STIM1–Orai1 Interaction Exacerbates LPS-Induced Inflammation and Endoplasmic Reticulum Stress in Bovine Hepatocytes through Store-Operated Calcium Entry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Hepatocyte Isolation and Cell Culture
2.3. Experimental Design
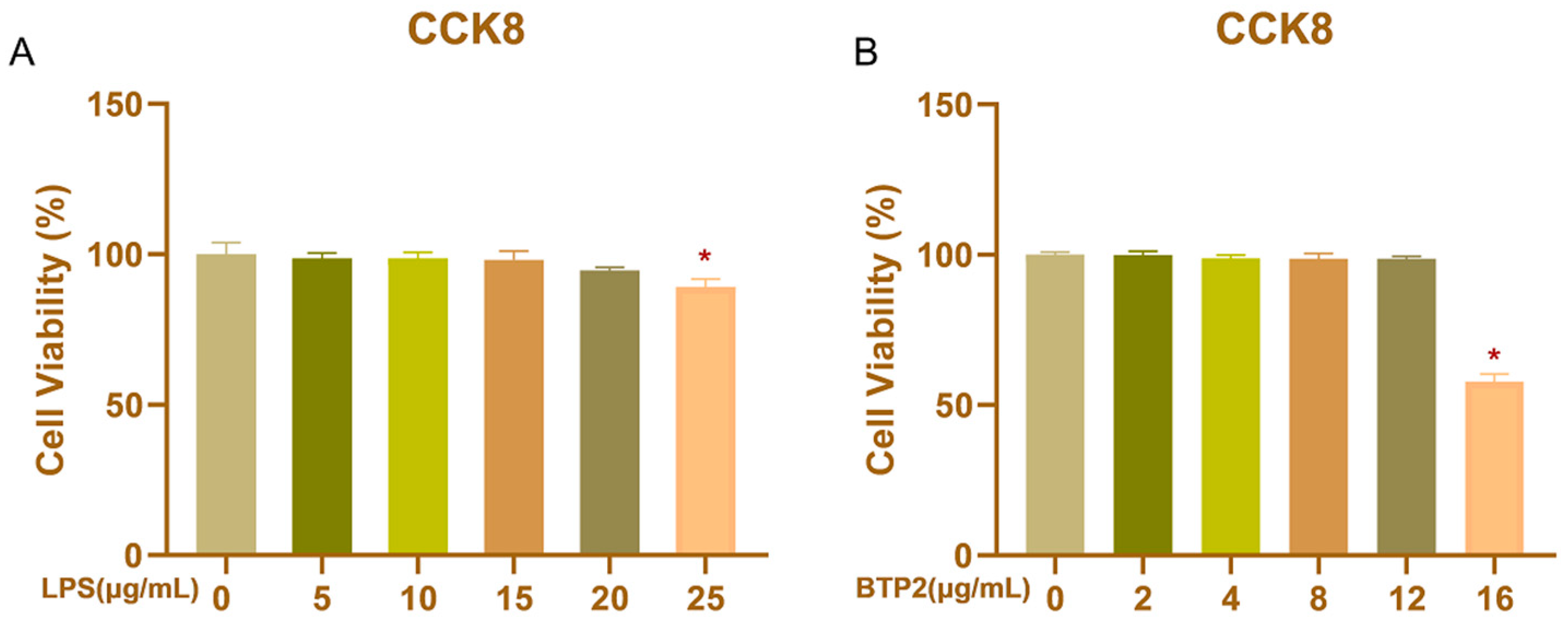
2.4. Cell Viability
2.5. RNA Isolation and Real-Time Polymerase Chain Reaction
2.6. Western Blot Analysis
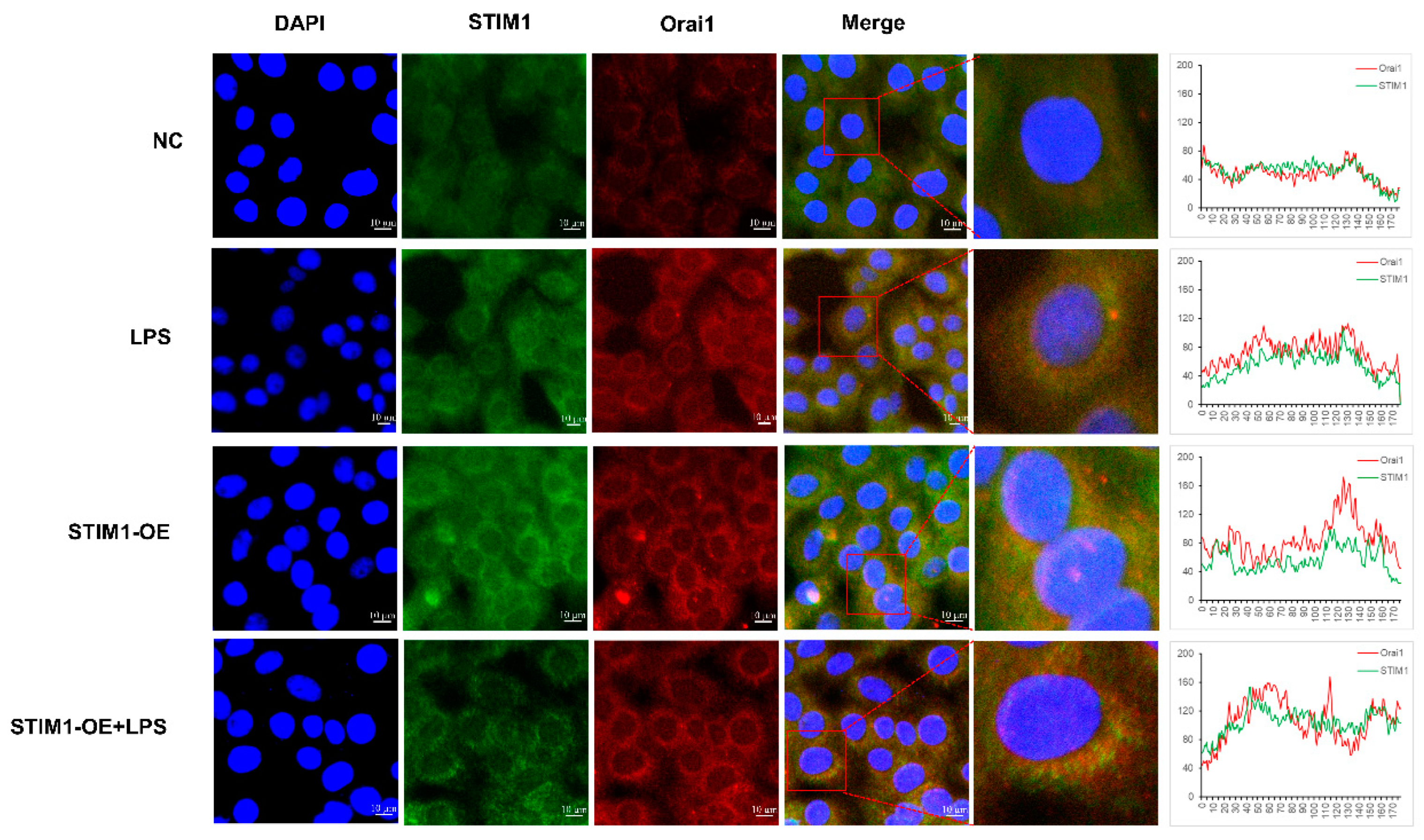
2.7. Immunofluorescence
2.8. Statistical Analysis
3. Results
3.1. Viability of BHEC Treated with LPS and BTP2 at Different Doses
3.2. Optimal Concentrations and Treatment Time Points of LPS
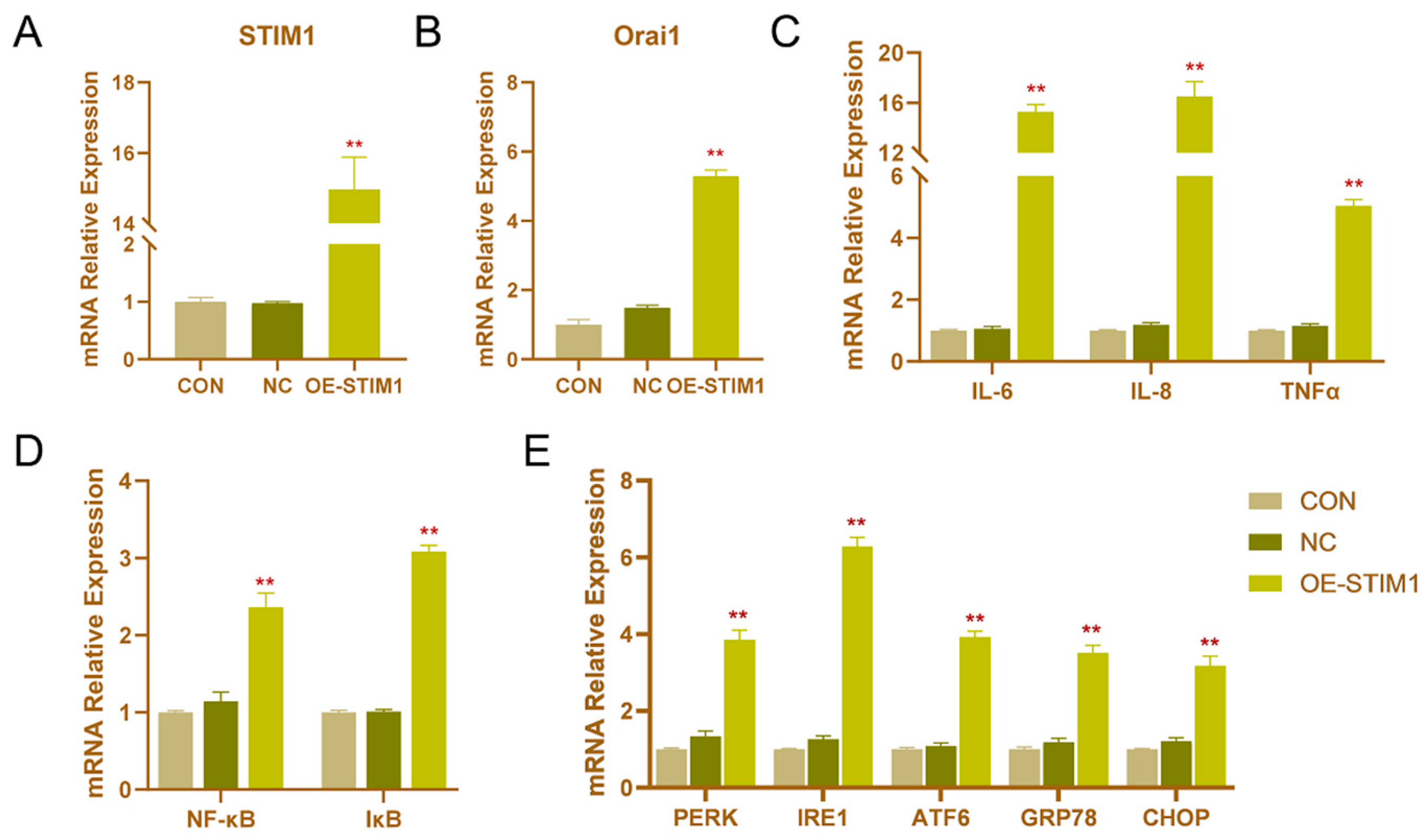
3.3. Effects of STIM1 Overexpression on BHEC
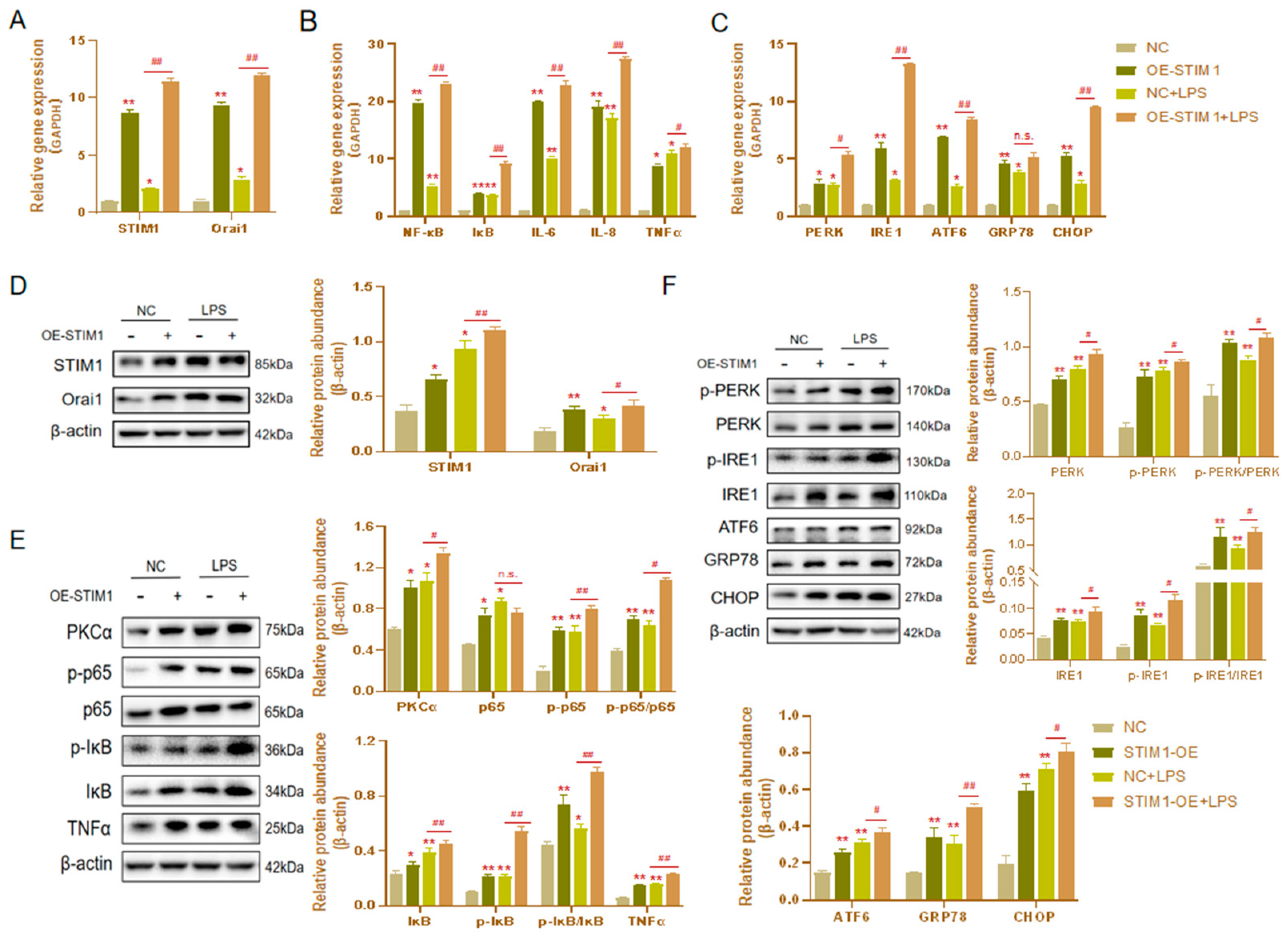
3.4. Effects of STIM1 Overexpression on LPS-Induced Inflammation and Endoplasmic Reticulum Stress in BHEC
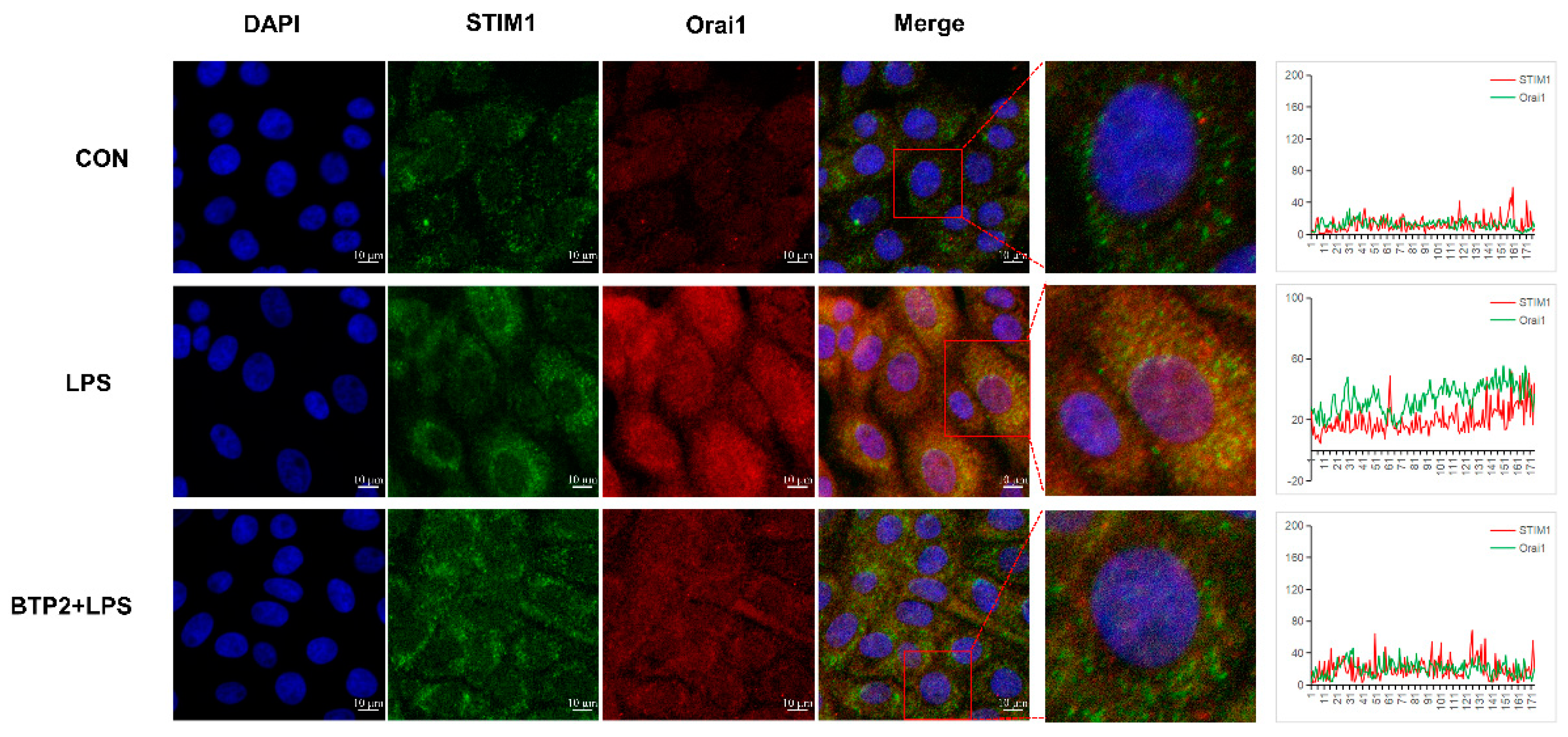
3.5. Interaction between STIM1/Orai1 in BHEC and the Effects of STIM1 Overexpression on the NF-κB Signaling Pathway
3.6. The Best BTP2 Pretreatment Effect
3.7. Effects of the Orai1 Inhibitor BTP2 on SOCE
3.8. The Orai1 Inhibitor BTP2 Alleviated LPS-Induced BHEC Inflammation and ERS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oakes, S.A.; Papa, F.R. The Role of Endoplasmic Reticulum Stress in Human Pathology. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 173–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidalevitz, T.; Stevens, F.; Argon, Y. Orchestration of secretory protein folding by ER chaperones. Biochim. Biophys. Acta 2013, 1833, 2410–2424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; Jiang, M.; Chen, W.; Zhao, T.; Wei, Y. Cancer and ER stress: Mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed. Pharmacother. 2019, 118, 109249. [Google Scholar] [CrossRef] [PubMed]
