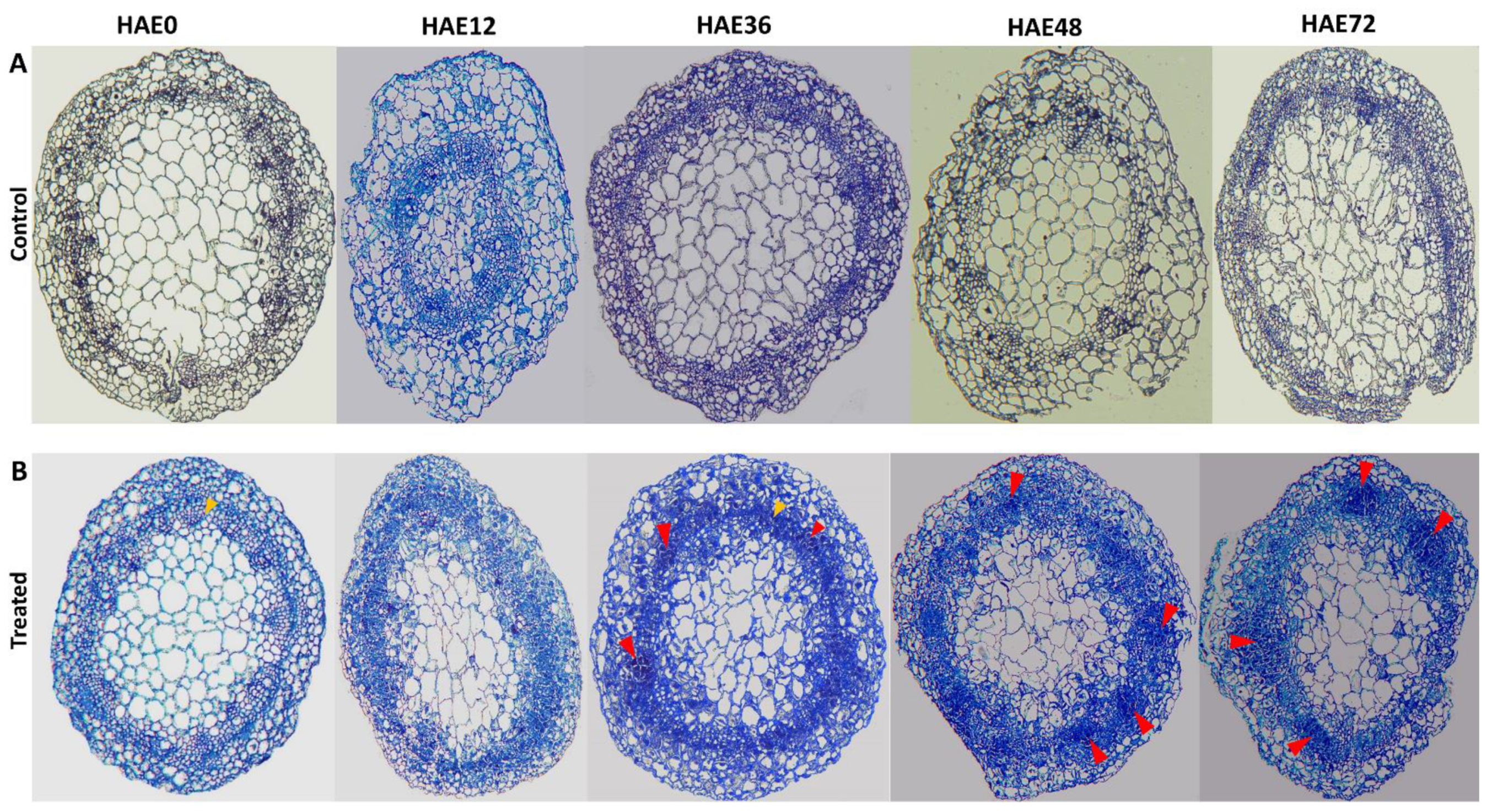
2.2. Paraffin Section Preparation and Microscopic Examination
2.3. RNA and Small RNA Library Construction and Sequencing
2.4. Analysis of Differentially Expressed miRNAs and Their Target Genes
2.5. miRNA Identification during AR Formation
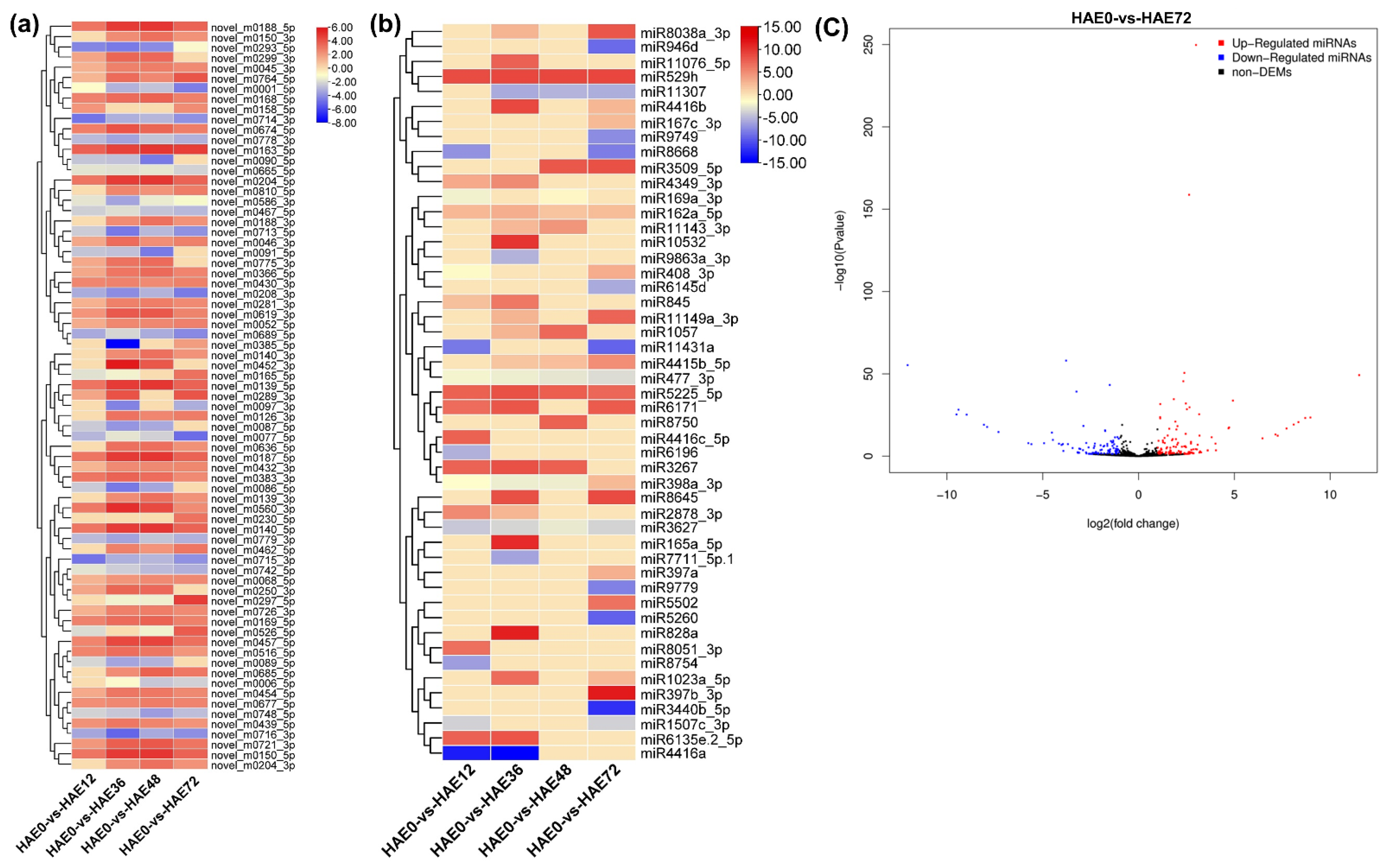
2.6. Differential Expression (DE) Analysis of miRNAs
2.7. Target Prediction of miRNAs
2.8. Relative Expression Analysis miRNA and Their Targets by Quantitative Real-Time PCR (qRT-PCR)
3. Results
3.1. The Effect of IBA on the Development of AR Formation in Dark-Pretreated Cuttings of Tetraploid R. pseudoacacia
