Proteomic Profiling and Pathway Analysis of Acid Stress-Induced Vasorelaxation of Mesenteric Arteries In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Collection of Mesenteric Artery
2.3. Preparation of Superior Mesenteric Artery and Tension Recording
2.4. Isometric Contraction Study
2.4.1. Histamine- and KCl-Induced Concentration-Related Contractile Responses at pHo 7.4, 6.8, and 6.0
2.4.2. NA- and KCl-Induced Sustained Contraction Relaxed with Ach and SNP
2.5. Preparation of Protein Extracts and Quantification
2.6. Gel Electrophoresis
2.7. Gel Image Analysis
2.8. MALDI TOF/MS and LC-MS/MS
2.9. Pathway Analysis
2.10. Statistical Analysis
3. Results
3.1. Isometric Contraction Study
Histamine- and KCl-Induced Concentration-Related Contractile Responses at pH0 7.4, 6.8, and 6.0
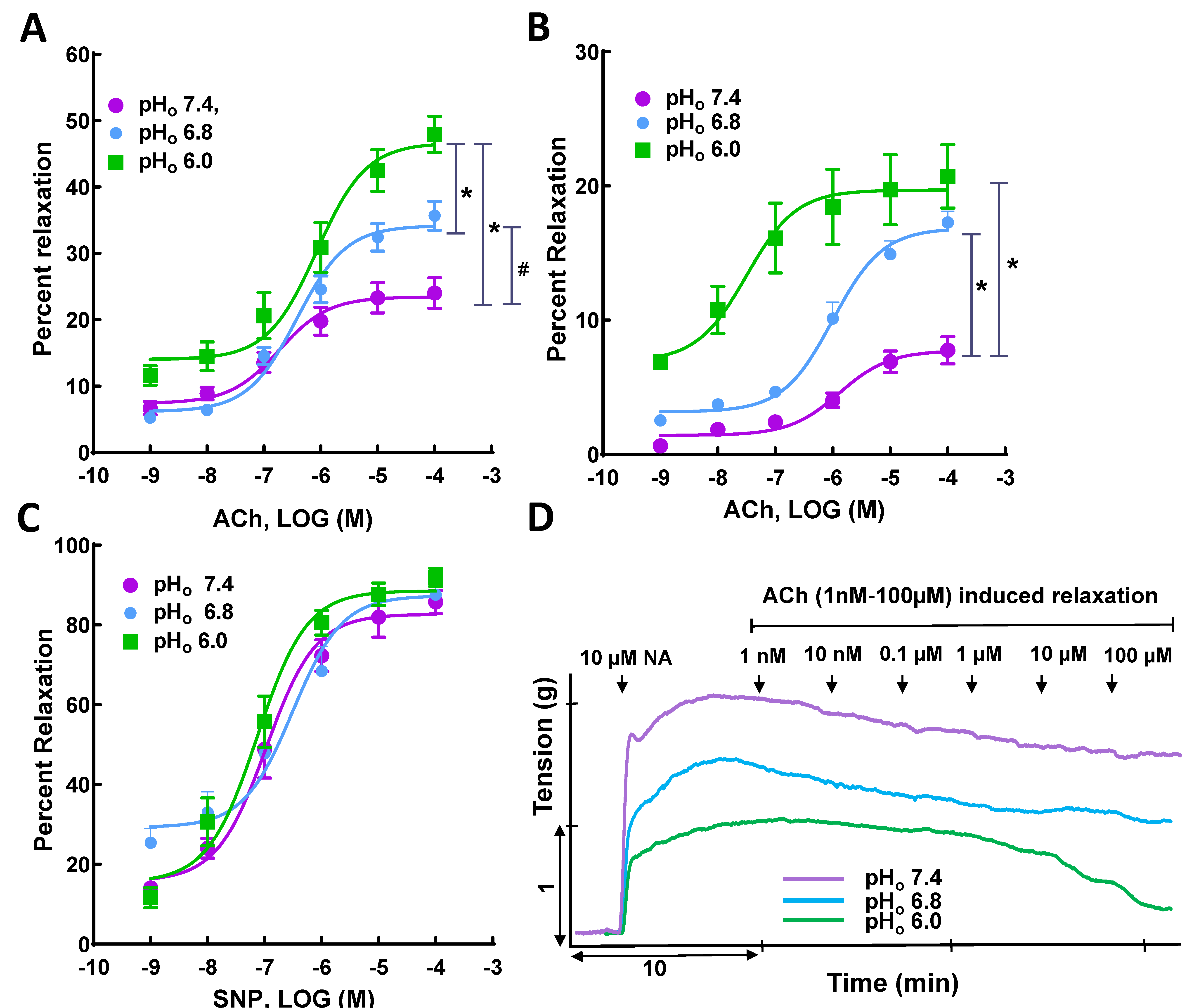
3.2. Ach- and SNP-Induced Vasorelaxation in NA- and KCl-Precontracted SMA Rings
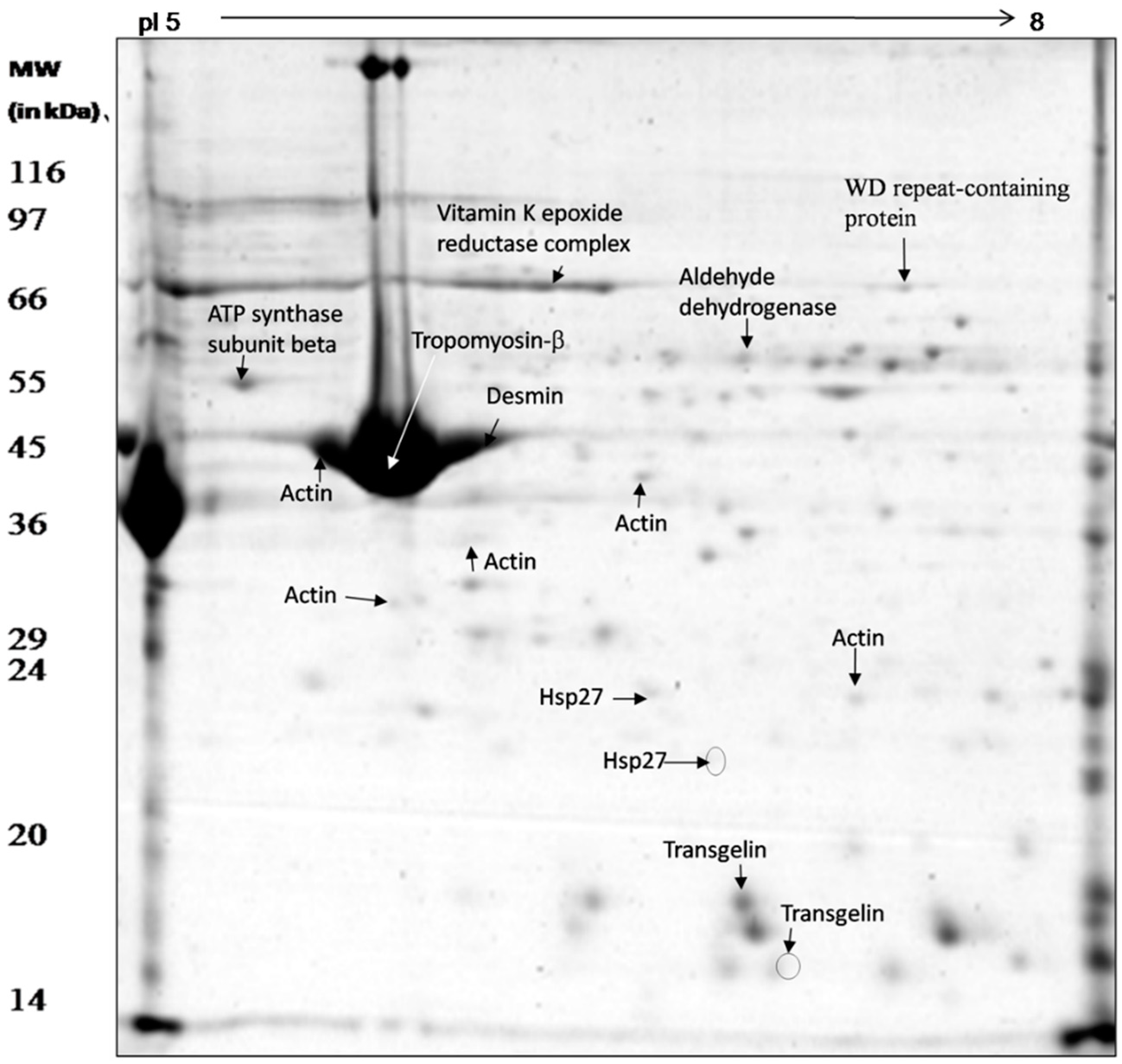
3.3. Proteomic Analysis of Whole Mesenteric Artery Wall Extracts by 2D-GE and MS
3.4. Pathway Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Schauer, K.L.; Freund, D.M.; Prenni, J.E.; Curthoys, N.P. Proteomic profiling and pathway analysis of the response of rat renal proximal convoluted tubules to metabolic acidosis. Am. J. Physiol.-Ren. Physiol. 2013, 305, F628–F640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, N. Non-Occlusive Mesenteric Ischemia during the Course of Heart Failure. J. Clin. Case Rep. 2012, 2, 220. [Google Scholar] [CrossRef]
- Lim, S.Y.; Park, Y.; Chin, H.J.; Na, K.Y.; Chae, D.-W.; Kim, S. Short-term and long-term effects of low serum bicarbonate level at admission in hospitalised patients. Sci. Rep. 2019, 9, 2798. [Google Scholar] [CrossRef] [PubMed]
- Căpuşă, C.; Ştefan, G.; Stancu, S.; Lipan, M.; Tsur, L.D.; Mircescu, G. Metabolic acidosis of chronic kidney disease and subclinical cardiovascular disease markers: Friend or foe? Medicine 2017, 96, e8802. [Google Scholar] [CrossRef]
- Kraut, J.A.; Madias, N.E. Metabolic acidosis: Pathophysiology, diagnosis and management. Nat. Rev. Nephrol. 2010, 6, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, A.; Yavuz, C.; Karahan, O.; Demirtas, S.; Yazici, S.; Guclu, O.; Mavitas, B. Serum ischaemia–modified albumin level is an irrelevant predictive factor for ischaemic duration in mesenteric ischaemia. Perfusion 2014, 29, 226–230. [Google Scholar] [CrossRef]
