mRNA Vaccines: Why Is the Biology of Retroposition Ignored?
Abstract
:1. Introduction
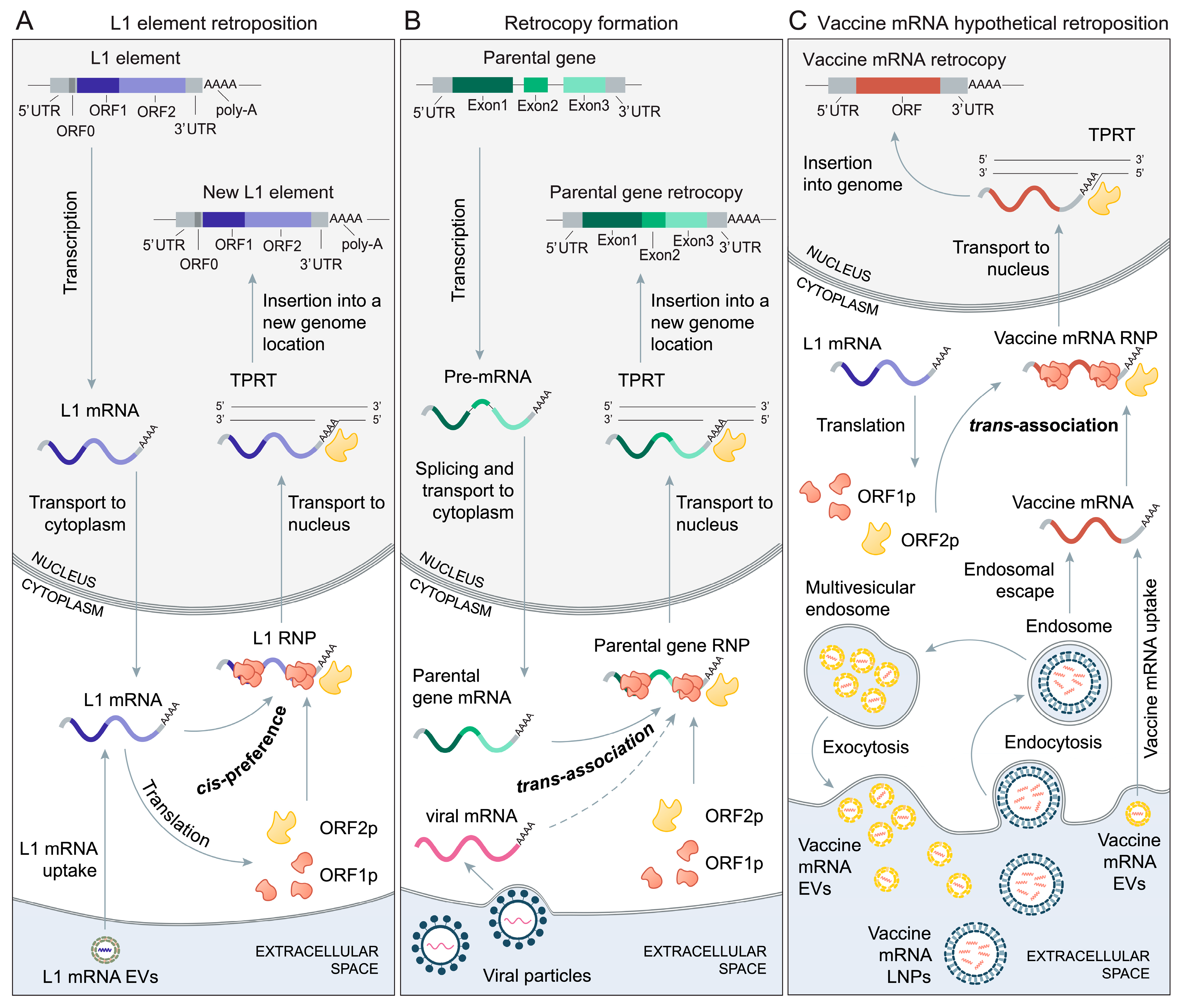
2. The Biology of Retroposition
3. The Mechanisms of Retrocopy Formation
4. L1 Elements in Germline and Soma
5. Vaccine mRNAs and Retroposition
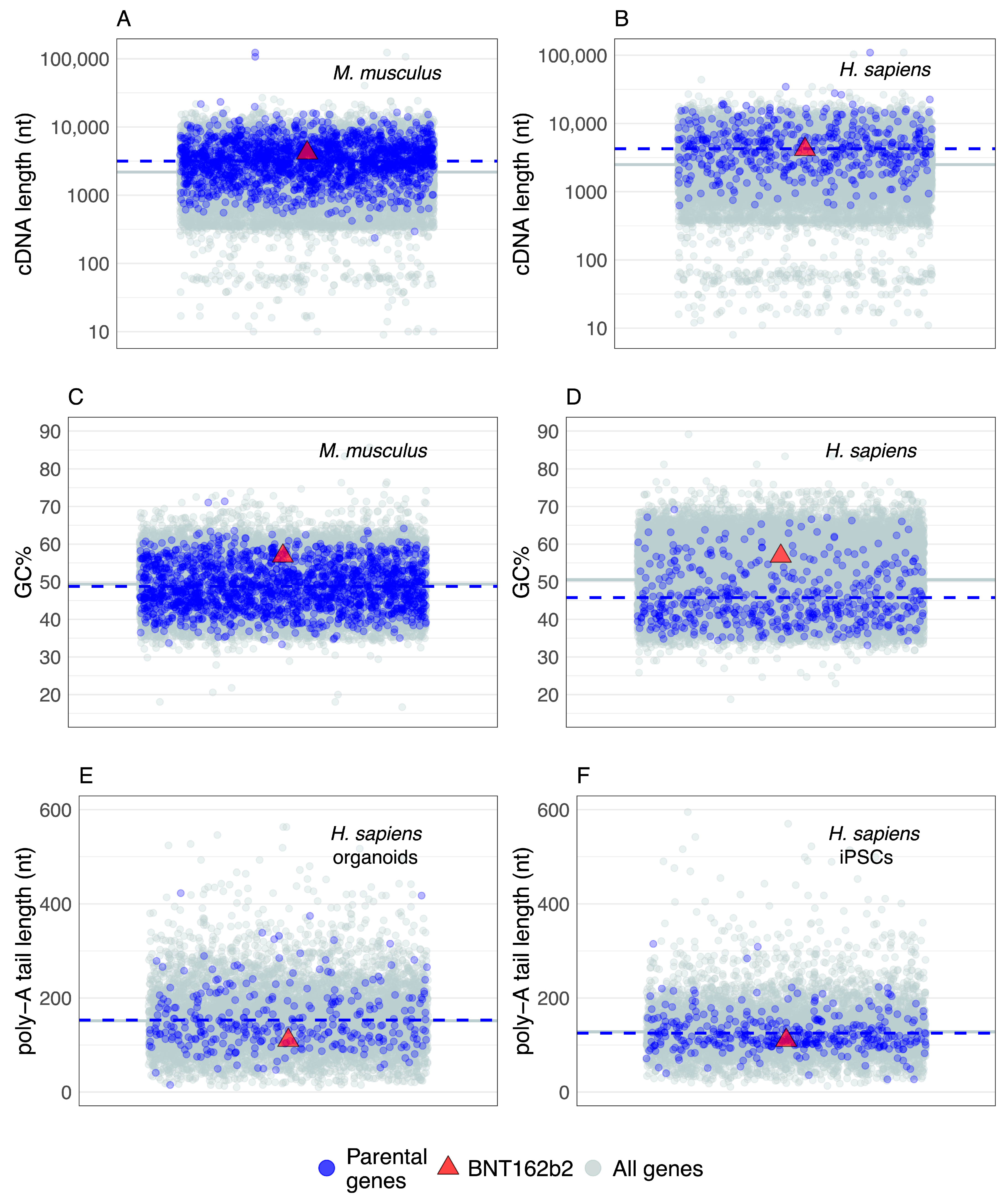
6. Parental Genes and BNT162b2
7. Pharmacology Aspects
8. Biodistribution Profiles
9. Final Remarks
10. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Medicines Agency. Comirnaty Assessment Report. 2020. EMA/707383/2020. Available online: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf (accessed on 30 December 2020).