3.2. Identification of DEGs and MicroRNAs during Dark-Pretreated IBA-Dependent AR Formation in Tetraploid R. pseudoacacia L.
3.3. Comparative Analysis of DEGs, MicroRNAs, and Their Expression Profiles during IBA-Dependent AR Development in Dark-Pretreated Cuttings of Tetraploid R. pseudoacacia L.
3.4. Annotation of Genes and MicroRNA during IBA-Dependent AR Formation in Dark-Pretreated Cuttings of Tetraploid R. pseudoacacia L.
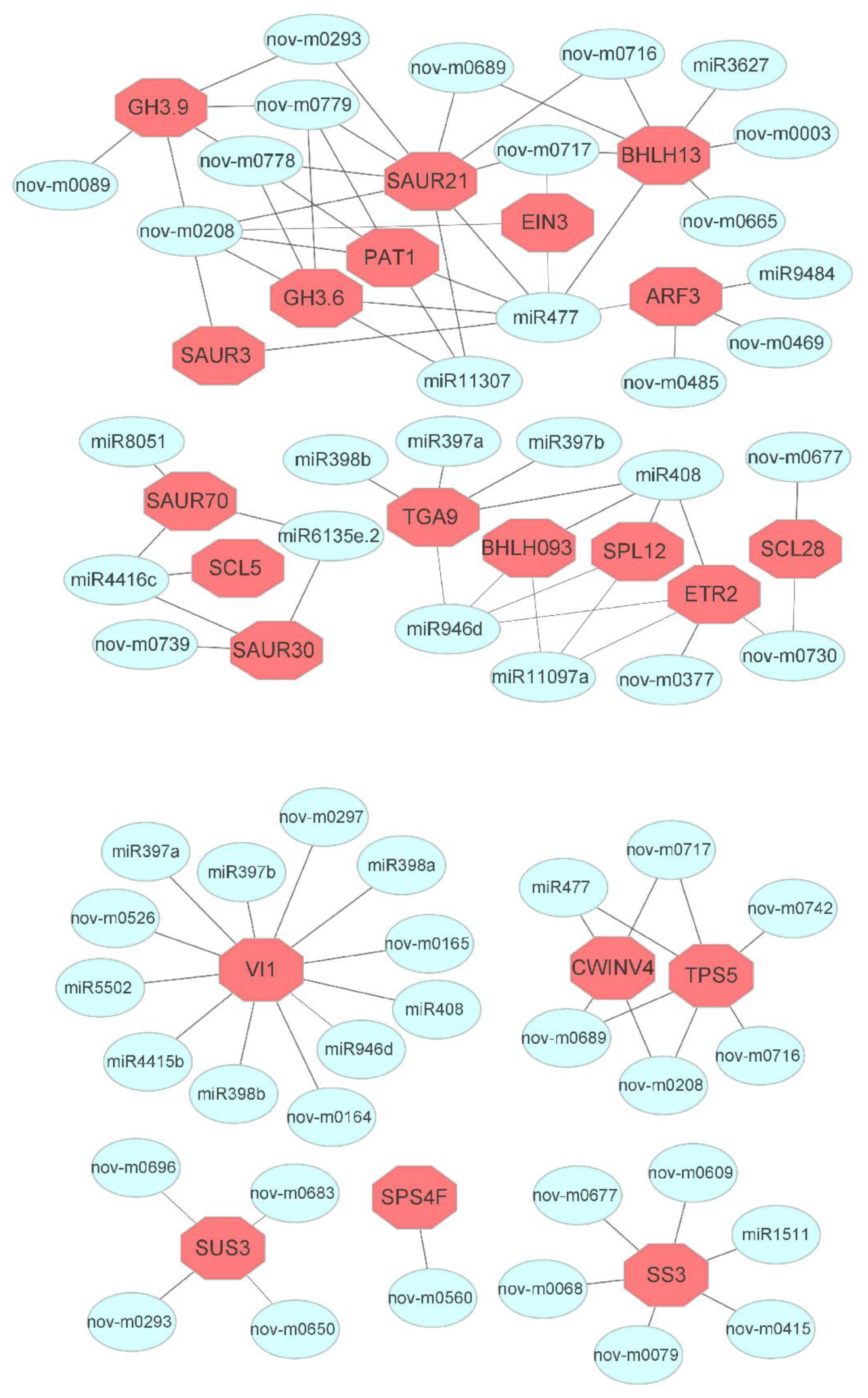
3.5. Association Analysis of miRNAs and Targeted Genes during IBA-Dependent AR Formation in Dark-Pretreated Cuttings
