Application of CRISPR/Cas Technology in Spermatogenesis Research and Male Infertility Treatment
Abstract
:1. Introduction
2. Spermatogenesis in Brief
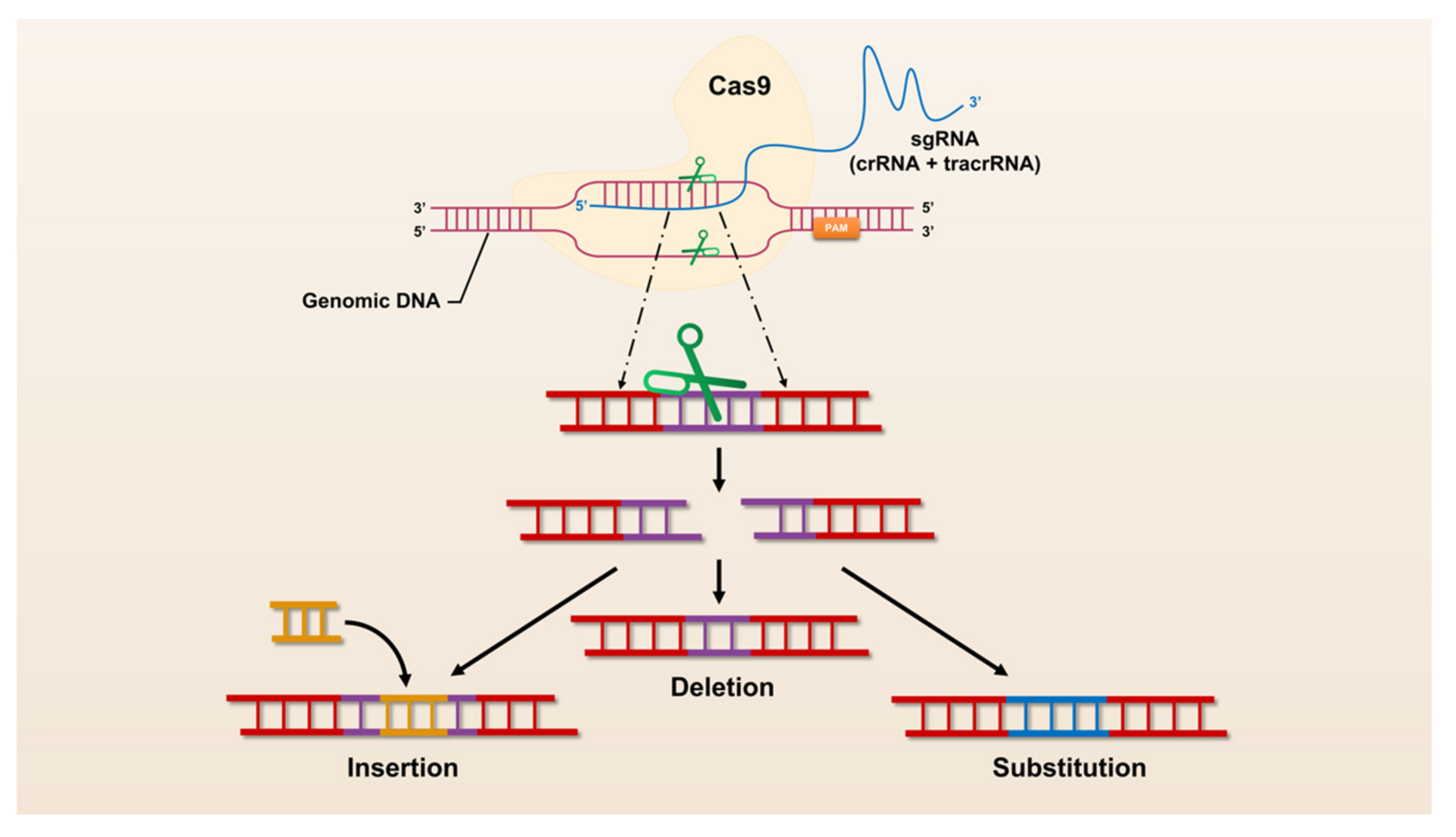
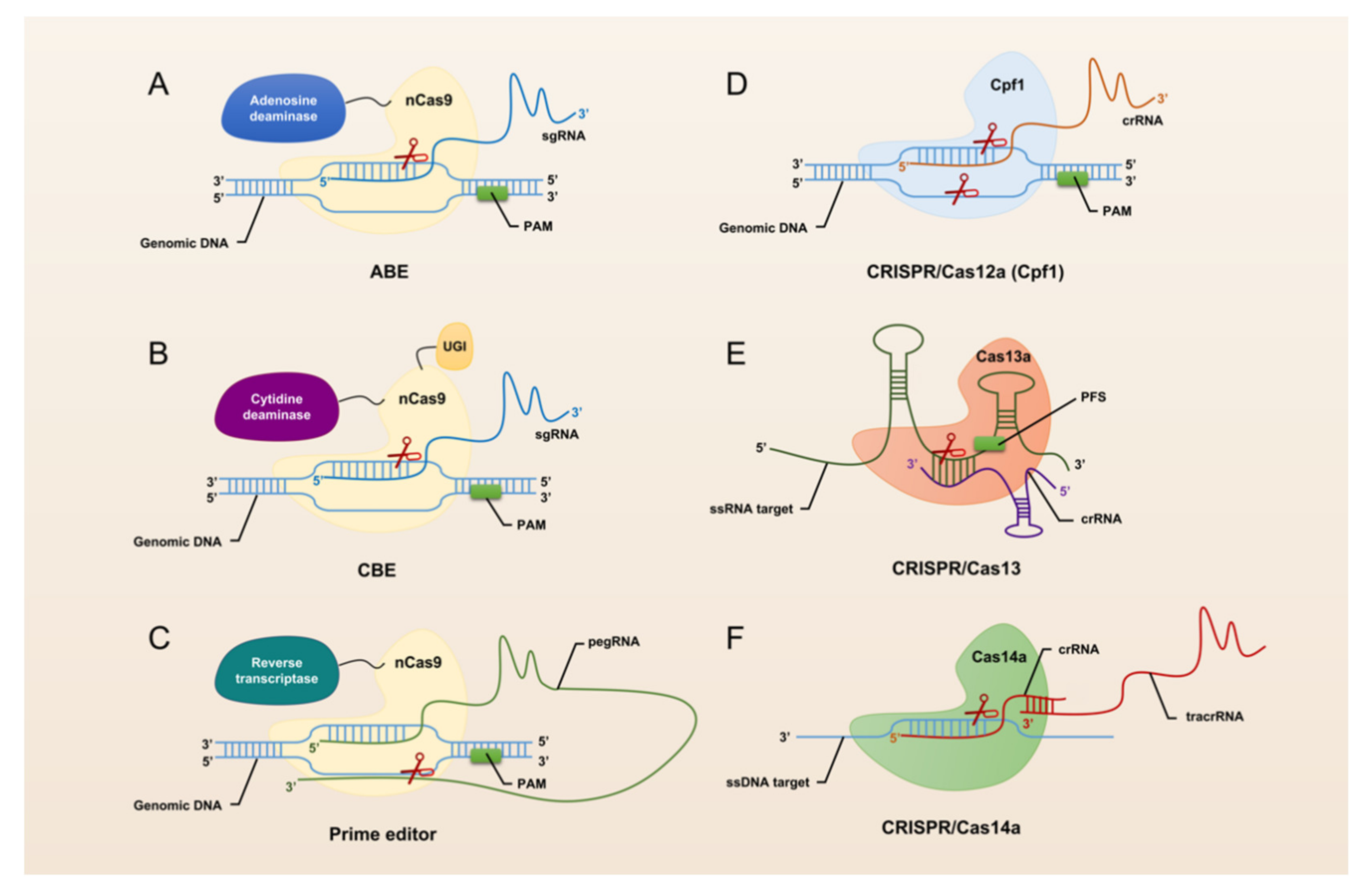
3. A Brief Overview of CRISPR/Cas Technology
4. CRISPR/Cas9: An In-Depth Exploration of Functional Genes for Spermatogenesis
4.1. Spermatogenesis Associated 16 (Spata16)
4.2. Doublesex and Mab-3 Related Transcription Factor 1 (Dmrt1)
4.3. Dpy-19-like 2 (Dpy19l2)
4.4. Testis Specific 10 (Tsga10)
5. CRISPR/Cas9: Potential New Tools for Treating Abnormal Spermatogenesis and Male Infertility
6. Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Statens Veterinärmedicinska Anstalt. Food and agriculture organization of the United Nations. 2013. [Google Scholar]
- Steinfeld, H.; Gerber, P.; Wassenaar, T.D.; Castel, V.; Rosales, M.; Rosales, M.; de Haan, C. Livestock’s Long Shadow: Environmental issues and Options; Food & Agriculture Organization: Rome, Italy, 2006. [Google Scholar]
- Bourdon, G.; Cadoret, V.; Charpigny, G.; Couturier-Tarrade, A.; Dalbies-Tran, R.; Flores, M.J.; Froment, P.; Raliou, M.; Reynaud, K.; Saint-Dizier, M.; et al. Progress and challenges in developing organoids in farm animal species for the study of reproduction and their applications to reproductive biotechnologies. Vet. Res. 2021, 52, 42. [Google Scholar] [CrossRef]
- Saha, S.; Roy, P.; Corbitt, C.; Kakar, S.S. Application of Stem Cell Therapy for Infertility. Cells 2021, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Giassetti, M.I.; Ciccarelli, M.; Oatley, J.M. Spermatogonial Stem Cell Transplantation: Insights and Outlook for Domestic Animals. Annu. Rev. Anim. Biosci. 2019, 7, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, M.; Hitit, M.; Kaya, A.; Jousan, F.D.; Memili, E. Sperm Functional Genome Associated With Bull Fertility. Front. Vet. Sci. 2021, 8, 610888. [Google Scholar] [CrossRef]
- Houdebine, L.M. Use of transgenic animals to improve human health and animal production. Reprod. Domest. Anim. 2005, 40, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.V.; Tiley, L.S.; Sang, H.M. Developments in transgenic technology: Applications for medicine. Trends Mol. Med. 2005, 11, 293–298. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol. Ther. 2003, 99, 261–282. [Google Scholar] [CrossRef]
- Lillico, S.G.; McGrew, M.J.; Sherman, A.; Sang, H.M. Transgenic chickens as bioreactors for protein-based drugs. Drug Discov. Today 2005, 10, 191–196. [Google Scholar] [CrossRef]