- European Medicines Agency. COVID-19 Vaccine Moderna Assessment Report. 2021. EMA/15689/2021. Available online: https://www.ema.europa.eu/en/documents/assessment-report/covid-19-vaccine-moderna-epar-public-assessment-report_en.pdf (accessed on 27 January 2021).
- Funk, C.D.; Laferrière, C.; Ardakani, A. A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic. Front. Pharmacol. 2020, 11, 937. [Google Scholar] [CrossRef]
- Knezevic, I.; Liu, M.A.; Peden, K.; Zhou, T.; Kang, H.-N. Development of MRNA Vaccines: Scientific and Regulatory Issues. Vaccines 2021, 9, 81. [Google Scholar] [CrossRef]
- Park, J.W.; Lagniton, P.N.P.; Liu, Y.; Xu, R.-H. MRNA Vaccines for COVID-19: What, Why and How. Int. J. Biol. Sci. 2021, 17, 1446–1460. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [Green Version]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The Promise of MRNA Vaccines: A Biotech and Industrial Perspective. NPJ Vaccines 2020, 5, 11. [Google Scholar] [CrossRef]
- Cannon, G.; Weissman, D. RNA Based Vaccines. DNA Cell Biol. 2002, 21, 953–961. [Google Scholar] [CrossRef]
- Geall, A.J.; Mandl, C.W.; Ulmer, J.B. RNA: The New Revolution in Nucleic Acid Vaccines. Semin. Immunol. 2013, 25, 152–159. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. MRNA-Based Therapeutics—Developing a New Class of Drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. Three Decades of Messenger RNA Vaccine Development. Nano Today 2019, 28, 100766. [Google Scholar] [CrossRef]
- Zhang, C.; Maruggi, G.; Shan, H.; Li, J. Advances in MRNA Vaccines for Infectious Diseases. Front. Immunol. 2019, 10, 594. [Google Scholar] [CrossRef] [Green Version]
- Tombácz, I.; Weissman, D.; Pardi, N. Vaccination with Messenger RNA: A Promising Alternative to DNA Vaccination. In DNA Vaccines; Sousa, Â., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2197, pp. 13–31. ISBN 978-1-07-160871-5. [Google Scholar]
- Kreiter, S.; Diken, M.; Selmi, A.; Türeci, Ö.; Sahin, U. Tumor Vaccination Using Messenger RNA: Prospects of a Future Therapy. Curr. Opin. Immunol. 2011, 23, 399–406. [Google Scholar] [CrossRef]
- Weissman, D. MRNA Transcript Therapy. Expert Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. MRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Dammes, N.; Peer, D. Paving the Road for RNA Therapeutics. Trends Pharmacol. Sci. 2020, 41, 755–775. [Google Scholar] [CrossRef]
- Fuller, D.H.; Berglund, P. Amplifying RNA Vaccine Development. N. Engl. J. Med. 2020, 382, 2469–2471. [Google Scholar] [CrossRef]
- Gerer, K.F.; Hoyer, S.; Dörrie, J.; Schaft, N. Electroporation of MRNA as Universal Technology Platform to Transfect a Variety of Primary Cells with Antigens and Functional Proteins. In RNA Vaccines; Kramps, T., Elbers, K., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1499, pp. 165–178. ISBN 978-1-4939-6479-6. [Google Scholar]
- Pardi, N.; Weissman, D. Nucleoside Modified MRNA Vaccines for Infectious Diseases. In RNA Vaccines; Kramps, T., Elbers, K., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1499, pp. 109–121. ISBN 978-1-4939-6479-6. [Google Scholar]
- Hinz, T.; Kallen, K.; Britten, C.M.; Flamion, B.; Granzer, U.; Hoos, A.; Huber, C.; Khleif, S.; Kreiter, S.; Rammensee, H.-G.; et al. The European Regulatory Environment of RNA-Based Vaccines. In RNA Vaccines; Kramps, T., Elbers, K., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1499, pp. 203–222. ISBN 978-1-4939-6479-6. [Google Scholar]
- Naik, R.; Peden, K. Regulatory Considerations on the Development of MRNA Vaccines. In Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- World Health Organization. Evaluation of the Quality, Safety and Efficacy of RNA-Based Prophylactic Vaccines for Infectious Diseases: Regulatory Considerations. (Draft). 2020. Available online: https://www.who.int/docs/default-source/biologicals/ecbs/reg-considerations-on-rna-vaccines_1st-draft_pc_tz_22122020.pdf (accessed on 11 January 2021).
- World Health Organization. Background Document on the mRNA Vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19. 2021. Available online: https://www.who.int/publications/i/item/background-document-on-mrna-vaccine-bnt162b2-(pfizer-biontech)-against-covid-19 (accessed on 12 February 2021).
- World Health Organization. Background Document on the mRNA-1273 Vaccine (Moderna) against COVID-19. 2021. Available online: https://www.who.int/publications/i/item/background-document-on-the-mrna-1273-vaccine-(moderna)-against-covid-19 (accessed on 12 February 2021).
- Youn, H.; Chung, J.-K. Modified MRNA as an Alternative to Plasmid DNA (PDNA) for Transcript Replacement and Vaccination Therapy. Expert Opin. Biol. Ther. 2015, 15, 1337–1348. [Google Scholar] [CrossRef]
- Orlandini von Niessen, A.G.; Poleganov, M.A.; Rechner, C.; Plaschke, A.; Kranz, L.M.; Fesser, S.; Diken, M.; Löwer, M.; Vallazza, B.; Beissert, T.; et al. Improving MRNA-Based Therapeutic Gene Delivery by Expression-Augmenting 3′ UTRs Identified by Cellular Library Screening. Mol. Ther. 2019, 27, 824–836. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, A.N.; Beissert, T.; Simon, P.; Vallazza, B.; Buck, J.; Davies, B.; Tureci, O.; Sahin, U. MRNA as a Versatile Tool for Exogenous Protein Expression. CGT 2012, 12, 347–361. [Google Scholar] [CrossRef]
- Phua, K.K.L.; Leong, K.W.; Nair, S.K. Transfection Efficiency and Transgene Expression Kinetics of MRNA Delivered in Naked and Nanoparticle Format. J. Control. Release 2013, 166, 227–233. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, A.; Kormann, M.; Rosenecker, J.; Rudolph, C. Current Prospects for MRNA Gene Delivery. Eur. J. Pharm. Biopharm. 2009, 71, 484–489. [Google Scholar] [CrossRef]
- Liu, A. Comparison of Plasmid DNA and MRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef] [Green Version]
- Cimolai, N. Do RNA Vaccines Obviate the Need for Genotoxicity Studies? Mutagenesis 2020, 35, 509–510. [Google Scholar] [CrossRef]
- Meurens, F. Flu RNA Vaccine: A Game Changer? Vaccines 2020, 8, 760. [Google Scholar] [CrossRef]
- Doerfler, W. Adenoviral Vector DNA- and SARS-CoV-2 MRNA-Based COVID-19 Vaccines: Possible Integration into the Human Genome—Are Adenoviral Genes Expressed in Vector-Based Vaccines? Virus Res. 2021, 302, 198466. [Google Scholar] [CrossRef]
- Wadhwa, A.; Aljabbari, A.; Lokras, A.; Foged, C.; Thakur, A. Opportunities and Challenges in the Delivery of MRNA-Based Vaccines. Pharmaceutics 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Kaessmann, H.; Vinckenbosch, N.; Long, M. RNA-Based Gene Duplication: Mechanistic and Evolutionary Insights. Nat. Rev. Genet. 2009, 10, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, S.; Abyzov, A.; Gerstein, M.B. Landscape and Variation of Novel Retroduplications in 26 Human Populations. PLoS Comput. Biol. 2017, 13, e1005567. [Google Scholar] [CrossRef]
