Glutamate Uptake Is Not Impaired by Hypoxia in a Culture Model of Human Fetal Neural Stem Cell-Derived Astrocytes
Abstract
:1. Introduction
2. Results
2.1. Isolation and Characterization of Human Fetal Neural Stem Cells
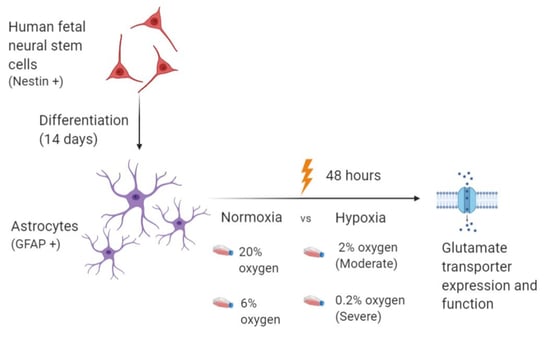
2.2. Differentiation of Human FNSCs into Astrocytes
2.3. Expression of Astrocytic Markers in Differentiating Cells
2.4. Expression of NSC-Marker Nestin and Neuronal Marker Doublecortin by Differentiating Cells
2.5. Exposing Differentiated Astrocytes to Hypoxia
2.6. Evaluation of Glutamate Transporter Expression in Astrocytes Exposed to Hypoxia
2.7. Evaluation of Glutamate Uptake in Astrocytes Exposed to Hypoxia
3. Discussion
4. Methodology
4.1. Sample Collection
4.2. Isolation of Human Fetal Neural Stem Cells (FNSCs)
4.3. Differentiation of Human Fetal Neural Stem Cells into Astrocytes
4.4. Exposure of Differentiated Human Astrocytes to Different Oxygen Concentrations
4.5. RNA Isolation, cDNA Synthesis and qPCR
4.6. Western Blotting
4.7. Flow Cytometry
4.8. Immunocytochemistry
4.9. Glutamate Uptake Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferriero, D.M. Neonatal brain injury. N. Engl. J. Med. 2004, 351, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Manuck, T.A.; Rice, M.M.; Bailit, J.L.; Grobman, W.A.; Reddy, U.M.; Wapner, R.J.; Thorp, J.M.; Caritis, S.N.; Prasad, M.; Tita, A.T.; et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am. J. Obstet Gynecol. 2016, 215, 103.e1–103.e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yates, N.; Gunn, A.J.; Bennet, L.; Dhillon, S.K.; Davidson, J.O. Preventing Brain Injury in the Preterm Infant-Current Controversies and Potential Therapies. Int. J. Mol. Sci. 2021, 22, 1671. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.V.; Trescher, W.H.; Ishida, A.; Nakajima, W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr. Res. 2001, 49, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Baburamani, A.A.; Ek, C.J.; Walker, D.W.; Castillo-Melendez, M. Vulnerability of the developing brain to hypoxic-ischemic damage: Contribution of the cerebral vasculature to injury and repair? Front. Physiol. 2012, 3, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossain, M.A. Hypoxic-ischemic injury in neonatal brain: Involvement of a novel neuronal molecule in neuronal cell death and potential target for neuroprotection. Int. J. Dev. Neurosci. 2008, 26, 93–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekaran, A.; Avci, H.X.; Leist, M.; Kobolák, J.; Dinnyés, A. Astrocyte Differentiation of Human Pluripotent Stem Cells: New Tools for Neurological Disorder Research. Front. Cell Neurosci. 2016, 10, 215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujikawa, D.G. The role of excitotoxic programmed necrosis in acute brain injury. Comput. Struct. Biotechnol. J. 2015, 13, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, R.D.; Bakken, T.E.; Miller, J.A.; Smith, K.A.; Barkan, E.R.; Graybuck, L.T.; Close, J.L.; Long, B.; Johansen, N.; Penn, O.; et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019, 573, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Khankan, R.R.; Caneda, C.; Godoy, M.I.; Haney, M.S.; Krawczyk, M.C.; Bassik, M.C.; Sloan, S.A.; Zhang, Y. Astrocyte-to-astrocyte contact and a positive feedback loop of growth factor signaling regulate astrocyte maturation. Glia 2019, 67, 1571–1597. [Google Scholar] [CrossRef]