- Xu, K.; Zhou, Y.; Mu, Y.; Liu, Z.; Hou, S.; Xiong, Y.; Fang, L.; Ge, C.; Wei, Y.; Zhang, X.; et al. CD163 and pAPN double-knockout pigs are resistant to PRRSV and TGEV and exhibit decreased susceptibility to PDCoV while maintaining normal production performance. eLife 2020, 9, e57132. [Google Scholar] [CrossRef]
- De Kretser, D.M.; Loveland, K.L.; Meinhardt, A.; Simorangkir, D.; Wreford, N. Spermatogenesis. Hum. Reprod. 1998, 13 (Suppl. 1), 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Rooij, D.G. The nature and dynamics of spermatogonial stem cells. Development 2017, 144, 3022–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Zhang, Y.; Qu, R.; He, Y.; Tian, X.; Zeng, W. Spermatogonial stem cells from domestic animals: Progress and prospects. Reproduction 2014, 147, R65–R74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griswold, M.D. Spermatogenesis: The Commitment to Meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griswold, M.D. 50 years of spermatogenesis: Sertoli cells and their interactions with germ cells. Biol. Reprod. 2018, 99, 87–100. [Google Scholar] [CrossRef]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, T.L.; Berndtson, W.E. Testicular weight, Sertoli cell number, daily sperm production, and sperm output of sexually mature rabbits after neonatal or prepubertal hemicastration. Biol. Reprod. 1993, 48, 952–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, L.D.; Ren, H.P.; Sinha Hikim, I.; Schulze, W.; Sinha Hikim, A.P. A comparative study in twelve mammalian species of volume densities, volumes, and numerical densities of selected testis components, emphasizing those related to the Sertoli cell. Am. J. Anat. 1990, 188, 21–30. [Google Scholar] [CrossRef]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef]
- Saez, J.M.; Avallet, O.; Naville, D.; Perrard-Sapori, M.H.; Chatelain, P.G. Sertoli-Leydig cell communications. Ann. N. Y. Acad. Sci. 1989, 564, 210–231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wu, J.; Liu, B.; Jiang, Y.; Chen, W.; Li, J.; He, Q.; He, Z. The roles and mechanisms of Leydig cells and myoid cells in regulating spermatogenesis. Cell Mol. Life Sci. 2019, 76, 2681–2695. [Google Scholar] [CrossRef]
- Ishino, Y.; Shinagawa, H.; Makino, K.; Amemura, M.; Nakata, A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J. Bacteriol. 1987, 169, 5429–5433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Lin, Q.; Jin, S.; Gao, C. The CRISPR-Cas toolbox and gene editing technologies. Mol. Cell 2022, 82, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Pickar-Oliver, A.; Gersbach, C.A. The next generation of CRISPR-Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarova, K.S.; Wolf, Y.I.; Alkhnbashi, O.S.; Costa, F.; Shah, S.A.; Saunders, S.J.; Barrangou, R.; Brouns, S.J.; Charpentier, E.; Haft, D.H.; et al. An updated evolutionary classification of CRISPR-Cas systems. Nat. Rev. Microbiol 2015, 13, 722–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shmakov, S.; Abudayyeh, O.O.; Makarova, K.S.; Wolf, Y.I.; Gootenberg, J.S.; Semenova, E.; Minakhin, L.; Joung, J.; Konermann, S.; Severinov, K.; et al. Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol. Cell 2015, 60, 385–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abudayyeh, O.O.; Gootenberg, J.S.; Essletzbichler, P.; Han, S.; Joung, J.; Belanto, J.J.; Verdine, V.; Cox, D.B.T.; Kellner, M.J.; Regev, A.; et al. RNA targeting with CRISPR-Cas13. Nature 2017, 550, 280–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR-Cas in agriculture and plant biotechnology. Nat. Rev. Mol. Cell Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef]
- Strich, J.R.; Chertow, D.S. CRISPR-Cas Biology and Its Application to Infectious Diseases. J. Clin. Microbiol 2019, 57, JCM-01307. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Huang, L.L.; Xu, J.; Ma, C.Q.; Chen, Z.H.; Zhang, Z.; Liao, C.H.; Zheng, S.X.; Huang, P.; Xu, W.M.; et al. Proteomics and single-cell RNA analysis of Akap4-knockout mice model confirm indispensable role of Akap4 in spermatogenesis. Dev. Biol. 2019, 454, 118–127. [Google Scholar] [CrossRef]
- Lin, Q.; Mei, J.; Li, Z.; Zhang, X.; Zhou, L.; Gui, J.F. Distinct and Cooperative Roles of amh and dmrt1 in Self-Renewal and Differentiation of Male Germ Cells in Zebrafish. Genetics 2017, 207, 1007–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutton, C.; Martinez, G.; Kherraf, Z.E.; Amiri-Yekta, A.; Boguenet, M.; Saut, A.; He, X.; Zhang, F.; Cristou-Kent, M.; Escoffier, J.; et al. Bi-allelic Mutations in ARMC2 Lead to Severe Astheno-Teratozoospermia Due to Sperm Flagellum Malformations in Humans and Mice. Am. J. Hum. Genet. 2019, 104, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Xu, J.; Zhou, Q.; Lin, M.; Lv, J.; Zhang, X.; Wu, Y.; Chen, X.; Yu, J.; Huang, X.; et al. E3 ubiquitin ligase ASB17 is required for spermiation in mice. Transl. Androl. Urol. 2021, 10, 4320–4332. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Y.; Qin, Y.; Xu, Q.; Zhou, R.; Cui, Y.; Zhu, Y.; Zhang, X.; Zhang, J.; Wei, X.; et al. Human X chromosome exome sequencing identifies BCORL1 as contributor to spermatogenesis. J. Med. Genet. 2021, 58, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, W.; Zhang, P.; Gao, F.; Zhao, X.; Shum, W.W.; Zeng, X. Cabs1 Maintains Structural Integrity of Mouse Sperm Flagella during Epididymal Transit of Sperm. Int. J. Mol. Sci. 2021, 22, 652. [Google Scholar] [CrossRef] [PubMed]