- Casola, C.; Betrán, E. The Genomic Impact of Gene Retrocopies: What Have We Learned from Comparative Genomics, Population Genomics, and Transcriptomic Analyses? Genome Biol. Evol. 2017, 9, 1351–1373. [Google Scholar] [CrossRef]
- Cheetham, S.W.; Faulkner, G.J.; Dinger, M.E. Overcoming Challenges and Dogmas to Understand the Functions of Pseudogenes. Nat. Rev. Genet. 2020, 21, 191–201. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, C.; Ullrich, K.; Zhang, Y.E.; Tautz, D. The Mutational Load in Natural Populations Is Significantly Affected by High Primary Rates of Retroposition. Proc. Natl. Acad. Sci. USA 2021, 118, e2013043118. [Google Scholar] [CrossRef]
- Carelli, F.N.; Hayakawa, T.; Go, Y.; Imai, H.; Warnefors, M.; Kaessmann, H. The Life History of Retrocopies Illuminates the Evolution of New Mammalian Genes. Genome Res. 2016, 26, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Horie, M.; Honda, T.; Suzuki, Y.; Kobayashi, Y.; Daito, T.; Oshida, T.; Ikuta, K.; Jern, P.; Gojobori, T.; Coffin, J.M.; et al. Endogenous Non-Retroviral RNA Virus Elements in Mammalian Genomes. Nature 2010, 463, 84–87. [Google Scholar] [CrossRef] [Green Version]
- Parrish, N.F.; Fujino, K.; Shiromoto, Y.; Iwasaki, Y.W.; Ha, H.; Xing, J.; Makino, A.; Kuramochi-Miyagawa, S.; Nakano, T.; Siomi, H.; et al. PiRNAs Derived from Ancient Viral Processed Pseudogenes as Transgenerational Sequence-Specific Immune Memory in Mammals. RNA 2015, 21, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Beck, C.R.; Garcia-Perez, J.L.; Badge, R.M.; Moran, J.V. LINE-1 Elements in Structural Variation and Disease. Annu. Rev. Genom. Hum. Genet. 2011, 12, 187–215. [Google Scholar] [CrossRef] [Green Version]
- Kazazian, H.H.; Moran, J.V. Mobile DNA in Health and Disease. N. Engl. J. Med. 2017, 377, 361–370. [Google Scholar] [CrossRef]
- Esnault, C.; Maestre, J.; Heidmann, T. Human LINE Retrotransposons Generate Processed Pseudogenes. Nat. Genet. 2000, 24, 363–367. [Google Scholar] [CrossRef]
- Mita, P.; Wudzinska, A.; Sun, X.; Andrade, J.; Nayak, S.; Kahler, D.J.; Badri, S.; LaCava, J.; Ueberheide, B.; Yun, C.Y.; et al. LINE-1 Protein Localization and Functional Dynamics during the Cell Cycle. eLife 2018, 7, e30058. [Google Scholar] [CrossRef]
- Naufer, M.N.; Furano, A.V.; Williams, M.C. Protein-Nucleic Acid Interactions of LINE-1 ORF1p. Semin. Cell Dev. Biol. 2019, 86, 140–149. [Google Scholar] [CrossRef]
- Hancks, D.C.; Kazazian, H.H. Roles for Retrotransposon Insertions in Human Disease. Mob. DNA 2016, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Boeke, J.D. LINEs and Alus—the PolyA Connection. Nat. Genet. 1997, 16, 6–7. [Google Scholar] [CrossRef]
- Doucet, A.J.; Wilusz, J.E.; Miyoshi, T.; Liu, Y.; Moran, J.V. A 3′ Poly(A) Tract Is Required for LINE-1 Retrotransposition. Mol. Cell 2015, 60, 728–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monot, C.; Kuciak, M.; Viollet, S.; Mir, A.A.; Gabus, C.; Darlix, J.-L.; Cristofari, G. The Specificity and Flexibility of L1 Reverse Transcription Priming at Imperfect T-Tracts. PLoS Genet. 2013, 9, e1003499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamura, Y.; Sanchez Calle, A.; Yamamoto, Y.; Sato, T.-A.; Ochiya, T. Extracellular Vesicles Mediate the Horizontal Transfer of an Active LINE-1 Retrotransposon. J. Extracell. Vesicles 2019, 8, 1643214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Richards, A.; Barrasa, M.I.; Hughes, S.H.; Young, R.A.; Jaenisch, R. Reverse-Transcribed SARS-CoV-2 RNA Can Integrate into the Genome of Cultured Human Cells and Can Be Expressed in Patient-Derived Tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2105968118. [Google Scholar] [CrossRef] [PubMed]
- Smits, N.; Rasmussen, J.; Bodea, G.O.; Amarilla, A.A.; Gerdes, P.; Sanchez-Luque, F.J.; Ajjikuttira, P.; Modhiran, N.; Liang, B.; Faivre, J.; et al. No Evidence of Human Genome Integration of SARS-CoV-2 Found by Long-Read DNA Sequencing. Cell. Rep. 2021, 36, 109530. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The Dawn of MRNA Vaccines: The COVID-19 Case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; et al. Linkage between Endosomal Escape of LNP-MRNA and Loading into EVs for Transport to Other Cells. Nat. Commun. 2019, 10, 4333. [Google Scholar] [CrossRef] [Green Version]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of MRNAs and MicroRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [Green Version]
- Pei, B.; Sisu, C.; Frankish, A.; Howald, C.; Habegger, L.; Mu, X.; Harte, R.; Balasubramanian, S.; Tanzer, A.; Diekhans, M.; et al. The GENCODE Pseudogene Resource. Genome Biol. 2012, 13, R51. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.R.; Salvador-Palomeque, C.; Faulkner, G.J. Diversity through Duplication: Whole-genome Sequencing Reveals Novel Gene Retrocopies in the Human Population. BioEssays 2014, 36, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Navarro, F.C.P.; Galante, P.A.F. A Genome-Wide Landscape of Retrocopies in Primate Genomes. Genome Biol. Evol. 2015, 7, 2265–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewing, A.D.; Ballinger, T.J.; Earl, D.; Broad Institute Genome Sequencing and Analysis Program and Platform; Harris, C.C.; Ding, L.; Wilson, R.K.; Haussler, D. Retrotransposition of Gene Transcripts Leads to Structural Variation in Mammalian Genomes. Genome Biol. 2013, 14, R22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrider, D.R.; Navarro, F.C.P.; Galante, P.A.F.; Parmigiani, R.B.; Camargo, A.A.; Hahn, M.W.; de Souza, S.J. Gene Copy-Number Polymorphism Caused by Retrotransposition in Humans. PLoS Genet. 2013, 9, e1003242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abyzov, A.; Iskow, R.; Gokcumen, O.; Radke, D.W.; Balasubramanian, S.; Pei, B.; Habegger, L.; The 1000 Genomes Project Consortium; Lee, C.; Gerstein, M. Analysis of Variable Retroduplications in Human Populations Suggests Coupling of Retrotransposition to Cell Division. Genome Res. 2013, 23, 2042–2052. [Google Scholar] [CrossRef] [Green Version]
- Chatron, N.; Cassinari, K.; Quenez, O.; Baert-Desurmont, S.; Bardel, C.; Buisine, M.; Calpena, E.; Capri, Y.; Corominas Galbany, J.; Diguet, F.; et al. Identification of Mobile Retrocopies during Genetic Testing: Consequences for Routine Diagnosis. Hum. Mutat. 2019, 40, 1993–2000. [Google Scholar] [CrossRef]