- Gokina, N.I.; Fairchild, R.I.; Bishop, N.M.; Dawson, T.E.; Prakash, K.; Bonney, E.A. Kinetics of Postpartum Mesenteric Artery Structure and Function Relative to Pregnancy and Lactation in Mice. Reprod. Sci. 2021, 28, 1200–1215. [Google Scholar] [CrossRef]
- Watters, A.; Gibson, D.; Dee, E.; Mascolo, M.; Mehler, P.S. Superior mesenteric artery syndrome in severe anorexia nervosa: A case series. Clin. Case Rep. 2020, 8, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Xing, H.; Chang, Y. Thermal damage width and hemostatic effect of bipolar electrocoagulation, LigaSure, and Ultracision techniques on goat mesenteric vessels and optimal power for bipolar electrocoagulation. BMC Surg. 2019, 19, 147. [Google Scholar] [CrossRef]
- Alvites, R.D.; Branquinho, M.V.; Sousa, A.C.; Lopes, B.; Sousa, P.; Mendonça, C.; Atayde, L.M.; Maurício, A.C. Small Ruminants and Its Use in Regenerative Medicine: Recent Works and Future Perspectives. Biology 2021, 10, 249. [Google Scholar] [CrossRef]
- Wu, X.; Han, X.; Li, L.; Fan, S.; Zhuang, P.; Yang, Z.; Zhang, Y. iTRAQ-based quantitative proteomics and target-fishing strategies reveal molecular signatures on vasodilation of Compound Danshen Dripping Pills. Chem.-Biol. Interact. 2020, 316, 108923. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Bernardes, G.J.L. Machine learning for target discovery in drug development. Curr. Opin. Chem. Biol. 2020, 56, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Saviola, A.J.; Negrão, F.; Yates, J.R. Proteomics of Select Neglected Tropical Diseases. Annu. Rev. Anal. Chem. 2020, 13, 315–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCall, M.N.; Kent, O.A.; Yu, J.; Fox-Talbot, K.; Zaiman, A.L.; Halushka, M.K. MicroRNA profiling of diverse endothelial cell types. BMC Med. Genom. 2011, 4, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, L.; Lorenzoni, P.; Bini, L.; Weber, E.; Tani, C.; Rossi, A.; Agliano, M.; Pallini, V.; Sacchi, G. Protein expression profiles ofBos taurus blood and lymphatic vessel endothelial cells. Proteomics 2007, 7, 1600–1614. [Google Scholar] [CrossRef]
- Walmsley, S.J.; Freund, D.M.; Curthoys, N.P. Proteomic profiling of the effect of metabolic acidosis on the apical membrane of the proximal convoluted tubule. Am. J. Physiol.-Ren. Physiol. 2012, 302, F1465–F1477. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, T.; Gu, H.; Momma, K. Developmental changes in the effect of acidosis on contraction, intracellular pH, and calcium in the rabbit mesenteric small artery. Pediatr. Res. 1997, 42, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, I.; Parija, S.C.; Suklabaidya, S.; Rattan, S. Acidosis potentiates endothelium-dependent vasorelaxation and gap junction communication in the superior mesenteric artery. Eur. J. Pharmacol. 2018, 827, 22–31. [Google Scholar] [CrossRef]