- Young, S.A.; Miyata, H.; Satouh, Y.; Kato, H.; Nozawa, K.; Isotani, A.; Aitken, R.J.; Baker, M.A.; Ikawa, M. CRISPR/Cas9-Mediated Rapid Generation of Multiple Mouse Lines Identified Ccdc63 as Essential for Spermiogenesis. Int. J. Mol. Sci. 2015, 16, 24732–24750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Tang, W.; Li, H.; Hua, R.; Yuan, Y.; Zhang, Y.; Zhu, Y.; Cui, Y.; Sha, J. T-complex protein 1 subunit zeta-2 (CCT6B) deficiency induces murine teratospermia. PeerJ 2021, 9, e11545. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhu, H.; Zhang, A.; Lin, J.; Zhang, G.; Liu, D.; Xiao, Y.; Ye, C.; Sun, D.; Wu, B.; et al. Cdc14a has a role in spermatogenesis, sperm maturation and male fertility. Exp. Cell Res. 2020, 395, 112178. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Miyata, H.; Kaneda, Y.; Castaneda, J.M.; Lu, Y.; Morohoshi, A.; Yu, Z.; Matzuk, M.M.; Ikawa, M. CIB4 is essential for the haploid phase of spermatogenesis in micedagger. Biol. Reprod. 2020, 103, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, X.; Liu, X.; Li, T.; Zhu, P.; Liu, Z.; Xue, H.; Wang, W.; Yang, X.; Liu, J.; et al. Integrated Analyses of Phenotype and Quantitative Proteome of CMTM4 Deficient Mice Reveal Its Association with Male Fertility. Mol. Cell Proteomics 2019, 18, 1070–1084. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, A.G.; Seroussi, U.; Lehrbach, N.J.; Renaud, M.S.; Sundby, A.E.; Molnar, R.I.; Lao, R.X.; Willis, A.R.; Woock, J.R.; Aber, M.J.; et al. Two isoforms of the essential C. elegans Argonaute CSR-1 differentially regulate sperm and oocyte fertility. Nucleic Acids Res. 2021, 49, 8836–8865. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xiao, H.; Shi, H.; Wang, T.; Sun, L.; Tao, W.; Kocher, T.D.; Li, M.; Wang, D. Loss of Cyp11c1 causes delayed spermatogenesis due to the absence of 11-ketotestosterone. J. Endocrinol. 2020, 244, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Le, H.T.; Hasegawa, Y.; Daitoku, Y.; Kato, K.; Miznuo-Iijima, S.; Dinh, T.T.H.; Kuba, Y.; Osawa, Y.; Mikami, N.; Morimoto, K.; et al. Generation of B6-Ddx4(em1(CreERT2)Utr), a novel CreERT2 knock-in line, for germ cell lineage by CRISPR/Cas9. Genesis 2020, 58, e23367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Y.; Xie, S.; Yin, Q.; Tang, C.; Ni, Z.; Fei, J.; Zhang, Y. CRISPR/Cas9-mediated genome editing reveals the synergistic effects of β-defensin family members on sperm maturation in rat epididymis. FASEB J. 2018, 32, 1354–1363. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, H.; Li, M.; Cheng, Y.; Jiang, D.; Sun, L.; Tao, W.; Zhou, L.; Wang, Z.; Wang, D. Isolation of doublesex- and mab-3-related transcription factor 6 and its involvement in spermatogenesis in tilapia. Biol. Reprod. 