- Frederiksen, K.; McKay, R.D. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J. Neurosci. 1988, 8, 1144–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.; Hwang, S.U.; Yoon, J.D.; Kim, H.; Lee, G.; Hyun, S.H. Isolation and characterization of GFAP-positive porcine neural stem/progenitor cells derived from a GFAP-CreER(T2) transgenic piglet. BMC Vet. Res. 2018, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Aja, S.; Kim, E.K.; Park, M.J.; Ramamurthy, S.; Jia, J.; Hu, X.; Geng, P.; Ronnett, G.V. Physiological oxygen level is critical for modeling neuronal metabolism in vitro. J. Neurosci. Res. 2012, 90, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, N.H.; Walker, K.; Fried, J.; Hackney, J.R.; McDonald, P.C.; Benavides, G.A.; Spina, R.; Audia, A.; Scott, S.E.; Libby, C.J.; et al. Addition of carbonic anhydrase 9 inhibitor SLC-0111 to temozolomide treatment delays glioblastoma growth in vivo. JCI Insight 2017, 2, e92928. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr. Res. 2004, 56, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Back, S.A.; Luo, N.L.; Borenstein, N.S.; Levine, J.M.; Volpe, J.J.; Kinney, H.C. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J. Neurosci. 2001, 21, 1302–1312. [Google Scholar] [CrossRef] [PubMed]
- Hüppi, P.S.; Amato, M. Advanced magnetic resonance imaging techniques in perinatal brain injury. Biol. Neonate. 2001, 80, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Berliocchi, L.; Bano, D.; Nicotera, P. Ca2+ signals and death programmes in neurons. Philos. Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 2255–2258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.G.; Tian, H.; Diamond, J.S. The relative roles of diffusion and uptake in clearing synaptically released glutamate change during early postnatal development. J. Neurosci. 2011, 31, 4743–4754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes Maintain Glutamate Homeostasis in the CNS by Controlling the Balance between Glutamate Uptake and Release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Holmseth, S.; Dehnes, Y.; Huang, Y.H.; Follin-Arbelet, V.V.; Grutle, N.J.; Mylonakou, M.N.; Plachez, C.; Zhou, Y.; Furness, D.N.; Bergles, D.E.; et al. The density of EAAC1 (EAAT3) glutamate transporters expressed by neurons in the mammalian CNS. J. Neurosci. 2012, 32, 6000–6013. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Lee, S.G.; Kegelman, T.P.; Su, Z.Z.; Das, S.K.; Dash, R.; Dasgupta, S.; Barral, P.M.; Hedvat, M.; Diaz, P.; et al. Role of excitatory amino acid transporter-2 (EAAT2) and glutamate in neurodegeneration: Opportunities for developing novel therapeutics. J. Cell Physiol. 2011, 226, 2484–2493. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.; Delgado-Esteban, M.; Bolaños, J.P.; Medina, J.M. Oxygen and glucose deprivation induces mitochondrial dysfunction and oxidative stress in neurones but not in astrocytes in primary culture. J. Neurochem. 2002, 81, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Yatomi, Y.; Tanaka, R.; Shimura, H.; Miyamoto, N.; Yamashiro, K.; Takanashi, M.; Urabe, T.; Hattori, N. Chronic brain ischemia induces the expression of glial glutamate transporter EAAT2 in subcortical white matter. Neuroscience 2013, 244, 113–121. [Google Scholar] [CrossRef]