- Mohanty, I.; Suklabaidya, S.; Parija, S.C. Acidosis reduces the function and expression of α1D -adrenoceptor in superior mesenteric artery of Capra hircus. Indian J. Pharmacol. 2016, 48, 399. [Google Scholar]
- Parija, S.C.; Mohanty, I. Acid stress reduces the function of Na+-K+ATPase in superior mesenteric artery of Capra hircus. Pharmacogn. Mag. 2018, 14, 558–563. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Mohanty, S.; Sharma, A.P.; Mohanty, B.P. Effect of storage temperature as a preanalytical variable on the lens crystallins protein quality for proteomic studies. Proteom. Clin. Appl. 2011, 5, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, P. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 1975, 250, 4007–4021. [Google Scholar] [CrossRef]
- Martin, S.A.M.; Mohanty, B.P.; Cash, P.; Houlihan, D.F.; Secombes, C.J. Proteome analysis of the Atlantic salmon (Salmo salar) cell line SHK-1 following recombinant IFN-γ stimulation. Proteomics 2007, 7, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Bringans, S.; Eriksen, S.; Kendrick, T.; Gopalakrishnakone, P.; Livk, A.; Lock, R.; Lipscombe, R. Proteomic analysis of the venom ofHeterometrus longimanus (Asian black scorpion). Proteomics 2008, 8, 1081–1096. [Google Scholar] [CrossRef] [PubMed]
- Mahanty, A.; Purohit, G.K.; Banerjee, S.; Karunakaran, D.; Mohanty, S.; Mohanty, B.P. Proteomic changes in the liver of Channa striatus in response to high temperature stress: Proteomics and 2-DE. Electrophoresis 2016, 37, 1704–1717. [Google Scholar] [CrossRef]
- Stark, R.J.; Choi, H.; Lamb, F.S. Neuronal ASIC1A as a Cerebral pH Sensor. Circ. Res. 2019, 125, 921–923. [Google Scholar] [CrossRef]
- Capellini, V.K.; Restini, C.B.A.; Bendhack, L.M.; Evora, P.R.B.; Celotto, A.C. The Effect of Extracellular pH Changes on Intracellular pH and Nitric Oxide Concentration in Endothelial and Smooth Muscle Cells from Rat Aorta. PLoS ONE 2013, 8, e62887. [Google Scholar] [CrossRef]
- Yartsev, V.N.; Karachentseva, O.V. Noradrenaline-Induced Restoration of Acidosis-Inhibited Neurogenic Vasoreactivity at Using Different Electrical Stimulation Frequencies. Neurosci. Behav. Physiol. 2017, 47, 179–185. [Google Scholar] [CrossRef]