3.6. MicroRNAs and Targeted Genes Involved during Sucrose Metabolism and Hormone Signaling Transduction Pathways
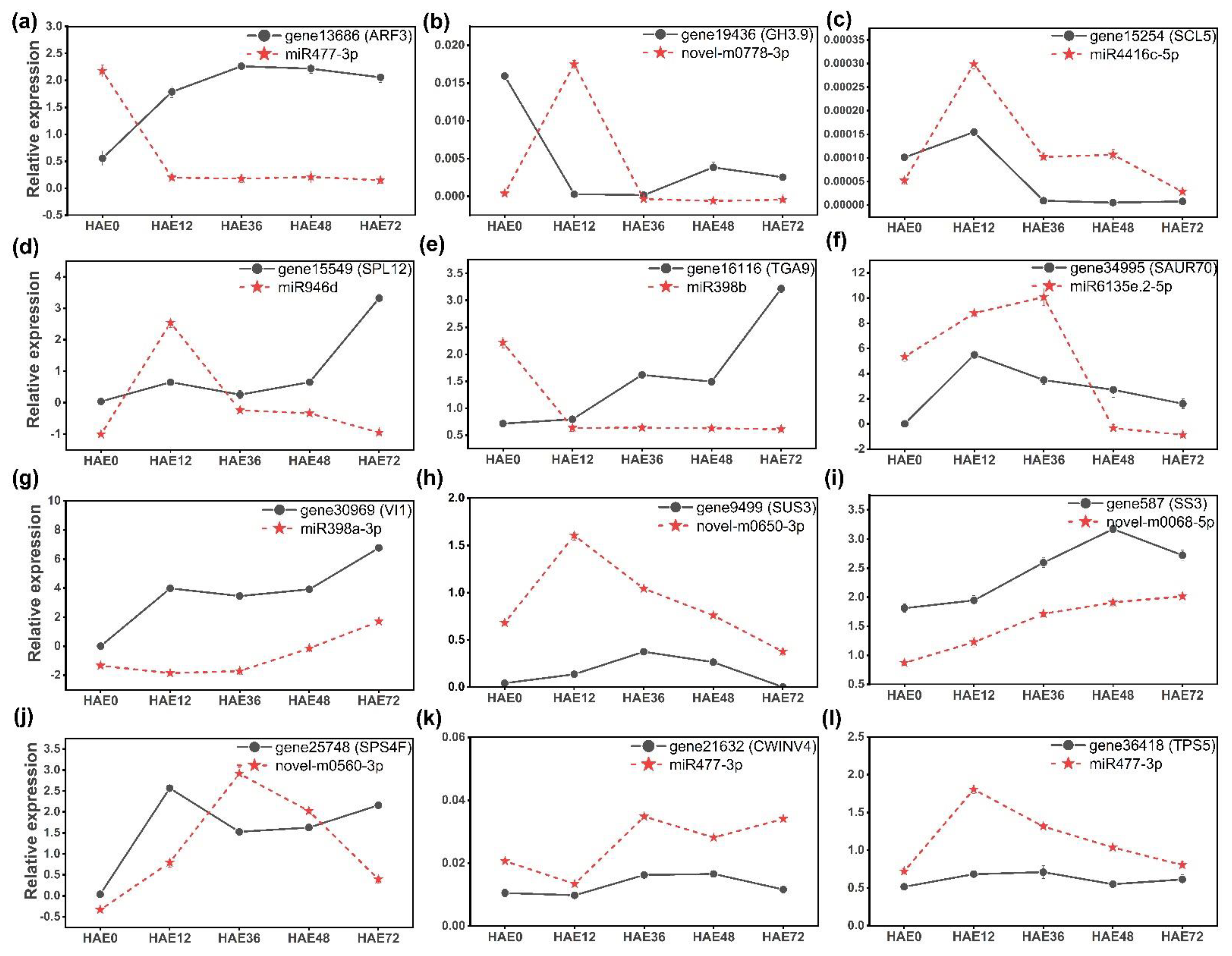
3.7. Relative Expression Analysis of Differentially Expressed miRNAs and Their Targeted Genes by qRT-PCR Assay
4. Discussion
4.1. Anatomical Observations during IBA-Dependent AR Formation in Dark-Pretreated Micro-Shoot Cuttings
4.2. Identification and Expression Profiling of miRNAs and Their Targets during IBA-Dependent AR Formation in Dark-Pretreated Tetraploid R. pseudoacacia L.