2014, 91, 136. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ouyang, J.; Li, X.; Xiao, X.; Sun, W.; Li, S.; Zhou, L.; Liao, Y.; Zhang, Q. DNAH17 is essential for rat spermatogenesis and fertility. J. Genet. 2021, 100, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, H.; Khan, T.; Ma, A.; Li, T.; Zhang, H.; Gao, J.; Zhou, J.; Li, Y.; Yu, C.; et al. A DNAH17 missense variant causes flagella destabilization and asthenozoospermia. J. Exp. Med. 2020, 217, e20182365. [Google Scholar] [CrossRef] [Green Version]
- Pierre, V.; Martinez, G.; Coutton, C.; Delaroche, J.; Yassine, S.; Novella, C.; Pernet-Gallay, K.; Hennebicq, S.; Ray, P.F.; Arnoult, C. Absence of Dpy19l2, a new inner nuclear membrane protein, causes globozoospermia in mice by preventing the anchoring of the acrosome to the nucleus. Development 2012, 139, 2955–2965. [Google Scholar] [CrossRef] [Green Version]
- N’Tumba-Byn, T.; Yamada, M.; Seandel, M. Loss of tyrosine kinase receptor Ephb2 impairs proliferation and stem cell activity of spermatogonia in culturedagger. Biol. Reprod. 2020, 102, 950–962. [Google Scholar] [CrossRef] [PubMed]
- Devlin, D.J.; Nozawa, K.; Ikawa, M.; Matzuk, M.M. Knockout of family with sequence similarity 170 member A (Fam170a) causes male subfertility, while Fam170b is dispensable in micedagger. Biol. Reprod. 2020, 103, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Gao, Q.; Feng, T.; Zheng, Y.; Guo, J.; Zeng, W. FTO Knockout Causes Chromosome Instability and G2/M Arrest in Mouse GC-1 Cells. Front. Genet. 2018, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ai, N.; Chen, W.; Wong, Q.W.; Ge, W. Loss of Growth Hormone Gene (gh1) in Zebrafish Arrests Folliculogenesis in Females and Delays Spermatogenesis in Males. Endocrinology 2019, 160, 568–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Zhao, W.; Liu, J.; Wang, Y.; Yuan, C.; Zhang, F.; Jin, G.; Qin, Q. Effects of HIF-1alpha on Spermatogenesis of Varicocele Rats by Regulating VEGF/PI3K/Akt Signaling Pathway. Reprod. Sci. 2021, 28, 1161–1174. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, J.; Wang, D.; Wang, Y.; Zhang, F.; Jin, G.; Yuan, C.; Wang, X.; Qin, Q. Effect of silencing HIF-1alpha gene on testicle spermatogenesis function in varicocele rats. Cell Tissue Res. 2019, 378, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Saju, J.M.; Hossain, M.S.; Liew, W.C.; Pradhan, A.; Thevasagayam, N.M.; Tan, L.S.E.; Anand, A.; Olsson, P.E.; Orban, L. Heat Shock Factor 5 Is Essential for Spermatogenesis in Zebrafish. Cell Rep. 2018, 25, 3252–3261.