- Workeneh, B.T.; Mitch, W.E. Review of muscle wasting associated with chronic kidney disease. Am. J. Clin. Nutr. 2010, 91, 1128S–1132S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitch, W.E.; Medina, R.; Grieber, S.; May, R.C.; England, B.K.; Price, S.R.; Bailey, J.L.; Goldberg, A.L. Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J. Clin. Investig. 1994, 93, 2127–2133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freund, D.M.; Prenni, J.E.; Curthoys, N.P. Response of the mitochondrial proteome of rat renal proximal convoluted tubules to chronic metabolic acidosis. Am. J. Physiol.-Ren. Physiol. 2013, 304, F145–F155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khacho, M.; Tarabay, M.; Patten, D.; Khacho, P.; MacLaurin, J.G.; Guadagno, J.; Bergeron, R.; Cregan, S.P.; Harper, M.-E.; Park, D.S.; et al. Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell survival. Nat. Commun. 2014, 5, 3550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakkar, V.; Meister-Broekema, M.; Minoia, M.; Carra, S.; Kampinga, H.H. Barcoding heat shock proteins to human diseases: Looking beyond the heat shock response. Dis. Models Mech. 2014, 7, 421–434. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Manns, M.P.; Seidler, U. Cytoprotective effects of acidosis via heat shock protein HSP27 against the anticancer drug doxorubicin. Cell. Mol. Life Sci. 2011, 68, 1041–1051. [Google Scholar] [CrossRef]
- Narasimhan, P.; Swanson, R.A.; Sagar, S.M.; Sharp, F.R. Astrocyte survival and HSP70 heat shock protein induction following heat shock and acidosis. Glia 1996, 17, 147–159. [Google Scholar] [CrossRef]
- Bardoń, A.; Ceder, O.; Roomans, G.M.; Kollberg, H. Effect of metabolic acidosis on glycolysis in rat submandibular glands. Res. Commun. Chem. Pathol. Pharmacol. 1985, 47, 449–452. [Google Scholar]
- Oriot, D.; Wood, C.; Gottesman, R.; Huault, G. Severe Lactic Acidosis Related to Acute Thiamine Deficiency. J. Parenter. Enter. Nutr. 1991, 15, 105–109. [Google Scholar] [CrossRef]
- Zosel, A.; Egelhoff, E.; Heard, K. Severe Lactic Acidosis after an Iatrogenic Propylene Glycol Overdose. Pharmacotherapy 2010, 30, 219. [Google Scholar] [CrossRef] [Green Version]