- Kleizen, B.; Braakman, I. Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 2004, 16, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, X.; Zhang, J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic beta cells. Cell Signal. 2016, 28, 1099–1104. [Google Scholar] [CrossRef]
- Jin, J.K.; Blackwood, E.A.; Azizi, K.; Thuerauf, D.J.; Fahem, A.G.; Hofmann, C.; Kaufman, R.J.; Doroudgar, S.; Glembotski, C.C. ATF6 Decreases Myocardial Ischemia/Reperfusion Damage and Links ER Stress and Oxidative Stress Signaling Pathways in the Heart. Circ. Res. 2017, 120, 862–875. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Ma, J.; Wang, X.; Yang, W.; Zhang, J.; Ji, Q. Free fatty acid induces endoplasmic reticulum stress and apoptosis of beta-cells by Ca2+/calpain-2 pathways. PLoS ONE 2013, 8, e59921. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Z.; Wu, Y.; Shi, Q.; Yan, H.; Mei, N.; Tolleson, W.H.; Guo, L. Endoplasmic Reticulum Stress and Store-Operated Calcium Entry Contribute to Usnic Acid-Induced Toxicity in Hepatic Cells. Toxicol. Sci. 2015, 146, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Li, M.; Yang, W.; Loor, J.J.; Liang, Y.; Wang, S.; Zhao, Y.; Guo, H.; Ma, X.; Yu, L.; et al. Mitochondrial dysfunction and endoplasmic reticulum stress in calf hepatocytes are associated with fatty acid-induced ORAI calcium release-activated calcium modulator 1 signaling. J. Dairy Sci. 2020, 103, 11945–11956. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Zou, Y.; Guo, H.; Zhang, B.; Xia, C.; Zhang, H.; Yang, W.; Xu, C. NFkappaB/Orai1 Facilitates Endoplasmic Reticulum Stress by Oxidative Stress in the Pathogenesis of Non-alcoholic Fatty Liver Disease. Front. Cell Dev. Biol. 2019, 7, 202. [Google Scholar] [CrossRef]