- Gardner, E.J.; Prigmore, E.; Gallone, G.; Danecek, P.; Samocha, K.E.; Handsaker, J.; Gerety, S.S.; Ironfield, H.; Short, P.J.; Sifrim, A.; et al. Contribution of Retrotransposition to Developmental Disorders. Nat. Commun. 2019, 10, 4630. [Google Scholar] [CrossRef] [Green Version]
- Parker, H.G.; VonHoldt, B.M.; Quignon, P.; Margulies, E.H.; Shao, S.; Mosher, D.S.; Spady, T.C.; Elkahloun, A.; Cargill, M.; Jones, P.G.; et al. An Expressed Fgf4 Retrogene Is Associated with Breed-Defining Chondrodysplasia in Domestic Dogs. Science 2009, 325, 995–998. [Google Scholar] [CrossRef] [Green Version]
- De Boer, M.; van Leeuwen, K.; Geissler, J.; Weemaes, C.M.; van den Berg, T.K.; Kuijpers, T.W.; Warris, A.; Roos, D. Primary Immunodeficiency Caused by an Exonized Retroposed Gene Copy Inserted in the CYBB Gene. Hum. Mutat. 2014, 35, 486–496. [Google Scholar] [CrossRef]
- Kazazian, H.H. Processed Pseudogene Insertions in Somatic Cells. Mob. DNA 2014, 5, 20. [Google Scholar] [CrossRef] [Green Version]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of Transcription in Human Cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Hangauer, M.J.; Vaughn, I.W.; McManus, M.T. Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs. PLoS Genet. 2013, 9, e1003569. [Google Scholar] [CrossRef] [PubMed]
- Neme, R.; Tautz, D. Fast Turnover of Genome Transcription across Evolutionary Time Exposes Entire Non-Coding DNA to de Novo Gene Emergence. eLife 2016, 5, e09977. [Google Scholar] [CrossRef]
- Tautz, D.; Domazet-Lošo, T. The Evolutionary Origin of Orphan Genes. Nat. Rev. Genet. 2011, 12, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Rohozinski, J.; Lamb, D.J.; Bishop, C.E. UTP14c Is a Recently Acquired Retrogene Associated with Spermatogenesis and Fertility in Man1. Biol. Reprod. 2006, 74, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Ciomborowska, J.; Rosikiewicz, W.; Szklarczyk, D.; Makalowski, W.; Makalowska, I. “Orphan” Retrogenes in the Human Genome. Mol. Biol. Evol. 2013, 30, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Domazet-Loso, T.; Tautz, D. An Ancient Evolutionary Origin of Genes Associated with Human Genetic Diseases. Mol. Biol. Evol. 2008, 25, 2699–2707. [Google Scholar] [CrossRef] [Green Version]
- ICGC Breast Cancer Group; Cooke, S.L.; Shlien, A.; Marshall, J.; Pipinikas, C.P.; Martincorena, I.; Tubio, J.M.C.; Li, Y.; Menzies, A.; Mudie, L.; et al. Processed Pseudogenes Acquired Somatically during Cancer Development. Nat. Commun. 2014, 5, 3644. [Google Scholar] [CrossRef]
- Scott, E.; Devine, S. The Role of Somatic L1 Retrotransposition in Human Cancers. Viruses 2017, 9, 131. [Google Scholar] [CrossRef]
- Bim, L.V.; Navarro, F.C.P.; Valente, F.O.F.; Lima-Junior, J.V.; Delcelo, R.; Dias-da-Silva, M.R.; Maciel, R.M.B.; Galante, P.A.F.; Cerutti, J.M. Retroposed Copies of RET Gene: A Somatically Acquired Event in Medullary Thyroid Carcinoma. BMC Med. Genom. 2019, 12, 104. [Google Scholar] [CrossRef]
- PCAWG Structural Variation Working Group; PCAWG Consortium; Rodriguez-Martin, B.; Alvarez, E.G.; Baez-Ortega, A.; Zamora, J.; Supek, F.; Demeulemeester, J.; Santamarina, M.; Ju, Y.S.; et al. Pan-Cancer Analysis of Whole Genomes Identifies Driver Rearrangements Promoted by LINE-1 Retrotransposition. Nat. Genet. 2020, 52, 306–319. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.; Cardoso-Moreira, M.; Shi, W.; Zhang, D.; Huang, J.; Mao, Y.; Jia, H.; Zhang, Y.; Chen, C.; Shao, Y.; et al. LTR-Mediated Retroposition as a Mechanism of RNA-Based Duplication in Metazoans. Genome Res. 2016, 26, 1663–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. International Human Genome Sequencing Consortium Initial Sequencing and Analysis of the Human Genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payer, L.M.; Burns, K.H. Transposable Elements in Human Genetic Disease. Nat. Rev. Genet. 2019, 20, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.H. Transposable Elements in Cancer. Nat. Rev. Cancer 2017, 17, 415–424. [Google Scholar] [CrossRef]
- Burns, K.H. Our Conflict with Transposable Elements and Its Implications for Human Disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 51–70. [Google Scholar] [CrossRef] [Green Version]
- Denli, A.M.; Narvaiza, I.; Kerman, B.E.; Pena, M.; Benner, C.; Marchetto, M.C.N.; Diedrich, J.K.; Aslanian, A.; Ma, J.; Moresco, J.J.; et al. Primate-Specific ORF0 Contributes to Retrotransposon-Mediated Diversity. Cell 2015, 163, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Taylor, M.S.; Altukhov, I.; Molloy, K.R.; Mita, P.; Jiang, H.; Adney, E.M.; Wudzinska, A.; Badri, S.; Ischenko, D.; Eng, G.; et al. Dissection of Affinity Captured LINE-1 Macromolecular Complexes. eLife 2018, 7, e30094. [Google Scholar] [CrossRef]
- Moldovan, J.B.; Wang, Y.; Shuman, S.; Mills, R.E.; Moran, J.V. RNA Ligation Precedes the Retrotransposition of U6/LINE-1 Chimeric RNA. Proc. Natl. Acad. Sci. USA 2019, 116, 20612–20622. [Google Scholar] [CrossRef] [Green Version]
- Legnini, I.; Alles, J.; Karaiskos, N.; Ayoub, S.; Rajewsky, N. FLAM-Seq: Full-Length MRNA Sequencing Reveals Principles of Poly(A) Tail Length Control. Nat. Methods 2019, 16, 879–886. [Google Scholar] [CrossRef]
- Wei, W.; Gilbert, N.; Ooi, S.L.; Lawler, J.F.; Ostertag, E.M.; Kazazian, H.H.; Boeke, J.D.; Moran, J.V. Human L1 Retrotransposition: Cis Preference versus Trans. Complementation. Mol. Cell. Biol. 2001, 21, 1429–1439. [Google Scholar] [CrossRef] [Green Version]
- Kulpa, D.A.; Moran, J.V. Cis-Preferential LINE-1 Reverse Transcriptase Activity in Ribonucleoprotein Particles. Nat. Struct. Mol. Biol. 2006, 13, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Ahl, V.; Keller, H.; Schmidt, S.; Weichenrieder, O. Retrotransposition and Crystal Structure of an Alu RNP in the Ribosome-Stalling Conformation. Mol. Cell 2015, 60, 715–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percharde, M.; Sultana, T.; Ramalho-Santos, M. What Doesn’t Kill You Makes You Stronger: Transposons as Dual Players in Chromatin Regulation and Genomic Variation. BioEssays 2020, 42, 1900232. [Google Scholar] [CrossRef] [Green Version]
- Beraldi, R.; Pittoggi, C.; Sciamanna, I.; Mattei, E.; Spadafora, C. Expression of LINE-1 Retroposons Is Essential for Murine Preimplantation Development. Mol. Reprod. Dev. 2006, 73, 279–287. [Google Scholar] [CrossRef]