- Bhatia, T.N.; Pant, D.B.; Eckhoff, E.A.; Gongaware, R.N.; Do, T.; Hutchison, D.F.; Gleixner, A.M.; Leak, R.K. Astrocytes Do Not Forfeit Their Neuroprotective Roles After Surviving Intense Oxidative Stress. Front. Mol. Neurosci. 2019, 12, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogal, B.; Li, J.; Lobner, D.; McCullough, L.D.; Hewett, S.J. System x(c)- activity and astrocytes are necessary for interleukin-1 beta-mediated hypoxic neuronal injury. J. Neurosci. 2007, 27, 10094–10105. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Pan, L.; Pembroke, W.G.; Rexach, J.E.; Godoy, M.I.; Condro, M.C.; Alvarado, A.G.; Harteni, M.; Chen, Y.W.; Stiles, L.; et al. Conservation and divergence of vulnerability and responses to stressors between human and mouse astrocytes. Nat. Commun. 2021, 12, 3958. [Google Scholar] [CrossRef] [PubMed]
- Dallas, M.; Boycott, H.E.; Atkinson, L.; Miller, A.; Boyle, J.P.; Pearson, H.A.; Peers, C. Hypoxia suppresses glutamate transport in astrocytes. J. Neurosci. 2007, 27, 3946–3955. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Bhat, N.R. Hypoxia/reoxygenation differentially modulates NF-kappaB activation and iNOS expression in astrocytes and microglia. Antioxid Redox Signal. 2006, 8, 911–918. [Google Scholar] [CrossRef]
- Lyons, S.A.; Kettenmann, H. Oligodendrocytes and microglia are selectively vulnerable to combined hypoxia and hypoglycemia injury in vitro. J. Cereb. Blood Flow Metab. 1998, 18, 521–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, R.A. Astrocyte glutamate uptake during chemical hypoxia in vitro. Neurosci. Lett. 1992, 147, 143–146. [Google Scholar] [CrossRef]
- Lukaszevicz, A.C.; Sampaïo, N.; Guégan, C.; Benchoua, A.; Couriaud, C.; Chevalier, E.; Sola, B.; Lacombe, P.; Onténiente, B. High sensitivity of protoplasmic cortical astroglia to focal ischemia. J. Cereb. Blood Flow Metab. 2002, 22, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.M.; Fonseca, L.L.; Peixoto, C.C.; Almeida, A.C.; Carrondo, M.J.; Santos, H. NMR studies on energy metabolism of immobilized primary neurons and astrocytes during hypoxia, ischemia and hypoglycemia. NMR Biomed. 2000, 13, 438–448. [Google Scholar] [CrossRef]
- Zhou, S.; Zhong, Z.; Huang, P.; Xiang, B.; Li, X.; Dong, H.; Zhang, G.; Wu, Y.; Li, P. IL-6/STAT3 Induced Neuron Apoptosis in Hypoxia by Downregulating ATF6 Expression. Front. Physiol. 2021, 12, 729925. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, S.; Huang, S.F.; Patkar, S.; Gassmann, M.; Ogunshola, O.O. Differential responses of blood-brain barrier associated cells to hypoxia and ischemia: A comparative study. Fluids Barriers CNS 2015, 12, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santello, M.; Volterra, A. Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience 2009, 158, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Tewari, M.; Monika Varghse, R.K.; Menon, M.; Seth, P. Astrocytes mediate HIV-1 Tat-induced neuronal damage via ligand-gated ion channel P2X7R. J. Neurochem. 2015, 132, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Jingar, P.; Agrawal, S.; Shrivastava, V.; Bhattacharya, A.; Manhas, J.; Garg, B.; Ansari, M.T.; Mridha, A.R.; Sreenivas, V.; et al. Symphytum officinale augments osteogenesis in human bone marrow-derived mesenchymal stem cells in vitro as they differentiate into osteoblasts. J. Ethnopharmacol. 2020, 248, 112329. [Google Scholar] [CrossRef] [PubMed]