- Zhang, B.; Li, M.; Yang, W.; Loor, J.J.; Wang, S.; Zhao, Y.; Guo, H.; Ma, X.; Xia, C.; Xu, C. Orai calcium release-activated calcium modulator 1 (ORAI1) plays a role in endoplasmic reticulum stress in bovine mammary epithelial cells challenged with physiological levels of ketone bodies. J. Dairy Sci. 2020, 103, 4691–4701. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.A.; Roe, M.W. Molecular physiology and pathophysiology of stromal interaction molecules. Exp. Biol. Med. (Maywood) 2018, 243, 451–472. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Tong, X.; Taleb, S.; Bone, R.N.; Iida, H.; Lee, C.C.; Sohn, P.; Gilon, P.; Roe, M.W.; Evans-Molina, C. Impaired Store-Operated Calcium Entry and STIM1 Loss Lead to Reduced Insulin Secretion and Increased Endoplasmic Reticulum Stress in the Diabetic beta-Cell. Diabetes 2018, 67, 2293–2304. [Google Scholar] [CrossRef] [Green Version]
- Palkowitsch, L.; Marienfeld, U.; Brunner, C.; Eitelhuber, A.; Krappmann, D.; Marienfeld, R.B. The Ca2+-dependent phosphatase calcineurin controls the formation of the Carma1-Bcl10-Malt1 complex during T cell receptor-induced NF-kappaB activation. J. Biol. Chem. 2011, 286, 7522–7534. [Google Scholar] [CrossRef] [Green Version]
- Lunz, V.; Romanin, C.; Frischauf, I. STIM1 activation of Orai1. Cell Calcium 2019, 77, 29–38. [Google Scholar] [CrossRef]
- Li, M.; Yang, W.; Wen, J.; Loor, J.J.; Aboragah, A.; Wang, J.; Wang, S.; Li, M.; Yu, L.; Hou, X.; et al. Intracellular Ca2+ signaling and ORAI calcium release-activated calcium modulator 1 are associated with hepatic lipidosis in dairy cattle. J. Anim. Sci. 2021, 99, skab184. [Google