- Carbonara, K.; Andonovski, M.; Coorssen, J.R. Proteomes Are of Proteoforms: Embracing the Complexity. Proteomes 2021, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Forgrave, L.M.; Wang, M.; Yang, D.; DeMarco, M.L. Proteoforms and their expanding role in laboratory medicine. Pract. Lab. Med. 2021, 28, e00260. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, B.P.; Mahanty, A.; Mitra, T.; Mohanty, S.; Naik, A.K.; Parija, S.C. Proteomic and transcriptomic changes in rat liver following oral feeding of formaldehyde. Chemosphere 2020, 245, 125599. [Google Scholar] [CrossRef] [PubMed]


| pHo 7.4 | pHo 6.8 | pHo 6.0 | ||
|---|---|---|---|---|
| Histamine | Emax | 1.57 ± 0.05 (n = 12) | 0.92 ± 0.02 * (n = 6) | 0.69 ± 0.07 * (n = 14) |
| KCl | Emax | 3.16 ± 0.20 (n = 7) | 2.67 ± 0.14 (n = 10) | 2.35 ± 0.20 * (n = 7) |
| Emax/EBmax (%) | −Log EC50 (pD2) | ||||||
|---|---|---|---|---|---|---|---|
| pHo 7.4 | pHo 6.8 | pHo 6.0 | pHo 7.4 | pHo 6.8 | pHo 6.0 | ||
| NA | Ach | 24.01 ± 2.31 (n = 19) | 35.66 ± 2.18 * (n = 13) | 47.94 ± 2.73 * (n = 19) | 6.73 ± 0.24 | 6.40 ± 0.18 | 6.01 ± 0.37 *# |
| SNP | 85.75 ± 2.98 (n = 8) | 87.57 ± 2.32 (n = 8) | 91.91 ± 2.31 (n = 6) | 6.98 ± 0.13 | 6.51 ± 0.17 | 7.13 ± 0.13 *# | |
| KCl | Ach | 4.06 ± 0.71 (n = 4) | 16.10 ± 2.67 * (n = 4) | 17.33 ± 2.97 * (n = 7) | 5.91 ± 0.26 | 6.04 ± 0.23 | 7.12 ± 0.44 *# |
| Spot Name | Protein ID | Protein Name | Species | Protein Mass | Coverage | Isoelectric Point | # of Unique Peptides | # of Unique Spectrums | Fold Change | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| G3 | gi|389620461|gb| AFK93550.1| | Hsp27 proteinpartial | C. hircus | 20,543.41 | 62.84 | 6.68 | 11 | 85 | 1.41 ↑ | 0.001 |
| G4 | gi|389620461|gb| AFK93550.1| | Hsp27 proteinpartial | C. hircus | 20,543.41 | 62.3 | 6.68 | 13 | 81 | Absent in exp | 0.001 |
| G23 | gi|548466133|ref|XP_005680388.1| | Predicted: ATP synthase subunit β mitochondrial | C. hircus | 56,148.48 | 80.87 | 4.92 | 34 | 537 | 2.14 ↑ | 0.005 |
| G13 | gi|548522357|ref|XP_005698249.1| | Predicted: actinaortic smooth muscle | C. hircus | 42,380.96 | 48.81 | 5.05 | 8 | 73 | 4.53 ↑ | 0.004 |
| G14 | gi|548485020|ref|XP_005686409.1| | Predicted: actin γ-enteric smooth muscle isoform X1 | C. hircus | 42,248.94 | 78.72 | 5.16 | 1 | 1 | 1.61 ↑ | 0.002 |
| G19 | gi|548522357|ref|XP_005698249.1| | Predicted: actin aortic smooth muscle | C. hircus | 42,380.96 | 70.82 | 5.05 | 5 | 49 | 2.99 ↑ | 0.001 |
| G10 | gi|548522357|ref|XP_005698249.1| | Predicted: actin aortic smooth muscle | C. hircus | 42,380.96 | 87.8 | 5.05 | 1 | 14 | No change | 0.38 |