- Jachowicz, J.W.; Bing, X.; Pontabry, J.; Bošković, A.; Rando, O.J.; Torres-Padilla, M.-E. LINE-1 Activation after Fertilization Regulates Global Chromatin Accessibility in the Early Mouse Embryo. Nat. Genet. 2017, 49, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, S.J.; Lee, S.; Grandi, F.C.; Gaysinskaya, V.; Rosser, J.M.; Vanden Berg, N.; Hogarth, C.A.; Marchetto, M.C.N.; Muotri, A.R.; Griswold, M.D.; et al. Intact PiRNA Pathway Prevents L1 Mobilization in Male Meiosis. Proc. Natl. Acad. Sci. USA 2017, 114, E5635–E5644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, S.R.; Faulkner, G.J. Heritable L1 Retrotransposition Events during Development: Understanding Their Origins: Examination of Heritable, Endogenous L1 Retrotransposition in Mice Opens up Exciting New Questions and Research Directions. BioEssays 2018, 40, 1700189. [Google Scholar] [CrossRef] [Green Version]
- Schwertz, H.; Rowley, J.W.; Schumann, G.G.; Thorack, U.; Campbell, R.A.; Manne, B.K.; Zimmerman, G.A.; Weyrich, A.S.; Rondina, M.T. Endogenous LINE-1 (Long Interspersed Nuclear Element-1) Reverse Transcriptase Activity in Platelets Controls Translational Events Through RNA–DNA Hybrids. ATVB 2018, 38, 801–815. [Google Scholar] [CrossRef]
- Levin, H.L.; Moran, J.V. Dynamic Interactions between Transposable Elements and Their Hosts. Nat. Rev. Genet. 2011, 12, 615–627. [Google Scholar] [CrossRef]
- Goodier, J.L. Restricting Retrotransposons: A Review. Mob. DNA 2016, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Pizarro, J.G.; Cristofari, G. Post-Transcriptional Control of LINE-1 Retrotransposition by Cellular Host Factors in Somatic Cells. Front. Cell Dev. Biol. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warkocki, Z.; Krawczyk, P.S.; Adamska, D.; Bijata, K.; Garcia-Perez, J.L.; Dziembowski, A. Uridylation by TUT4/7 Restricts Retrotransposition of Human LINE-1s. Cell 2018, 174, 1537–1548.e29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Luque, F.J.; Kempen, M.-J.H.C.; Gerdes, P.; Vargas-Landin, D.B.; Richardson, S.R.; Troskie, R.-L.; Jesuadian, J.S.; Cheetham, S.W.; Carreira, P.E.; Salvador-Palomeque, C.; et al. LINE-1 Evasion of Epigenetic Repression in Humans. Mol. Cell 2019, 75, 590–604.e12. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, M.; Criscione, S.W.; Peterson, A.L.; Neretti, N.; Sedivy, J.M.; Kreiling, J.A. Transposable Elements Become Active and Mobile in the Genomes of Aging Mammalian Somatic Tissues. Aging 2013, 5, 867–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostertag, E.M.; DeBerardinis, R.J.; Goodier, J.L.; Zhang, Y.; Yang, N.; Gerton, G.L.; Kazazian, H.H. A Mouse Model of Human L1 Retrotransposition. Nat. Genet. 2002, 32, 655–660. [Google Scholar] [CrossRef]
- Belancio, V.P.; Roy-Engel, A.M.; Pochampally, R.R.; Deininger, P. Somatic Expression of LINE-1 Elements in Human Tissues. Nucleic Acids Res. 2010, 38, 3909–3922. [Google Scholar] [CrossRef] [Green Version]
- Kano, H.; Godoy, I.; Courtney, C.; Vetter, M.R.; Gerton, G.L.; Ostertag, E.M.; Kazazian, H.H. L1 Retrotransposition Occurs Mainly in Embryogenesis and Creates Somatic Mosaicism. Genes Dev. 2009, 23, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Kohlrausch, F.B.; Berteli, T.S.; Wang, F.; Navarro, P.A.; Keefe, D.L. Control of LINE-1 Expression Maintains Genome Integrity in Germline and Early Embryo Development. Reprod. Sci. 2021. [Google Scholar] [CrossRef]
- Ergün, S.; Buschmann, C.; Heukeshoven, J.; Dammann, K.; Schnieders, F.; Lauke, H.; Chalajour, F.; Kilic, N.; Strätling, W.H.; Schumann, G.G. Cell Type-Specific Expression of LINE-1 Open Reading Frames 1 and 2 in Fetal and Adult Human Tissues. J. Biol. Chem. 2004, 279, 27753–27763. [Google Scholar] [CrossRef] [Green Version]
- Lazaros, L.; Kitsou, C.; Kostoulas, C.; Bellou, S.; Hatzi, E.; Ladias, P.; Stefos, T.; Markoula, S.; Galani, V.; Vartholomatos, G.; et al. Retrotransposon Expression and Incorporation of Cloned Human and Mouse Retroelements in Human Spermatozoa. Fertil. Steril. 2017, 107, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Giordano, R.; Magnano, A.R.; Zaccagnini, G.; Pittoggi, C.; Moscufo, N.; Lorenzini, R.; Spadafora, C. Reverse Transcriptase Activity in Mature Spermatozoa of Mouse. J. Cell Biol. 2000, 148, 1107–1114. [Google Scholar] [CrossRef]
- Georgiou, I.; Noutsopoulos, D.; Dimitriadou, E.; Markopoulos, G.; Apergi, A.; Lazaros, L.; Vaxevanoglou, T.; Pantos, K.; Syrrou, M.; Tzavaras, T. Retrotransposon RNA Expression and Evidence for Retrotransposition Events in Human Oocytes. Hum. Mol. Genet. 2009, 18, 1221–1228. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.R.; Gerdes, P.; Gerhardt, D.J.; Sanchez-Luque, F.J.; Bodea, G.-O.; Muñoz-Lopez, M.; Jesuadian, J.S.; Kempen, M.-J.H.C.; Carreira, P.E.; Jeddeloh, J.A.; et al. Heritable L1 Retrotransposition in the Mouse Primordial Germline and Early Embryo. Genome Res. 2017, 27, 1395–1405. [Google Scholar] [CrossRef] [Green Version]
- Del Re, B.; Giorgi, G. Long INterspersed Element-1 Mobility as a Sensor of Environmental Stresses. Environ. Mol. Mutagen. 2020, 61, 465–493. [Google Scholar] [CrossRef]
- Rangwala, S.H.; Zhang, L.; Kazazian, H.H. Many LINE1 Elements Contribute to the Transcriptome of Human Somatic Cells. Genome Biol. 2009, 10, R100. [Google Scholar] [CrossRef] [Green Version]
- Banaz-Yaşar, F.; Steffen, G.; Hauschild, J.; Bongartz, B.M.; Schumann, G.G.; Ergün, S. LINE-1 Retrotransposition Events Affect Endothelial Proliferation and Migration. Histochem. Cell Biol. 2010, 134, 581–589. [Google Scholar] [CrossRef]
- Thomas, C.A.; Paquola, A.C.M.; Muotri, A.R. LINE-1 Retrotransposition in the Nervous System. Annu. Rev. Cell Dev. Biol. 2012, 28, 555–573. [Google Scholar] [CrossRef]
- Upton, K.R.; Gerhardt, D.J.; Jesuadian, J.S.; Richardson, S.R.; Sánchez-Luque, F.J.; Bodea, G.O.; Ewing, A.D.; Salvador-Palomeque, C.; van der Knaap, M.S.; Brennan, P.M.; et al. Ubiquitous L1 Mosaicism in Hippocampal Neurons. Cell 2015, 161, 228–239. [Google Scholar] [CrossRef] [Green Version]
- Erwin, J.A.; Paquola, A.C.M.; Singer, T.; Gallina, I.; Novotny, M.; Quayle, C.; Bedrosian, T.A.; Alves, F.I.A.; Butcher, C.R.; Herdy, J.R.; et al. L1-Associated Genomic Regions Are Deleted in Somatic Cells of the Healthy Human Brain. Nat. Neurosci. 2016, 19, 1583–1591. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, G.J.; Garcia-Perez, J.L. L1 Mosaicism in Mammals: Extent, Effects, and Evolution. Trends Genet. 2017, 33, 802–816. [Google Scholar] [CrossRef] [Green Version]
- Terry, D.M.; Devine, S.E. Aberrantly High Levels of Somatic LINE-1 Expression and Retrotransposition in Human Neurological Disorders. Front. Genet. 2020, 10, 1244. [Google Scholar] [CrossRef] [Green Version]
- Bodea, G.O.; McKelvey, E.G.Z.; Faulkner, G.J. Retrotransposon-Induced Mosaicism in the Neural Genome. Open Biol. 2018, 8, 180074. [Google Scholar] [CrossRef] [Green Version]