Scholar] [CrossRef]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [Green Version]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, X.; Zhao, D.; Wang, X.; Gurley, E.C.; Liu, R.; Li, X.; Hylemon, P.B.; Chen, W.; Zhou, H. Berberine inhibits free fatty acid and LPS-induced inflammation via modulating ER stress response in macrophages and hepatocytes. PLoS ONE 2020, 15, e0232630. [Google Scholar] [CrossRef]
- Meng, M.; Wang, L.; Wang, Y.; Ma, N.; Xie, W.; Chang, G.; Shen, X. A high-concentrate diet provokes inflammation, endoplasmic reticulum stress, and apoptosis in mammary tissue of dairy cows through the upregulation of STIM1/ORAI1. J. Dairy Sci. 2022, 105, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ma, N.; Wang, Y.; Shi, X.; Chang, G.; Loor, J.J.; Shen, X. Sodium Butyrate Supplementation Alleviates the Adaptive Response to Inflammation and Modulates Fatty Acid Metabolism in Lipopolysaccharide-Stimulated Bovine Hepatocytes. J. Agric. Food Chem. 2018, 66, 6281–6290. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tan, Y.; Chen, S.; Xiao, X.; Zhang, M.; Wu, Q.; Dong, M. Irisin alleviates LPS-induced liver injury and inflammation through inhibition of NLRP3 inflammasome and NF-kappaB signaling. J. Recept. Signal Transduct. Res. 2021, 41, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Aabdin, Z.U.; Wang, Y.; Ma, N.; Dai, H.; Shi, X.; Shen, X. Glutamine pretreatment protects bovine mammary epithelial cells from inflammation and oxidative stress induced by gamma-d-glutamyl-meso-diaminopimelic acid (iE-DAP). J. Dairy Sci. 2021, 104, 2123–2139. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, F.; Zhang, H.; Wen, T.; Piao, Z.; Zhou, L.; Zheng, S.; Zhang, J.; Chen, Y.; Han, Y.; et al. Inhibition of glycogen synthase kinase 3beta ameliorates D-GalN/LPS-induced liver injury by reducing endoplasmic reticulum stress-triggered apoptosis. PLoS ONE 2012, 7, e45202. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Chen, L.; Zhang, X.; Xu, L.; Xie, B.; Shi, H.; Duan, Z.; Zhang, H.; Ren, F. Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed. Pharmacother. 2019, 111, 468–475. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Baker, R.G.; Hayden, M.S.; Ghosh, S. NF-kappaB, inflammation, and metabolic disease. Cell Metab. 2011, 13, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Wang, Y.; Ma, N.; Dai, H.; Roy, A.C.; Chang, G.; Shi, X.; Shen, X. Sodium valproate attenuates the iE-DAP induced inflammatory response by inhibiting the NOD1-NF-kappaB pathway and histone modifications in bovine mammary epithelial cells. Int. Immunopharmacol. 2020, 83, 106392. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-kappaB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Secondo, A.; Petrozziello, T.; Tedeschi, V.; Boscia, F.; Vinciguerra, A.; Ciccone, R.; Pannaccione, A.; Molinaro, P.; Pignataro, G.; Annunziato, L. ORAI1/STIM1 Interaction Intervenes in Stroke and in Neuroprotection Induced by Ischemic Preconditioning Through Store-Operated Calcium Entry. Stroke 2019, 50, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Gehring, K. Calnexin cycle-structural features of the ER chaperone system. FEBS J. 2020, 287, 4322–4340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, T.; Song, W.; Xu, R. Autophagy and ER stress in LPS/GalNinduced acute liver injury. Mol. Med. Rep. 2017, 16, 7001–7005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, X.; Wei, Z.; Mao, H.; Gao, M.; Liu, Y.; Ma, Y.; Liu, X.; Guo, C.; Zhang, L.; et al. Pretreatment with wortmannin alleviates lipopolysaccharide/d-galactosamine-induced acute liver injury. Biochem. Biophys. Res. Commun. 