4.3. miRNAs and Their Target Genes during Plant Hormone Signaling Transduction Pathway
4.4. miRNAs and Their Target Genes in Sucrose Metabolic Pathways during AR Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, G.; Li, Y.; Li, F.; Xu, Z.; Sun, Y. Effects of root age on biomass and leaf nutrition in tetraploid Robinia pseudoacacia. J. Beijing For. Univ. 2009, 31, 37–41. [Google Scholar]
- Zhang, S.; Zhao, Z.; Zhang, L.; Zhou, Q. Comparative proteomic analysis of tetraploid black locust (Robinia pseudoacacia L.) cuttings in different phases of adventitious root development. Trees 2015, 29, 367–384. [Google Scholar] [CrossRef]
- Legué, V.; Rigal, A.; Bhalerao, R.P. Adventitious root formation in tree species: Involvement of transcription factors. Physiol. Plant 2014, 151, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, M.; Xie, W.; Huang, M. Overexpression of PeRHD3 alters the root architecture in Populus. Biochem. Biophys. Res. Commun. 2012, 424, 239–244. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Liu, L.; Meng, F. Physiological and proteomic responses of diploid and tetraploid black locust (Robinia pseudoacacia L.) subjected to salt stress. Int. J. Mol. Sci. 2013, 14, 20299–20325. [Google Scholar] [CrossRef] [Green Version]
- Ling, W.X.; Zhong, Z. Seasonal variation in rooting of the cuttings from Tetraploid Locust in relation to nutrients and endogenous plant hormones of the shoot. Turk. J. Agric. For. 2012, 36, 257–266. [Google Scholar]
- Wang, X.; Zhao, Z.; Quan, J. Indole-3-butyric acid on rooting and endogenous plant hormones in tetraploid and diploid Robinia pseudoacacia hardwood cuttings. Phyton-Rev. Int. Bot. Exp. 2011, 23, 93–99. [Google Scholar]
- Rédei, K.; Keserű, Z.; Csiha, I.; Rásó, J.; Bakti, B.; Takács, M. Improvement of black locust (Robinia pseudoacacia L.) growing under marginal site conditions in Hungary: Case studies. Acta Agrar. Debr. 2018, 74, 129–133. [Google Scholar] [CrossRef]
- Ragonezi, C.; Klimaszewska, K.; Castro, M.R.; Lima, M.; de Oliveira, P.; Zavattieri, M.A. Adventitious rooting of conifers: Influence of physical and chemical factors. Trees 2010, 24, 975–992. [Google Scholar] [CrossRef] [Green Version]
- Shu, W.; Zhou, H.; Jiang, C.; Zhao, S.; Wang, L.; Li, Q.; Yang, Z.; Groover, A.; Lu, M.Z. The auxin receptor TIR 1 homolog (Pag FBL 1) regulates adventitious rooting through interactions with Aux/IAA 28 in Populus. Plant Biotechnol. J. 2019, 17, 338–349. [Google Scholar] [CrossRef] [Green Version]
- Massoumi, M.; Krens, F.A.; Visser, R.G.; De Klerk, G.-J.M. Etiolation and flooding of donor plants enhance the capability of Arabidopsis explants to root. Plant Cell Tissue Organ. Cult. 2017, 130, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Lu, N.; Dai, L.; Luo, Z.; Wang, S.; Wen, Y.; Duan, H.; Hou, R.; Sun, Y.; Li, Y. Characterization of the transcriptome and gene expression of tetraploid black locust cuttings in response to etiolation. Genes 2017, 8, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husen, A. Rejuvenation and adventitious rooting in coppice-shoot cuttings of Tectona grandis as affected by stock-plant etiolation. Am. J. Plant Sci. 2011, 2, 370–374. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Gao, Y.; Song, X.; Ma, Q.; Zhang, J.; Pei, D. A novel rejuvenation approach to induce endohormones and improve rhizogenesis in mature Juglans tree. Plant Methods 2018, 14, 13. [Google Scholar] [CrossRef]
- Pacholczak, A.; Szydło, W.; Łukaszewska, A. The effect of etiolation and shading of stock plants on rhizogenesis in stem cuttings of Cotinus coggygria. Acta Physiol. Plant 2005, 27, 417–428. [Google Scholar] [CrossRef]
- Sorin, C.; Bussell, J.D.; Camus, I.; Ljung, K.; Kowalczyk, M.; Geiss, G.; McKhann, H.; Garcion, C.; Vaucheret, H.; Sandberg, G. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell 2005, 17, 1343–1359. [Google Scholar] [CrossRef] [Green Version]
- Lau, O.S.; Deng, X.W. Plant hormone signaling lightens up: Integrators of light and hormones. Curr. Opin. Plant Biol. 2010, 13, 571–577. [Google Scholar] [CrossRef]
- Keuskamp, D.H.; Pollmann, S.; Voesenek, L.A.; Peeters, A.J.; Pierik, R. Auxin transport through PIN-FORMED 3 (PIN3) controls shade avoidance and fitness during competition. Proc. Natl. Acad. Sci. USA 2010, 107, 22740–22744. [Google Scholar] [CrossRef] [Green Version]
- Kozuka, T.; Kobayashi, J.; Horiguchi, G.; Demura, T.; Sakakibara, H.; Tsukaya, H.; Nagatani, A. Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 2010, 153, 1608–1618. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Fan, R.; Guo, S.; Wang, P.; Zhu, X.; Fan, Y.; Chen, Y.; He, K.; Kumar, A.; Shi, J. The Arabidopsis MYB transcription factor, MYB111 modulates salt responses by regulating flavonoid biosynthesis. Environ. Exp. Bot. 2019, 166, 103807. [Google Scholar] [CrossRef]