| G26 | gi|548485020|ref|XP_005686409.1| | Predicted: actin γ-enteric smooth muscle isoform X1 | C. hircus | 42,248.94 | 89.89 | 5.16 | 1 | 1 | No change | 0.21 |
| G8 | gi|548494696|ref|XP_005689548.1| | Predicted: transgelin | C. hircus | 22,609.45 | 78.61 | 9.29 | 22 | 285 | 1.67 ↑ | 0.002 |
| G15 | gi|548494696|ref|XP_005689548.1| | Predicted: transgelin | C. hircus | 22,609.45 | 65.67 | 9.29 | 14 | 102 | Absent in exp | 0.001 |
| G17 | gi|548471260|ref|XP_005682023.1| | Predicted: WD repeat-containing protein 1(partial) | C. hircus | 65,402.72 | 47.55 | 6.47 | 22 | 230 | 5.4 ↑ | 0.001 |
| G18 | gi|548501256|ref|XP_005691616.1| | Predicted: aldehyde dehydrogenase mitochondrial | C. hircus | 46,336.44 | 56.4 | 6.08 | 15 | 120 | 2.39 ↑ | 0.003 |
| G20 | gi|548481183|ref|XP_005685233.1| | Predicted: pyruvate kinase isoform X1 | C. hircus | 58,513.25 | 36.16 | 7.76 | 17 | 58 | Absent in cont | 0.001 |
| Spot Name | Protein ID | Protein Mass (Daltons) | Protein Score | pI | % Sequence Coverage | Peptide Searched/Matched | Proteins Identified | Species | Fold Change | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| G16 | gi|358420988|ref|XP_003584789.1| | 17,854.71 | 51.2 | 9.3 | 17.31 | 27/156 | Vitamin K epoxide reductase complex, subunit 1-like 1 | B. taurus | 3.38 ↑ | 0.001 |
| G11 | gi|28189827|dbj|BAC56528.1| | 215,154.23 | 323 | 4.49 | 46.70 | 85/182 | Desmin | B. taurus | 2.34 ↑ | 0.002 |
| G12 | gi|87196510|ref|NP_001010995.2| | 33,048.59 | 219 | 4.34 | 32.04 | 91/284 | Tropomyosin-β chain | B. taurus | No change | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohanty, I.; Banerjee, S.; Mahanty, A.; Mohanty, S.; Nayak, N.R.; Parija, S.C.; Mohanty, B.P. Proteomic Profiling and Pathway Analysis of Acid Stress-Induced Vasorelaxation of Mesenteric Arteries In Vitro. Genes 2022, 13, 801. https://doi.org/10.3390/genes13050801
Mohanty I, Banerjee S, Mahanty A, Mohanty S, Nayak NR, Parija SC, Mohanty BP. Proteomic Profiling and Pathway Analysis of Acid Stress-Induced Vasorelaxation of Mesenteric Arteries In Vitro. Genes. 2022; 13(5):801. https://doi.org/10.3390/genes13050801
Chicago/Turabian StyleMohanty, Ipsita, Sudeshna Banerjee, Arabinda Mahanty, Sasmita Mohanty, Nihar Ranjan Nayak, Subas Chandra Parija, and Bimal Prasanna Mohanty. 2022. "Proteomic Profiling and Pathway Analysis of Acid Stress-Induced Vasorelaxation of Mesenteric Arteries In Vitro" Genes 13, no. 5: 801. https://doi.org/10.3390/genes13050801