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oura, S.; Miyata, H.; Noda, T.; Shimada, K.; Matsumura, T.; Morohoshi, A.; Isotani, A.; Ikawa, M. Chimeric analysis with newly established EGFP/DsRed2-tagged ES cells identify HYDIN as essential for spermiogenesis in mice. Exp. Anim. 2019, 68, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Liu, X.; Dai, S.; Xiao, H.; Qi, S.; Li, Y.; Zheng, Q.; Jie, M.; Cheng, C.H.K.; Wang, D. Regulation of spermatogenesis and reproductive capacity by Igf3 in tilapia. Cell Mol. Life Sci. 2020, 77, 4921–4938. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, D.; Zhu, H.; Sun, X.; Xiao, Y.; Lin, Z.; Zhang, A.; Ye, C.; Gao, J. Deficiency for Lcn8 causes epididymal sperm maturation defects in mice. Biochem. Biophys. Res. Commun. 2021, 548, 7–13. [Google Scholar] [CrossRef]
- Gong, Q.Q.; Dou, Z.L.; Wang, X.; Zhang, K.Y.; Chen, H.; Gao, J.G.; Sun, X.Y. Epididymal initial segment-specific Cre recombinase activity in Lcn8-Cre knock-in mice. Mol. Biol. Rep. 2021, 48, 6015–6023. [Google Scholar] [CrossRef]
- Bae, H.S.; Jin, Y.K.; Ham, S.; Kim, H.K.; Shin, H.; Cho, G.B.; Lee, K.J.; Lee, H.; Kim, K.M.; Koo, O.J.; et al. CRISRP/Cas9-mediated knockout of Mct8 reveals a functional involvement of Mct8 in testis and sperm development in a rat. Sci. Rep. 2020, 10, 11148. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, Q.; Zhang, L.; Liu, H.; Zhang, D.; Yuan, S.; Yap, Y.; Qu, W.; Shiang, R.; Song, S.; et al. A single amino acid mutation in the mouse MEIG1 protein disrupts a cargo transport system necessary for sperm formation. J. Biol. Chem. 2021, 297, 101312. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Kam, C.; Shen, C.; Jin, W.; Wang, J.; Lee, K.M.; Jiang, L.; Xia, J. PICK1 deficiency causes male infertility in mice by disrupting acrosome formation. J. Clin. Invest. 2009, 119, 802–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Xu, J.; Chen, K.; Liu, Y.; Yang, X.; Tang, L.; Luo, X.; Liu, Z.; Li, M.; Walters, J.R.; et al. BmPMFBP1 regulates the development of eupyrene sperm in the silkworm, Bombyx mori. PLoS Genet. 2022, 18, e1010131. [Google Scholar] [CrossRef]
- Kobayashi, K.; Endo, T.; Matsumura, T.; Lu, Y.; Yu, Z.; Matzuk, M.M.; Ikawa, M. Prss55 but not Prss51 is required for male fertility in micedagger. Biol. Reprod. 2020, 103, 223–234. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Wu, Y.; Sun, S.; Song, Q.; Wei, J.; Sun, L.; Li, M.; Wang, D.; Zhou, L. Rln3a is a prerequisite for spermatogenesis and fertility in male fish. J. Steroid. Biochem. Mol. Biol. 2020, 197, 105517. [Google Scholar] [CrossRef]