2014, 455, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Cursio, R.; Colosetti, P.; Codogno, P.; Cuervo, A.M.; Shen, H.M. The role of autophagy in liver diseases: Mechanisms and potential therapeutic targets. Biomed. Res. Int. 2015, 2015, 480508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, J.; Zhang, C.; Wang, P.; Lu, L.; Qian, X.; Qin, J.; Pan, X.; Li, G.; Wang, X.; Zhang, F. C/EBP homologous protein (CHOP) contributes to hepatocyte death via the promotion of ERO1alpha signalling in acute liver failure. Biochem. J. 2015, 466, 369–378. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Liang, T.; Lu, X.; Zhang, C.; Liu, X.; Jiang, X.; Martin, R.C.; Cheng, M.; Cai, L. ER stress and autophagy dysfunction contribute to fatty liver in diabetic mice. Int. J. Biol. Sci. 2015, 11, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harbor Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Berry, C.T.; May, M.J.; Freedman, B.D. STIM- and Orai-mediated calcium entry controls NF-kappaB activity and function in lymphocytes. Cell Calcium 2018, 74, 131–143. [Google Scholar] [CrossRef]
- Nascimento Da Conceicao, V.; Sun, Y.; Zboril, E.K.; De la Chapa, J.J.; Singh, B.B. Loss of Ca(2+) entry via Orai-TRPC1 induces ER stress, initiating immune activation in macrophages. J. Cell Sci. 2019, 133, jcs.237610. [Google Scholar] [CrossRef] [Green Version]