- Li, C.; Zheng, L.; Wang, X.; Hu, Z.; Zheng, Y.; Chen, Q.; Hao, X.; Xiao, X.; Wang, X.; Wang, G. Comprehensive expression analysis of Arabidopsis GA2-oxidase genes and their functional insights. Plant Sci. 2019, 285, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef]
- Li, S.-W.; Shi, R.-F.; Leng, Y.; Zhou, Y. Transcriptomic analysis reveals the gene expression profile that specifically responds to IBA during adventitious rooting in mung bean seedlings. BMC Genom. 2016, 17, 43. [Google Scholar] [CrossRef] [Green Version]
- Ludwig-Müller, J. Synthesis and hydrolysis of auxins and their conjugates with different side-chain lengths: Are all products active auxins? Spiridion Brusina lecture. Period. Biol. 2020, 121–122, 81–96. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Javid, M.G.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heloir, M.-C.; Kevers, C.; Hausman, J.-F.; Gaspar, T. Changes in the concentrations of auxins and polyamines during rooting of in-vitro-propagated walnut shoots. Tree Physiol. 1996, 16, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Lindroth, A.M.; Kvarnheden, A.; von Arnold, S. Isolation of a PSTAIRE CDC2 cDNA from Pinus contorta and its expression during adventitious root development. Plant Physiol. Biochem. 2001, 39, 107–114. [Google Scholar] [CrossRef]
- Lindroth, A.M.; Saarikoski, P.; Flygh, G.; Clapham, D.; Grönroos, R.; Thelander, M.; Ronne, H.; von Arnold, S. Two S-adenosylmethionine synthetase-encoding genes differentially expressed during adventitious root development in Pinus contorta. Plant Mol. Biol. 2001, 46, 335–346. [Google Scholar] [CrossRef]
- Sedira, M.; Butler, E.; Gallagher, T.; Welander, M. Verification of auxin-induced gene expression during adventitious rooting in rolB-transformed and untransformed apple Jork 9. Plant Sci. 2005, 168, 1193–1198. [Google Scholar] [CrossRef]
- Ricci, A.; Rolli, E.; Dramis, L.; Diaz-Sala, C. N, N′-bis-(2, 3-Methylenedioxyphenyl) urea and N, N′-bis-(3, 4-methylenedioxyphenyl) urea enhance adventitious rooting in Pinus radiata and affect expression of genes induced during adventitious rooting in the presence of exogenous auxin. Plant Sci. 2008, 175, 356–363. [Google Scholar] [CrossRef]
- Da Costa, C.T.; De Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.D.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhubing, H.; Shuyan, L. Comparative transcriptome analysis revealed the cooperative regulation of sucrose and IAA on adventitious root formation in lotus (Nelumbo nucifera Gaertn). BMC Genom. 2020, 21, 653. [Google Scholar]
- Li, K.; Liu, Z.; Xing, L.; Wei, Y.; Mao, J.; Meng, Y.; Bao, L.; Han, M.; Zhao, C.; Zhang, D. miRNAs associated with auxin signaling, stress response, and cellular activities mediate adventitious root formation in apple rootstocks. Plant Physiol. Biochem. 2019, 139, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Yang, C.; Liu, S.; Qi, H.; Wu, L.; Xu, L.-A.; Xu, M. MiRNA-target pairs regulate adventitious rooting in Populus: A functional role for miR167a and its target Auxin response factor 8. Tree Physiol. 2019, 39, 1922–1936. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Mao, J.; Tahir, M.M.; Wang, H.; Wei, Y.; Zhao, C.; Li, K.; Ma, D.; Zhao, C.; Zhang, D. Mdm-miR160 participates in auxin-induced adventitious root formation of apple rootstock. Sci. Hortic. 2020, 270, 109442. [Google Scholar] [CrossRef]
- Gutierrez, L.; Bussell, J.D.; Pacurar, D.I.; Schwambach, J.; Pacurar, M.; Bellini, C. Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 2009, 21, 3119–3132. [Google Scholar] [CrossRef] [Green Version]
- Marin, E.; Jouannet, V.; Herz, A.; Lokerse, A.S.; Weijers, D.; Vaucheret, H.; Nussaume, L.; Crespi, M.D.; Maizel, A. miR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell 2010, 22, 1104–1117. [Google Scholar] [CrossRef] [Green Version]
- Carlsbecker, A.; Lee, J.-Y.; Roberts, C.J.; Dettmer, J.; Lehesranta, S.; Zhou, J.; Lindgren, O.; Moreno-Risueno, M.A.; Vatén, A.; Thitamadee, S. Cell signalling by microRNA165/6 directs gene dose-dependent root cell fate. Nature 2010, 465, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Jiang, F.; Zheng, X.; Wu, Z. Identification and analysis of oxygen responsive microRNAs in the root of wild tomato (S. habrochaites). BMC Plant Biol. 2019, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Xu, Y.; Guo, C.; Zheng, J.; Zhou, B.; Zhang, Y.; Ding, Y.; Zhang, L.; Zhu, Z.; Wang, H. Modulation of miR156 to identify traits associated with vegetative phase change in tobacco (Nicotiana tabacum). J. Exp. Bot. 2016, 67, 1493–1504. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Ma, X.; Chen, D.; Wu, P.; Chen, M. MicroRNA-mediated signaling involved in plant root development. Biochem. Biophys. Res. Commun. 2010, 393, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.; Murashige, T.; Thorpe, T.; Vasil, I. Plant tissue culture media. In Vitro 1976, 12, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.Z.; Ud Din, S.; Imran, M.; Zhang, Z.; Pervaiz, T.; Han, C.; Un Nisa, Z.; Bakhsh, A.; Atif Muneer, M.; Sun, Y. Transcriptomic and Anatomic Profiling Reveal Etiolation Promotes Adventitious Rooting by Exogenous Application of 1-Naphthalene Acetic Acid in Robinia pseudoacacia L. Forests 2021, 12, 789. [Google Scholar] [CrossRef]