- Li, D.; Li, F.; Meng, L.; Wei, H.; Zhang, Q.; Jiang, F.; Chen, D.N.; Li, W.; Tan, Y.Q.; Li, J.D. RNF216 regulates meiosis and PKA stability in the testes. FASEB J. 2021, 35, e21460. [Google Scholar] [CrossRef]
- Melnick, A.F.; Gao, Y.; Liu, J.; Ding, D.; Predom, A.; Kelly, C.; Hess, R.A.; Chen, C. RNF216 is essential for spermatogenesis and male fertilitydagger. Biol. Reprod. 2019, 100, 1132–1134. [Google Scholar] [CrossRef]
- Wei, L.; Tang, Y.; Zeng, X.; Li, Y.; Zhang, S.; Deng, L.; Wang, L.; Wang, D. The transcription factor Sox30 is involved in Nile tilapia spermatogenesis. J. Genet. Genomics 2021. [Google Scholar] [CrossRef]
- Feng, C.A.; Spiller, C.; Merriner, D.J.; O’Bryan, M.K.; Bowles, J.; Koopman, P. SOX30 is required for male fertility in mice. Sci. Rep. 2017, 7, 17619. [Google Scholar] [CrossRef] [Green Version]
- Fujihara, Y.; Oji, A.; Larasati, T.; Kojima-Kita, K.; Ikawa, M. Human Globozoospermia-Related Gene Spata16 Is Required for Sperm Formation Revealed by CRISPR/Cas9-Mediated Mouse Models. Int. J. Mol. Sci 2017, 18, 2208. [Google Scholar] [CrossRef] [Green Version]
- Girault, M.S.; Dupuis, S.; Ialy-Radio, C.; Stouvenel, L.; Viollet, C.; Pierre, R.; Favier, M.; Ziyyat, A.; Barbaux, S. Deletion of the Spata3 Gene Induces Sperm Alterations and In Vitro Hypofertility in Mice. Int. J. Mol. Sci. 2021, 22, 1959. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kwon, J.T.; Jeong, J.; Kim, J.; Hong, S.H.; Kim, J.; Park, Z.Y.; Chung, K.H.; Eddy, E.M.; Cho, C. SPATC1L maintains the integrity of the sperm head-tail junction. EMBO. Rep. 2018, 19, e45991. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, K.; Zhang, Q.; Miyata, H.; Devlin, D.J.; Yu, Z.; Oura, S.; Koyano, T.; Matsuyama, M.; Ikawa, M.; Matzuk, M.M. Knockout of serine-rich single-pass membrane protein 1 (Ssmem1) causes globozoospermia and sterility in male micedagger. Biol. Reprod. 2020, 103, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Khan, R.; Yu, C.; Alsheimer, M.; Jiang, X.; Ma, H.; Shi, Q. The testis-specific LINC component SUN3 is essential for sperm head shaping during mouse spermiogenesis. J. Biol. Chem. 2020, 295, 6289–6298. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Zhang, Y.; Qin, D.; Gui, Y.; Wang, S.; Du, G.; Yang, F.; Li, L.; Yuan, S.; Wang, M.; et al. Transcription factor-like 5 is a potential DNA- and RNA-binding protein essential for maintaining male fertility in mice. J. Cell Sci. 2022, 135, jcs259036. [Google Scholar] [CrossRef]
- Feng, M.; Bai, Y.; Chen, Y.; Wang, K. Knockout of the Transducin-Like Enhancer of Split 6 Gene Affects the Proliferation and Cell Cycle Process of Mouse Spermatogonia. Int. J. Mol. Sci. 2020, 21, 5827. [Google Scholar] [CrossRef]