- Vaeth, M.; Eckstein, M.; Shaw, P.J.; Kozhaya, L.; Yang, J.; Berberich-Siebelt, F.; Clancy, R.; Unutmaz, D.; Feske, S. Store-Operated Ca(2+) Entry in Follicular T Cells Controls Humoral Immune Responses and Autoimmunity. Immunity 2016, 44, 1350–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, A.; Ordóñez, R.; González-Gallego, R.J.R.J.; Mauriz, J.L. Melatonin and endoplasmic reticulum stress: Relation to autophagy and apoptosis. J. Pineal Res. 2015, 59, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Waldron, R.T.; Chen, Y.; Pham, H.; Go, A.; Su, H.Y.; Hu, C.; Wen, L.; Husain, S.Z.; Sugar, C.A.; Roos, J.; et al. The Orai Ca(2+) channel inhibitor CM4620 targets both parenchymal and immune cells to reduce inflammation in experimental acute pancreatitis. J. Physiol. 2019, 597, 3085–3105. [Google Scholar] [CrossRef] [PubMed]
- Vaeth, M.; Kahlfuss, S.; Feske, S. CRAC Channels and Calcium Signaling in T Cell-Mediated Immunity. Trends Immunol. 2020, 41, 878–901. [Google Scholar] [CrossRef]
- Meizoso-Huesca, A.; Launikonis, B.S. The Orai1 inhibitor BTP2 has multiple effects on Ca2+ handling in skeletal muscle. J. Gen. Physiol. 2021, 153. [Google Scholar] [CrossRef]



| Gene | Gene Bank Accession | Primer Sequence (5′-3′) | Length (bp) | |
|---|---|---|---|---|
| STIM1 | NM_001035409.1 | Forward | AGTCCACTGCAGCAGAGTTT | 250 |
| Reverse | TTCCATGCCTTCCACAGGTC | |||
| Orai1 | NM_001099002.1 | Forward | GTCCTGGCGCAAACTCTACT | 125 |
| Reverse | GGTAGTCGTGGTCAGCATCC | |||
| IL-6 | NM_173923.2 | Forward | CGAAGCTCTCATTAAGCACATC | 221 |
| Reverse | CCAGGTATATCTGATACTCCAG | |||
| IL-8 | NM_173925.2 | Forward | CTGAGAGTTATTGAGAGTGGGC | 259 |
| Reverse | CAGTACTCAAGGCACTGAAGTAG | |||
| TNF-α | NM_173966.3 | Forward | CAACAGGCCTCTGGTTCAGAC | 209 |
| Reverse | GGACCTGCGAGTAGATGAGG | |||
| IL-10 | NM_174088.1 | Forward | AGCACTACTCTGTTGCCTGG | 230 |
| Reverse | TTGGGGTAGACTTTGGGGTCT | |||
| NF-κB | NM_001045868.1 | Forward | CTTCCATCCTGGAACCACTAAA | 108 |
| Reverse | ACCTCTCTGTCGTCACTCTT | |||