- Suárez, E.; Alfayate, C.; Pérez-Francés, J.F.; Rodríguez-Pérez, J.A. Structural and ultrastructural variations in in vitro and ex vitro rooting of microcuttings from two micropropagated Leucospermum (Proteaceae). Sci. Hortic. 2018, 239, 300–307. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Lyu, S.; Yu, Y.; Xu, S.; Cai, W.; Chen, G.; Chen, J.; Pan, D.; She, W. Identification of Appropriate Reference Genes for Normalizing miRNA Expression in Citrus Infected by Xanthomonas citri subsp. citri. Genes 2020, 11, 17. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.M.; Wang, H.; Ahmad, B.; Liu, Y.; Fan, S.; Li, K.; Lei, C.; Shah, K.; Li, S.; Zhang, D. Identification and characterization of NRT gene family reveals their critical response to nitrate regulation during adventitious root formation and development in apple rootstock. Sci. Hortic. 2021, 275, 109642. [Google Scholar] [CrossRef]
- Min, L.; Li, Y.; Hu, Q.; Zhu, L.; Gao, W.; Wu, Y.; Ding, Y.; Liu, S.; Yang, X.; Zhang, X. Sugar and auxin signaling pathways respond to high-temperature stress during anther development as revealed by transcript profiling analysis in cotton. Plant Physiol. 2014, 164, 1293–1308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Henderson, I.R.; Lu, C.; Green, P.J.; Jacobsen, S.E. Role of RNA polymerase IV in plant small RNA metabolism. Proc. Natl. Acad. Sci. USA 2007, 104, 4536–4541. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Yang, T.; Tang, Y.; Aisimutuola, P.; Zhang, G.; Wang, B.; Li, N.; Wang, J.; Yu, Q. Transcriptomic profile analysis of non-coding RNAs involved in Capsicum chinense Jacq. fruit ripening. Sci. Hortic. 2020, 264, 109158. [Google Scholar] [CrossRef]
- Gao, C.; Ju, Z.; Cao, D.; Zhai, B.; Qin, G.; Zhu, H.; Fu, D.; Luo, Y.; Zhu, B. Micro RNA profiling analysis throughout tomato fruit development and ripening reveals potential regulatory role of RIN on micro RNA s accumulation. Plant Biotechnol. J. 2015, 13, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, R.; Vaucheret, H.; Trejo, J.; Bartel, D.P. A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev. 2006, 20, 3407–3425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Li, D.; Cong, L.; Lu, X. Identification of microRNAs involved in crosstalk between nitrogen, phosphorus and potassium under multiple nutrient deficiency in sorghum. Crop. J. 2021, 9, 465–475. [Google Scholar]
- Morin, R.D.; Aksay, G.; Dolgosheina, E.; Ebhardt, H.A.; Magrini, V.; Mardis, E.R.; Sahinalp, S.C.; Unrau, P.J. Comparative analysis of the small RNA transcriptomes of Pinus contorta and Oryza sativa. Genome Res. 2008, 18, 571–584. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, W.; Chang, H.; Zhou, J.; Luo, Y.; Zhang, K.; Wang, B. Sweet cherry fruit miRNAs and effect of high CO2 on the profile associated with ripening. Planta 2019, 249, 1799–1810. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Li, D.; Chen, H. Identification and characterization of maize microRNAs involved in the very early stage of seed germination. BMC Genom. 2011, 12, 154. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Chen, J.; Li, Z.; Li, X.; Hu, X.; Huang, Y.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L. Integrated profiling of microRNAs and mRNAs: MicroRNAs located on Xq27. 3 associate with clear cell renal cell carcinoma. PLoS ONE 2010, 5, e15224. [Google Scholar] [CrossRef]
- Druege, U.; Hilo, A.; Pérez-Pérez, J.M.; Klopotek, Y.; Acosta, M.; Shahinnia, F.; Zerche, S.; Franken, P.; Hajirezaei, M.R. Molecular and physiological control of adventitious rooting in cuttings: Phytohormone action meets resource allocation. Ann. Bot. 2019, 123, 929–949. [Google Scholar] [CrossRef] [Green Version]
- Guan, L.; Murphy, A.S.; Peer, W.A.; Gan, L.; Li, Y.; Cheng, Z.-M. Physiological and molecular regulation of adventitious root formation. Crit. Rev. Plant Sci. 2015, 34, 506–521. [Google Scholar] [CrossRef]
- Niemi, K.; Scagel, C. Adventitious Root Formation of Forest Trees and Horticultural Plants-from Genes to Applications; Research Signpost: Kerala, India, 2009; 408p. [Google Scholar]
- De Klerk, G.-J.; Van Der Krieken, W.; de Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. Vitr. Cell. Dev. Biol.-Plant 1999, 35, 189–199. [Google Scholar]