- Muotri, A.R.; Chu, V.T.; Marchetto, M.C.N.; Deng, W.; Moran, J.V.; Gage, F.H. Somatic Mosaicism in Neuronal Precursor Cells Mediated by L1 Retrotransposition. Nature 2005, 435, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Coufal, N.G.; Garcia-Perez, J.L.; Peng, G.E.; Yeo, G.W.; Mu, Y.; Lovci, M.T.; Morell, M.; O’Shea, K.S.; Moran, J.V.; Gage, F.H. L1 Retrotransposition in Human Neural Progenitor Cells. Nature 2009, 460, 1127–1131. [Google Scholar] [CrossRef] [Green Version]
- Baillie, J.K.; Barnett, M.W.; Upton, K.R.; Gerhardt, D.J.; Richmond, T.A.; De Sapio, F.; Brennan, P.M.; Rizzu, P.; Smith, S.; Fell, M.; et al. Somatic Retrotransposition Alters the Genetic Landscape of the Human Brain. Nature 2011, 479, 534–537. [Google Scholar] [CrossRef] [Green Version]
- Macia, A.; Widmann, T.J.; Heras, S.R.; Ayllon, V.; Sanchez, L.; Benkaddour-Boumzaouad, M.; Muñoz-Lopez, M.; Rubio, A.; Amador-Cubero, S.; Blanco-Jimenez, E.; et al. Engineered LINE-1 Retrotransposition in Nondividing Human Neurons. Genome Res. 2017, 27, 335–348. [Google Scholar] [CrossRef] [Green Version]
- Shukla, R.; Upton, K.R.; Muñoz-Lopez, M.; Gerhardt, D.J.; Fisher, M.E.; Nguyen, T.; Brennan, P.M.; Baillie, J.K.; Collino, A.; Ghisletti, S.; et al. Endogenous Retrotransposition Activates Oncogenic Pathways in Hepatocellular Carcinoma. Cell 2013, 153, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Ewing, A.D.; Gacita, A.; Wood, L.D.; Ma, F.; Xing, D.; Kim, M.-S.; Manda, S.S.; Abril, G.; Pereira, G.; Makohon-Moore, A.; et al. Widespread Somatic L1 Retrotransposition Occurs Early during Gastrointestinal Cancer Evolution. Genome Res. 2015, 25, 1536–1545. [Google Scholar] [CrossRef] [Green Version]
- Doucet-O’Hare, T.T.; Rodić, N.; Sharma, R.; Darbari, I.; Abril, G.; Choi, J.A.; Young Ahn, J.; Cheng, Y.; Anders, R.A.; Burns, K.H.; et al. LINE-1 Expression and Retrotransposition in Barrett’s Esophagus and Esophageal Carcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, E4894–E4900. [Google Scholar] [CrossRef] [Green Version]
- Doucet-O’Hare, T.T.; Sharma, R.; Rodić, N.; Anders, R.A.; Burns, K.H.; Kazazian, H.H. Somatically Acquired LINE-1 Insertions in Normal Esophagus Undergo Clonal Expansion in Esophageal Squamous Cell Carcinoma: HUMAN MUTATION. Hum. Mutat. 2016, 37, 942–954. [Google Scholar] [CrossRef] [Green Version]
- De Cecco, M.; Ito, T.; Petrashen, A.P.; Elias, A.E.; Skvir, N.J.; Criscione, S.W.; Caligiana, A.; Brocculi, G.; Adney, E.M.; Boeke, J.D.; et al. L1 Drives IFN in Senescent Cells and Promotes Age-Associated Inflammation. Nature 2019, 566, 73–78. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Messenger RNA Encoding the Full-Length SARS-CoV-2 Spike Glycoprotein. 2020. Entry 11889. Available online: https://web.archive.org/web/20210105162941/https://mednet-communities.net/inn/db/media/docs/11889.doc (accessed on 30 April 2021).
- Vogel, A.B.; Kanevsky, I.; Che, Y.; Swanson, K.A.; Muik, A.; Vormehr, M.; Kranz, L.M.; Walzer, K.C.; Hein, S.; Güler, A.; et al. BNT162b Vaccines Protect Rhesus Macaques from SARS-CoV-2. Nature 2021, 592, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N1-Methylpseudouridine-Incorporated MRNA Outperforms Pseudouridine-Incorporated MRNA by Providing Enhanced Protein Expression and Reduced Immunogenicity in Mammalian Cell Lines and Mice. J. Control. Release 2015, 217, 337–344. [Google Scholar] [CrossRef]
- Svitkin, Y.V.; Cheng, Y.M.; Chakraborty, T.; Presnyak, V.; John, M.; Sonenberg, N. N1-Methyl-Pseudouridine in MRNA Enhances Translation through EIF2α-Dependent and Independent Mechanisms by Increasing Ribosome Density. Nucleic Acids Res. 2017, 45, 6023–6036. [Google Scholar] [CrossRef] [Green Version]
- Parr, C.J.C.; Wada, S.; Kotake, K.; Kameda, S.; Matsuura, S.; Sakashita, S.; Park, S.; Sugiyama, H.; Kuang, Y.; Saito, H. N 1-Methylpseudouridine Substitution Enhances the Performance of Synthetic MRNA Switches in Cells. Nucleic Acids Res. 2020, 48, e35. [Google Scholar] [CrossRef] [Green Version]
- Corbett, K.S.; Edwards, D.K.; Leist, S.R.; Abiona, O.M.; Boyoglu-Barnum, S.; Gillespie, R.A.; Himansu, S.; Schäfer, A.; Ziwawo, C.T.; DiPiazza, A.T.; et al. SARS-CoV-2 MRNA Vaccine Design Enabled by Prototype Pathogen Preparedness. Nature 2020, 586, 567–571. [Google Scholar] [CrossRef]
- Shi, K.; Liu, T.; Fu, H.; Li, W.; Zheng, X. Genome-Wide Analysis of LncRNA Stability in Human. PLoS Comput. Biol. 2021, 17, e1008918. [Google Scholar] [CrossRef]
- Mauger, D.M.; Cabral, B.J.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B.; Link, K.; Khatwani, N.; Reynders, J.; Moore, M.J.; et al. MRNA Structure Regulates Protein Expression through Changes in Functional Half-Life. Proc. Natl. Acad. Sci. USA 2019, 116, 24075–24083. [Google Scholar] [CrossRef] [Green Version]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Lopez Bernal, J. Effectiveness of COVID-19 Booster Vaccines against COVID-19-Related Symptoms, Hospitalization and Death in England. Nat. Med. 2022. [Google Scholar] [CrossRef]
- Watson, C. Three, Four or More: What’s the Magic Number for Booster Shots? Nature 2022, 602, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for MRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Song, H.; Xu, Y.; Garrison, K.E.; Buzdin, A.A.; Anwar, N.; Hunter, D.V.; Mujib, S.; Mihajlovic, V.; Martin, E.; et al. LINE-1 Retrotransposable Element DNA Accumulates in HIV-1-Infected Cells. J. Virol. 2013, 87, 13307–13320. [Google Scholar] [CrossRef] [Green Version]
- Macchietto, M.G.; Langlois, R.A.; Shen, S.S. Virus-Induced Transposable Element Expression up-Regulation in Human and Mouse Host Cells. Life Sci. Alliance 2020, 3, e201900536. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, X.; He, X.; Zhou, L. Exogenous Coronavirus Interacts with Endogenous Retrotransposon in Human Cells. Front. Cell. Infect. Microbiol. 2021, 11, 609160. [Google Scholar] [CrossRef]
- Trepotec, Z.; Geiger, J.; Plank, C.; Aneja, M.K.; Rudolph, C. Segmented Poly(A) Tails Significantly Reduce Recombination of Plasmid DNA without Affecting MRNA Translation Efficiency or Half-Life. RNA 2019, 25, 507–518. [Google Scholar] [CrossRef]
- Holtkamp, S.; Kreiter, S.; Selmi, A.; Simon, P.; Koslowski, M.; Huber, C.; Türeci, Ö.; Sahin, U. Modification of Antigen-Encoding RNA Increases Stability, Translational Efficacy, and T-Cell Stimulatory Capacity of Dendritic Cells. Blood 2006, 108, 4009–4017. [Google Scholar] [CrossRef]
- Potapov, V.; Fu, X.; Dai, N.; Corrêa, I.R.; Tanner, N.A.; Ong, J.L. Base Modifications Affecting RNA Polymerase and Reverse Transcriptase Fidelity. Nucleic Acids Res. 2018, 46, 5753–5763. [Google Scholar] [CrossRef]