- Lutgen, V.; Narasipura, S.D.; Sharma, A.; Min, S.; Al-Harthi, L. β-Catenin signaling positively regulates glutamate uptake and metabolism in astrocytes. J. Neuroinflamm. 2016, 13, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Gene | Primer Sequence (5′ to 3′) |
|---|---|
| 18s rRNA | FP: GTAACCCGTTGAACCCCATT RP: CCATCCAATCGGTAGTAGCG |
| GFAP | FP: AGCCCACTCCTTCATAAAGCC RP: ATGCGTCTCCTCTCCATCCT |
| Nestin | FP: CCTCAAGATGTCCCTCAGCC RP: TCCAGCTTGGGGTCCTGAAA |
| Doublecortin | FP: GGGGGTGTGGGCATAAAGAA RP: CCTGCTCTTTACCAGCCTCC |
| EAAT1 | FP: GAATGGCGGCGCTAGATAGT RP: CCAGGCTTCTACCAGATTTG |
| EAAT2 | FP: CAGGGAAAGCAACTCTAATC RP: CAAGGTTCTTCCTCAACA |
| CA9 | FP: CTTTGAATGGGCGAGTGATT RP: CTTCTGTGCTGCCTTCTCATCT |
| VEGF | FP: ACCATGAACTTTCTGCTGTCTTG RP: ATGGCTTGAAGATGTACTCGATCTC |
| PGK-1 | FP: CCGAGCCAGCCAAAATAGA RP: ACTTTAGCTCCGCCCAGGAT |
| Antibody | Molecular Weight | Antibody Specifications | Antibody Dilution | Procured From |
|---|---|---|---|---|
| Anti-GFAP (Catalog #ABP54511) | 50 kDa | Polyclonal Rabbit IgG | 1:1000 | Abbkine, Wuhan, China |
| Anti-EAAT2 (Catalog #ABP57320) | 60 kDa | Polyclonal Rabbit IgG | 1:1000 | Abbkine, Wuhan, China |
| Anti-HIF1α (Catalog #36169S) | 120 kDa | Monoclonal Rabbit IgG | 1:1000 | CST, Danvers, MA, USA |
| Anti-GAPDH (loading control for differentiation experiments) Catalog #10-10011 | 37 kDa | Monoclonal Mouse IgG | 1:2000 | Abgenex, Bhubaneswar, India |
| Anti β-actin (loading control for hypoxia experiments) Catalog #STJ94020 | 42 kDa | Polyclonal Rabbit IgG | 1:1000 | St. John’s Laboratory, London, UK |
| HRP-tagged Anti-rabbit secondary antibody (Catalog #7074S) | - | Goat Anti-rabbit IgG | 1:2000 | CST, Danvers, MA, USA |
| HRP-tagged anti-mouse secondary antibody (Catalog #7076S) | - | Horse Anti-mouse IgG | 1:2000 | CST, Danvers, MA, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrivastava, V.; Dey, D.; Singal, C.M.S.; Jaiswal, P.; Singh, A.; Sharma, J.B.; Chattopadhyay, P.; Nayak, N.R.; Palanichamy, J.K.; Sinha, S.; et al. Glutamate Uptake Is Not Impaired by Hypoxia in a Culture Model of Human Fetal Neural Stem Cell-Derived Astrocytes. Genes 2022, 13, 506. https://doi.org/10.3390/genes13030506
Shrivastava V, Dey D, Singal CMS, Jaiswal P, Singh A, Sharma JB, Chattopadhyay P, Nayak NR, Palanichamy JK, Sinha S, et al. Glutamate Uptake Is Not Impaired by Hypoxia in a Culture Model of Human Fetal Neural Stem Cell-Derived Astrocytes. Genes. 2022; 13(3):506. https://doi.org/10.3390/genes13030506
Chicago/Turabian StyleShrivastava, Vadanya, Devanjan Dey, Chitra Mohinder Singh Singal, Paritosh Jaiswal, Ankit Singh, Jai Bhagwan Sharma, Parthaprasad Chattopadhyay, Nihar Ranjan Nayak, Jayanth Kumar Palanichamy, Subrata Sinha, and et al. 2022. "Glutamate Uptake Is Not Impaired by Hypoxia in a Culture Model of Human Fetal Neural Stem Cell-Derived Astrocytes" Genes 13, no. 3: 506. https://doi.org/10.3390/genes13030506