- San-José, M.; Vidal, N.; Ballester, A. Anatomical and biochemical changes during root formation in oak and apple shoots cultured in vitro. Agronomie 1992, 12, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Geiss, G.; Gutierrez, L.; Bellini, C. Adventitious root formation: New insights and perspectives. Annu. Plant Rev. Online 2018, 37, 127–156. [Google Scholar]
- Wang, Y.; Tao, X.; Tang, X.-M.; Xiao, L.; Sun, J.-l.; Yan, X.-F.; Li, D.; Deng, H.-Y.; Ma, X.-R. Comparative transcriptome analysis of tomato (Solanum lycopersicum) in response to exogenous abscisic acid. BMC Genom. 2013, 14, 841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrot-Rechenmann, C. Cellular responses to auxin: Division versus expansion. Cold Spring Harb. Perspect. Biol. 2010, 2, a001446. [Google Scholar] [CrossRef] [PubMed]
- Libao, C.; Runzhi, J.; Jianjun, Y.; Xiaoyong, X.; Haitao, Z.; Shuyan, L. Transcriptome profiling reveals an IAA-regulated response to adventitious root formation in lotus seedling. Z. Nat. C 2018, 73, 229–240. [Google Scholar]
- Cheng, L.; Liu, H.; Jiang, R.; Li, S. A proteomics analysis of adventitious root formation after leaf removal in lotus (Nelumbo nucifera Gaertn.). Z. Nat. C 2018, 73, 375–389. [Google Scholar]
- Marioni, J.C.; Mason, C.E.; Mane, S.M.; Stephens, M.; Gilad, Y. RNA-seq: An assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 2008, 18, 1509–1517. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef]
- Rigal, A.; Yordanov, Y.S.; Perrone, I.; Karlberg, A.; Tisserant, E.; Bellini, C.; Busov, V.B.; Martin, F.; Kohler, A.; Bhalerao, R. The AINTEGUMENTA LIKE1 homeotic transcription factor PtAIL1 controls the formation of adventitious root primordia in poplar. Plant Physiol. 2012, 160, 1996–2006. [Google Scholar] [CrossRef] [Green Version]
- Ludwig-Müller, J.; Vertocnik, A.; Town, C.D. Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. J. Exp. Bot. 2005, 56, 2095–2105. [Google Scholar] [CrossRef] [Green Version]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, J.-P.; Zhang, D.; Zhang, X.; Li, K.; Liu, Z.; Meng, Y.; Lei, C.; Han, M.-Y. Effect of exogenous indole-3-butanoic acid (IBA) application on the morphology, hormone status, and gene expression of developing lateral roots in Malus hupehensis. Sci. Hortic. 2018, 232, 112–120. [Google Scholar] [CrossRef]
- Gautam, V.; Singh, A.; Verma, S.; Kumar, A.; Kumar, P.; Singh, S.; Mishra, V.; Sarkar, A.K. Role of miRNAs in root development of model plant Arabidopsis thaliana. Indian J. Plant Physiol. 2017, 22, 382–392. [Google Scholar] [CrossRef]
- Yu, N.; Niu, Q.W.; Ng, K.H.; Chua, N.H. The role of miR156/SPL s modules in Arabidopsis lateral root development. Plant J. 2015, 83, 673–685. [Google Scholar] [CrossRef]
- Mallory, A.C.; Bartel, D.P.; Bartel, B. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 2005, 17, 1360–1375. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, R.E.; Ercoli, M.F.; Debernardi, J.M.; Breakfield, N.W.; Mecchia, M.A.; Sabatini, M.; Cools, T.; De Veylder, L.; Benfey, P.N.; Palatnik, J.F. MicroRNA miR396 regulates the switch between stem cells and transit-amplifying cells in Arabidopsis roots. Plant Cell 2015, 27, 3354–3366. [Google Scholar] [CrossRef]
- Garg, V.; Khan, A.W.; Kudapa, H.; Kale, S.M.; Chitikineni, A.; Qiwei, S.; Sharma, M.; Li, C.; Zhang, B.; Xin, L. Integrated transcriptome, small RNA and degradome sequencing approaches provide insights into Ascochyta blight resistance in chickpea. Plant Biotechnol. J. 2019, 17, 914–931. [Google Scholar] [CrossRef] [Green Version]
- Wei, K.; Ruan, L.; Wang, L.; Cheng, H. Auxin-induced adventitious root formation in nodal cuttings of Camellia sinensis. Int. J. Mol. Sci. 2019, 20, 4817. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Păcurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Păcurar, M.; Demailly, H.; Geiss, G. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Chen, S.; Wang, S.; Liu, G.; Li, H.; Huang, H.; Jiang, J. BpGH3. 5, an early auxin-response gene, regulates root elongation in Betula platyphylla× Betula pendula. Plant Cell Tissue Organ. Cult. 2015, 120, 239–250. [Google Scholar] [CrossRef]
- Lakehal, A.; Chaabouni, S.; Cavel, E.; Le Hir, R.; Ranjan, A.; Raneshan, Z.; Novák, O.; Păcurar, D.I.; Perrone, I.; Jobert, F. A molecular framework for the control of adventitious rooting by TIR1/AFB2-Aux/IAA-dependent auxin signaling in Arabidopsis. Mol. Plant 2019, 12, 1499–1514. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xing, L.; Li, K.; Wei, Y.; Wang, H.; Mao, J.; Dong, F.; Ma, D.; Zhang, Z.; Han, M. Genome-wide identification, characterization and expression analysis of novel long non-coding RNAs that mediate IBA-induced adventitious root formation in apple rootstocks. Plant Growth Regul. 2019, 87, 287–302. [Google Scholar] [CrossRef]