- Linares-Fernández, S.; Lacroix, C.; Exposito, J.-Y.; Verrier, B. Tailoring MRNA Vaccine to Balance Innate/Adaptive Immune Response. Trends Mol. Med. 2020, 26, 311–323. [Google Scholar] [CrossRef]
- Zhang, N.-N.; Li, X.-F.; Deng, Y.-Q.; Zhao, H.; Huang, Y.-J.; Yang, G.; Huang, W.-J.; Gao, P.; Zhou, C.; Zhang, R.-R.; et al. A Thermostable MRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef]
- Rauch, S.; Roth, N.; Schwendt, K.; Fotin-Mleczek, M.; Mueller, S.O.; Petsch, B. MRNA Based SARS-CoV-2 Vaccine Candidate CVnCoV Induces High Levels of Virus Neutralizing Antibodies and Mediates Protection in Rodents. NPJ Vaccines 2020, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marinov, G.K.; Williams, B.A.; McCue, K.; Schroth, G.P.; Gertz, J.; Myers, R.M.; Wold, B.J. From Single-Cell to Cell-Pool Transcriptomes: Stochasticity in Gene Expression and RNA Splicing. Genome Res. 2014, 24, 496–510. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-Assembled MRNA Vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef]
- Wu, Z.; Li, T. Nanoparticle-Mediated Cytoplasmic Delivery of Messenger RNA Vaccines: Challenges and Future Perspectives. Pharm. Res. 2021, 38, 473–478. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for Translation: Non-Viral Materials for Therapeutic MRNA Delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjugate Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef]
- Garneau, N.L.; Wilusz, J.; Wilusz, C.J. The Highways and Byways of MRNA Decay. Nat. Rev. Mol. Cell Biol. 2007, 8, 113–126. [Google Scholar] [CrossRef]
- Houseley, J.; Tollervey, D. The Many Pathways of RNA Degradation. Cell 2009, 136, 763–776. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.-J.; Crank, M.C.; Shiver, J.; Graham, B.S.; Mascola, J.R.; Nabel, G.J. Next-Generation Influenza Vaccines: Opportunities and Challenges. Nat. Rev. Drug Discov. 2020, 19, 239–252. [Google Scholar] [CrossRef]
- Hubstenberger, A.; Courel, M.; Bénard, M.; Souquere, S.; Ernoult-Lange, M.; Chouaib, R.; Yi, Z.; Morlot, J.-B.; Munier, A.; Fradet, M.; et al. P-Body Purification Reveals the Condensation of Repressed MRNA Regulons. Mol. Cell 2017, 68, 144–157.e5. [Google Scholar] [CrossRef] [Green Version]
- Corbet, G.A.; Parker, R. RNP Granule Formation: Lessons from P-Bodies and Stress Granules. Cold Spring Harb. Symp. Quant. Biol. 2019, 84, 203–215. [Google Scholar] [CrossRef]
- Mandal, P.K.; Ewing, A.D.; Hancks, D.C.; Kazazian, H.H. Enrichment of Processed Pseudogene Transcripts in L1-Ribonucleoprotein Particles. Hum. Mol. Genet. 2013, 22, 3730–3748. [Google Scholar] [CrossRef] [Green Version]
- Roberson, P.A.; Romero, M.A.; Osburn, S.C.; Mumford, P.W.; Vann, C.G.; Fox, C.D.; McCullough, D.J.; Brown, M.D.; Roberts, M.D. Skeletal Muscle LINE-1 ORF1 MRNA Is Higher in Older Humans but Decreases with Endurance Exercise and Is Negatively Associated with Higher Physical Activity. J. Appl. Physiol. 2019, 127, 895–904. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression Kinetics of Nucleoside-Modified MRNA Delivered in Lipid Nanoparticles to Mice by Various Routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Reply to Open Letter Concerning COVID-19 Vaccines. 2021. EMA/140520/2021. Available online: https://www.ema.europa.eu/en/documents/other/reply-open-letter-concerning-vaccines-covid-19_en.pdf (accessed on 29 May 2021).
- Akinc, A.; Querbes, W.; De, S.; Qin, J.; Frank-Kamenetsky, M.; Jayaprakash, K.N.; Jayaraman, M.; Rajeev, K.G.; Cantley, W.L.; Dorkin, J.R.; et al. Targeted Delivery of RNAi Therapeutics with Endogenous and Exogenous Ligand-Based Mechanisms. Mol. Ther. 2010, 18, 1357–1364. [Google Scholar] [CrossRef]
- Cagigi, A.; Loré, K. Immune Responses Induced by MRNA Vaccination in Mice, Monkeys and Humans. Vaccines 2021, 9, 61. [Google Scholar] [CrossRef]
- Hussain, M.M.; Strickland, D.K.; Bakillah, A. The Mammalian Low-Density Lipoprotein Receptor Family. Annu. Rev. Nutr. 1999, 19, 141–172. [Google Scholar] [CrossRef]
- Mahley, R.W.; Rall, S.C. A POLIPOPROTEIN E: Far More Than a Lipid Transport Protein. Annu. Rev. Genom. Hum. Genet. 2000, 1, 507–537. [Google Scholar] [CrossRef]
- Probst, J.; Weide, B.; Scheel, B.; Pichler, B.J.; Hoerr, I.; Rammensee, H.-G.; Pascolo, S. Spontaneous Cellular Uptake of Exogenous Messenger RNA in Vivo Is Nucleic Acid-Specific, Saturable and Ion Dependent. Gene Ther. 2007, 14, 1175–1180. [Google Scholar] [CrossRef] [Green Version]
- Lazzaro, S.; Giovani, C.; Mangiavacchi, S.; Magini, D.; Maione, D.; Baudner, B.; Geall, A.J.; De Gregorio, E.; D’Oro, U.; Buonsanti, C. CD8 T-Cell Priming upon MRNA Vaccination Is Restricted to Bone-Marrow-Derived Antigen-Presenting Cells and May Involve Antigen Transfer from Myocytes. Immunology 2015, 146, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; Deletic, P.; McKay, P.F.; Bouton, C.R.; Ashford, M.; Shattock, R.J.; Sabirsh, A. Effect of Complexing Lipids on Cellular Uptake and Expression of Messenger RNA in Human Skin Explants. J. Control. Release 2021, 330, 1250–1261. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of MRNA and Protein Delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma Microvesicles Transport RNA and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular Microvesicles/Exosomes: Discovery, Disbelief, Acceptance, and the Future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Van den Boorn, J.G.; Schlee, M.; Coch, C.; Hartmann, G. SiRNA Delivery with Exosome Nanoparticles. Nat. Biotechnol. 2011, 29, 325–326. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M. Dendritic Cell Extracellular Vesicles. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 349, pp. 213–249. ISBN 978-0-12-818357-1. [Google Scholar]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal MiRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384.e12. [Google Scholar] [CrossRef] [Green Version]
- Ostermeier, G.C.; Miller, D.; Huntriss, J.D.; Diamond, M.P.; Krawetz, S.A. Delivering Spermatozoan RNA to the Oocyte. Nature 2004, 429, 154. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, W.; Duan, E. Epigenetic Inheritance of Acquired Traits through Sperm RNAs and Sperm RNA Modifications. Nat. Rev. Genet. 2016, 17, 733–743. [Google Scholar] [CrossRef]
- Sun, Y.H.; Wang, A.; Song, C.; Shankar, G.; Srivastava, R.K.; Au, K.F.; Li, X.Z. Single-Molecule Long-Read Sequencing Reveals a Conserved Intact Long RNA Profile in Sperm. Nat. Commun. 2021, 12, 1361. [Google Scholar] [CrossRef]