| IκB | XM_0056999961 | Forward | GGTGAAGGAGCTGCGAGAG | 326 |
| Reverse | GCTCACAGGCAAGGTGTAGG | |||
| PERK | NM_001098086.1 | Forward | GCCGCTCAGCTCTCCTAGTCC | 165 |
| Reverse | TGGCTCTCGGATGAACTGGTCTG | |||
| IRE1 | XM_024980954.1 | Forward | TCCTCCCAGATCCCAACGAT | 127 |
| Reverse | ATGCCATCTGAACTTCGGCA | |||
| ATF6 | XM_024989876.1 | Forward | AGCCCTGATGGTGCTAACTGA | 100 |
| Reverse | TTCATGATTTAACCTGAGAGATTCTGTT | |||
| GRP78 | XM_024998380.1 | Forward | AACGACCCCTGACGAAAGAC | 100 |
| Reverse | TCAAAGGTGACTTCAATCTGTGG | |||
| CHOP | XM_019960966.1 | Forward | CCTGAGGAGAGAGTGTTCCAG | 160 |
| Reverse | CCTGCAGGTCCTCATACCAG | |||
| GAPDH | NM_001034034.2 | Forward | GGGTCATCATCTCTGCACCT | 180 |
| Reverse | GGTCATAAGTCCCTCCACGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Zhou, S.; Xie, W.; Meng, M.; Ma, N.; Zhang, H.; Wang, Y.; Chang, G.; Shen, X. STIM1–Orai1 Interaction Exacerbates LPS-Induced Inflammation and Endoplasmic Reticulum Stress in Bovine Hepatocytes through Store-Operated Calcium Entry. Genes 2022, 13, 874. https://doi.org/10.3390/genes13050874
Xue Y, Zhou S, Xie W, Meng M, Ma N, Zhang H, Wang Y, Chang G, Shen X. STIM1–Orai1 Interaction Exacerbates LPS-Induced Inflammation and Endoplasmic Reticulum Stress in Bovine Hepatocytes through Store-Operated Calcium Entry. Genes. 2022; 13(5):874. https://doi.org/10.3390/genes13050874
Chicago/Turabian StyleXue, Yang, Shendong Zhou, Wan Xie, Meijuan Meng, Nana Ma, Hongzhu Zhang, Yan Wang, Guangjun Chang, and Xiangzhen Shen. 2022. "STIM1–Orai1 Interaction Exacerbates LPS-Induced Inflammation and Endoplasmic Reticulum Stress in Bovine Hepatocytes through Store-Operated Calcium Entry" Genes 13, no. 5: 874. https://doi.org/10.3390/genes13050874