- Yang, H.; Klopotek, Y.; Hajirezaei, M.R.; Zerche, S.; Franken, P.; Druege, U. Role of auxin homeostasis and response in nitrogen limitation and dark stimulation of adventitious root formation in petunia cuttings. Ann. Bot. 2019, 124, 1053–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-García, M.-P.; Vilarrasa-Blasi, J.; Zhiponova, M.; Divol, F.; Mora-García, S.; Russinova, E.; Caño-Delgado, A.I. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 2011, 138, 849–859. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Guo, W.; Zhang, B. Genome-wide identification and characterization of SPL transcription factor family and their evolution and expression profiling analysis in cotton. Sci. Rep. 2018, 8, 762. [Google Scholar] [CrossRef]
- Li, A.; Lakshmanan, P.; He, W.; Tan, H.; Liu, L.; Liu, H.; Liu, J.; Huang, D.; Chen, Z. Transcriptome profiling provides molecular insights into auxin-induced adventitious root formation in sugarcane (Saccharum spp. interspecific hybrids) Microshoots. Plants 2020, 9, 931. [Google Scholar] [CrossRef]
- Ruedell, C.M.; de Almeida, M.R.; Fett-Neto, A.G. Concerted transcription of auxin and carbohydrate homeostasis-related genes underlies improved adventitious rooting of microcuttings derived from far-red treated Eucalyptus globulus Labill mother plants. Plant Physiol. Biochem. 2015, 97, 11–19. [Google Scholar] [CrossRef]
- Kötting, O.; Kossmann, J.; Zeeman, S.C.; Lloyd, J.R. Regulation of starch metabolism: The age of enlightenment? Curr. Opin. Plant Biol. 2010, 13, 320–328. [Google Scholar] [CrossRef]
- Ahkami, A.H.; Lischewski, S.; Haensch, K.T.; Porfirova, S.; Hofmann, J.; Rolletschek, H.; Melzer, M.; Franken, P.; Hause, B.; Druege, U. Molecular physiology of adventitious root formation in Petunia hybrida cuttings: Involvement of wound response and primary metabolism. New Phytol. 2009, 181, 613–625. [Google Scholar] [CrossRef]
- Klopotek, Y.; Franken, P.; Klaering, H.-P.; Fischer, K.; Hause, B.; Hajirezaei, M.-R.; Druege, U. A higher sink competitiveness of the rooting zone and invertases are involved in dark stimulation of adventitious root formation in Petunia hybrida cuttings. Plant Sci. 2016, 243, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Ahkami, A.; Scholz, U.; Steuernagel, B.; Strickert, M.; Haensch, K.-T.; Druege, U.; Reinhardt, D.; Nouri, E.; von Wirén, N.; Franken, P. Comprehensive transcriptome analysis unravels the existence of crucial genes regulating primary metabolism during adventitious root formation in Petunia hybrida. PLoS ONE 2014, 9, e100997. [Google Scholar] [CrossRef] [PubMed]
- Agulló-Antón, M.Á.; Ferrández-Ayela, A.; Fernández-García, N.; Nicolás, C.; Albacete, A.; Pérez-Alfocea, F.; Sánchez-Bravo, J.; Pérez-Pérez, J.M.; Acosta, M. Early steps of adventitious rooting: Morphology, hormonal profiling and carbohydrate turnover in carnation stem cuttings. Physiol. Plant. 2014, 150, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liang, Y.; Xing, L.; Mao, J.; Liu, Z.; Dong, F.; Meng, Y.; Han, M.; Zhao, C.; Bao, L. Transcriptome analysis reveals multiple hormones, wounding and sugar signaling pathways mediate adventitious root formation in apple rootstock. Int. J. Mol. Sci. 2018, 19, 2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villacorta-Martín, C.; Sánchez-García, A.B.; Villanova, J.; Cano, A.; van de Rhee, M.; de Haan, J.; Acosta, M.; Passarinho, P.; Pérez-Pérez, J.M. Gene expression profiling during adventitious root formation in carnation stem cuttings. BMC Genom. 2015, 16, 789. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uddin, S.; Munir, M.Z.; Gull, S.; Khan, A.H.; Khan, A.; Khan, D.; Khan, M.A.; Wu, Y.; Sun, Y.; Li, Y. Transcriptome Profiling Reveals Role of MicroRNAs and Their Targeted Genes during Adventitious Root Formation in Dark-Pretreated Micro-Shoot Cuttings of Tetraploid Robinia pseudoacacia L. Genes 2022, 13, 441. https://doi.org/10.3390/genes13030441
Uddin S, Munir MZ, Gull S, Khan AH, Khan A, Khan D, Khan MA, Wu Y, Sun Y, Li Y. Transcriptome Profiling Reveals Role of MicroRNAs and Their Targeted Genes during Adventitious Root Formation in Dark-Pretreated Micro-Shoot Cuttings of Tetraploid Robinia pseudoacacia L. Genes. 2022; 13(3):441. https://doi.org/10.3390/genes13030441
Chicago/Turabian StyleUddin, Saleem, Muhammad Zeeshan Munir, Sadia Gull, Aamir Hamid Khan, Aimal Khan, Dilawar Khan, Muhammad Asif Khan, Yue Wu, Yuhan Sun, and Yun Li. 2022. "Transcriptome Profiling Reveals Role of MicroRNAs and Their Targeted Genes during Adventitious Root Formation in Dark-Pretreated Micro-Shoot Cuttings of Tetraploid Robinia pseudoacacia L." Genes 13, no. 3: 441. https://doi.org/10.3390/genes13030441
APA StyleUddin, S., Munir, M. Z., Gull, S., Khan, A. H., Khan, A., Khan, D., Khan, M. A., Wu, Y., Sun, Y., & Li, Y. (2022). Transcriptome Profiling Reveals Role of MicroRNAs and Their Targeted Genes during Adventitious Root Formation in Dark-Pretreated Micro-Shoot Cuttings of Tetraploid Robinia pseudoacacia L. Genes, 13(3), 441. https://doi.org/10.3390/genes13030441