- Sciamanna, I.; Serafino, A.; Shapiro, J.A.; Spadafora, C. The Active Role of Spermatozoa in Transgenerational Inheritance. Proc. R. Soc. B 2019, 286, 20191263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cossetti, C.; Lugini, L.; Astrologo, L.; Saggio, I.; Fais, S.; Spadafora, C. Soma-to-Germline Transmission of RNA in Mice Xenografted with Human Tumour Cells: Possible Transport by Exosomes. PLoS ONE 2014, 9, e101629. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, J.-Y.; Yang, J.-S.; Kim, J.W.; Kim, V.N.; Chang, H. The Architecture of SARS-CoV-2 Transcriptome. Cell 2020, 181, 914–921.e10. [Google Scholar] [CrossRef] [PubMed]
- Flasch, D.A.; Macia, Á.; Sánchez, L.; Ljungman, M.; Heras, S.R.; García-Pérez, J.L.; Wilson, T.E.; Moran, J.V. Genome-Wide de Novo L1 Retrotransposition Connects Endonuclease Activity with Replication. Cell 2019, 177, 837–851.e28. [Google Scholar] [CrossRef] [PubMed]
- Sultana, T.; van Essen, D.; Siol, O.; Bailly-Bechet, M.; Philippe, C.; Zine El Aabidine, A.; Pioger, L.; Nigumann, P.; Saccani, S.; Andrau, J.-C.; et al. The Landscape of L1 Retrotransposons in the Human Genome Is Shaped by Pre-Insertion Sequence Biases and Post-Insertion Selection. Mol. Cell 2019, 74, 555–570.e7. [Google Scholar] [CrossRef]
- Aldén, M.; Olofsson Falla, F.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 MRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. [Google Scholar] [CrossRef]
- Bartha, I.; di Iulio, J.; Venter, J.C.; Telenti, A. Human Gene Essentiality. Nat. Rev. Genet. 2018, 19, 51–62. [Google Scholar] [CrossRef]
- Zhang, L.; Vijg, J. Somatic Mutagenesis in Mammals and Its Implications for Human Disease and Aging. Annu. Rev. Genet. 2018, 52, 397–419. [Google Scholar] [CrossRef]
- Sender, R.; Milo, R. The Distribution of Cellular Turnover in the Human Body. Nat. Med. 2021, 27, 45–48. [Google Scholar] [CrossRef]
- Tomasetti, C.; Poling, J.; Roberts, N.J.; London, N.R.; Pittman, M.E.; Haffner, M.C.; Rizzo, A.; Baras, A.; Karim, B.; Kim, A.; et al. Cell Division Rates Decrease with Age, Providing a Potential Explanation for the Age-Dependent Deceleration in Cancer Incidence. Proc. Natl. Acad. Sci. USA 2019, 116, 20482–20488. [Google Scholar] [CrossRef] [Green Version]
- Pucella, J.N.; Upadhaya, S.; Reizis, B. The Source and Dynamics of Adult Hematopoiesis: Insights from Lineage Tracing. Annu. Rev. Cell Dev. Biol. 2020, 36, 529–550. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Iwasaki, Y.W.; Lin, Z.Y.-C.; Imamura, M.; Seki, N.M.; Sasaki, E.; Saito, K.; Okano, H.; Siomi, M.C.; Siomi, H. Small RNA Profiling and Characterization of PiRNA Clusters in the Adult Testes of the Common Marmoset, a Model Primate. RNA 2014, 20, 1223–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Lin, H. Roles of PiRNAs in Transposon and Pseudogene Regulation of Germline MRNAs and LncRNAs. Genome Biol. 2021, 22, 27. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. Detailed Dissection and Critical Evaluation of the Pfizer/BioNTech and Moderna MRNA Vaccines. Vaccines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Weissman, D. Recent Advances in MRNA Vaccine Technology. Curr. Opin. Immunol. 2020, 65, 14–20. [Google Scholar] [CrossRef]
- Jain, R.; Frederick, J.P.; Huang, E.Y.; Burke, K.E.; Mauger, D.M.; Andrianova, E.A.; Farlow, S.J.; Siddiqui, S.; Pimentel, J.; Cheung-Ong, K.; et al. MicroRNAs Enable MRNA Therapeutics to Selectively Program Cancer Cells to Self-Destruct. Nucleic Acid Ther. 2018, 28, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Mei, Y.-F. SARS-CoV-2 Spike Impairs DNA Damage Repair and Inhibits V(D)J Recombination In Vitro. Viruses 2021, 13, 2056. [Google Scholar] [CrossRef]
- Finlay, B.B.; Amato, K.R.; Azad, M.; Blaser, M.J.; Bosch, T.C.G.; Chu, H.; Dominguez-Bello, M.G.; Ehrlich, S.D.; Elinav, E.; Geva-Zatorsky, N.; et al. The Hygiene Hypothesis, the COVID Pandemic, and Consequences for the Human Microbiome. Proc. Natl. Acad. Sci. USA 2021, 118, e2010217118. [Google Scholar] [CrossRef]
- Romano-Keeler, J.; Zhang, J.; Sun, J. COVID-19 and the Neonatal Microbiome: Will the Pandemic Cost Infants Their Microbes? Gut Microbes 2021, 13, 1912562. [Google Scholar] [CrossRef]
- Ghanemi, A.; Yoshioka, M.; St-Amand, J. Coronavirus Disease 2019 (COVID-19) Crisis: Losing Our Immunity When We Need It the Most. Biology 2021, 10, 545. [Google Scholar] [CrossRef]
- Feliciello, I.; Procino, A. MRNA Vaccines: Why and How They Should Be Modified. J. Biol. Res. 2021, 94, 10072. [Google Scholar] [CrossRef]
- Adams, J.W.; Kaufman, R.E.; Kretschmer, P.J.; Harrison, M.; Nienhuis, A.W. A Family of Long Reiterated DNA Sequences, One Copy of Which Is next to the Human Beta Globin Gene. Nucl. Acids Res. 1980, 8, 6113–6128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skowronski, J.; Singer, M.F. Expression of a Cytoplasmic LINE-1 Transcript Is Regulated in a Human Teratocarcinoma Cell Line. Proc. Natl. Acad. Sci. USA 1985, 82, 6050–6054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Sun, F.; Ping, Z.; Ouyang, Q.; Qian, L. DNA Storage: Research Landscape and Future Prospects. Natl. Sci. Rev. 2020, 7, 1092–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Medicines Agency. Comirnaty: EPAR—Product Information. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 27 January 2021).
- European Medicines Agency. Spikevax (Previously COVID-19 Vaccine Moderna): EPAR—Product Information. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/spikevax-previously-covid-19-vaccine-moderna-epar-product-information_en.pdf (accessed on 28 January 2021).
| mRNA Vaccine Features | References 1 |
| Native mRNA architecture | [6,10,28,133] |
| 3′ poly-A tail | [51,52,132] |
| m1Ψ modification | [20,134,135,136] |
| Improved stability, half-life and translational efficiency | [6,10,27,56,133,138,139] |
| mRNA concentration per dose | [1,140] |
| Recurrent application | [1,2,56,141,142] |
| Lipid nanoparticle formulation | [1,2,10,56,143] |
| Cytosol delivery | [5,6,10,56] |
| Biodistribution | [1,2] |
| Extracellular vesicles repackaging | [53,57] |
| Other Factors | References1 |
| Increased cell proliferation rates | [37,64,84] |
| Aging | [104,131] |
| Viral infection | [54,55,144,145,146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domazet-Lošo, T. mRNA Vaccines: Why Is the Biology of Retroposition Ignored? Genes 2022, 13, 719. https://doi.org/10.3390/genes13050719
Domazet-Lošo T. mRNA Vaccines: Why Is the Biology of Retroposition Ignored? Genes. 2022; 13(5):719. https://doi.org/10.3390/genes13050719
Chicago/Turabian StyleDomazet-Lošo, Tomislav. 2022. "mRNA Vaccines: Why Is the Biology of Retroposition Ignored?" Genes 13, no. 5: 719. https://doi.org/10.3390/genes13050719
APA StyleDomazet-Lošo, T. (2022). mRNA Vaccines: Why Is the Biology of Retroposition Ignored? Genes, 13(5), 719. https://doi.org/10.3390/genes13050719