- Larasati, T.; Noda, T.; Fujihara, Y.; Shimada, K.; Tobita, T.; Yu, Z.; Matzuk, M.M.; Ikawa, M. Tmprss12 is required for sperm motility and uterotubal junction migration in micedagger. Biol. Reprod. 2020, 103, 254–263. [Google Scholar] [CrossRef]
- Luo, G.; Hou, M.; Wang, B.; Liu, Z.; Liu, W.; Han, T.; Zhang, D.; Zhou, X.; Jia, W.; Tan, Y.; et al. Tsga10 is essential for arrangement of mitochondrial sheath and male fertility in mice. Andrology 2021, 9, 368–375. [Google Scholar] [CrossRef]
- Nayyab, S.; Gervasi, M.G.; Tourzani, D.A.; Caraballo, D.A.; Jha, K.N.; Teves, M.E.; Cui, W.; Georg, G.I.; Visconti, P.E.; Salicioni, A.M. TSSK3, a novel target for male contraception, is required for spermiogenesis. Mol. Reprod. Dev. 2021, 88, 718–730. [Google Scholar] [CrossRef]
- Liu, W.; He, X.; Yang, S.; Zouari, R.; Wang, J.; Wu, H.; Kherraf, Z.E.; Liu, C.; Coutton, C.; Zhao, R.; et al. Bi-allelic Mutations in TTC21A Induce Asthenoteratospermia in Humans and Mice. Am. J. Hum. Genet. 2019, 104, 738–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.; Liu, Z.; Zheng, Y.; Feng, T.; Gao, Q.; Zeng, W. YTHDF2 promotes spermagonial adhesion through modulating MMPs decay via m(6)A/mRNA pathway. Cell Death Dis. 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gustafson, E.A.; Seymour, K.A.; Sigrist, K.; Rooij, D.; Freiman, R.N. ZFP628 Is a TAF4b-Interacting Transcription Factor Required for Mouse Spermiogenesis. Mol. Cell Biol. 2020, 40, e00228-19. [Google Scholar] [CrossRef] [PubMed]
- Nakasuji, T.; Ogonuki, N.; Chiba, T.; Kato, T.; Shiozawa, K.; Yamatoya, K.; Tanaka, H.; Kondo, T.; Miyado, K.; Miyasaka, N.; et al. Complementary Critical Functions of Zfy1 and Zfy2 in Mouse Spermatogenesis and Reproduction. PLoS Genet. 2017, 13, e1006578. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Matsumura, T.; Bakse, J.; Holmlund, H.; Blanchet, G.; Carrot, E.; Ikawa, M.; Ward, M.A. Loss of mouse Y chromosome gene Zfy1 and Zfy2 leads to spermatogenesis impairment, sperm defects, and infertility. Biol. Reprod. 2022, ioac057. [Google Scholar] [CrossRef]
- Hu, X.; Shen, B.; Liao, S.; Ning, Y.; Ma, L.; Chen, J.; Lin, X.; Zhang, D.; Li, Z.; Zheng, C.; et al. Gene knockout of Zmym3 in mice arrests spermatogenesis at meiotic metaphase with defects in spindle assembly checkpoint. Cell Death Dis. 2017, 8, e2910. [Google Scholar] [CrossRef]
- Wu, X.L.; Yun, D.M.; Gao, S.; Liang, A.J.; Duan, Z.Z.; Wang, H.S.; Wang, G.S.; Sun, F. The testis-specific gene 1700102P08Rik is essential for male fertility. Mol. Reprod. Dev. 2020, 87, 231–240. [Google Scholar] [CrossRef]
- Xu, M.; Xiao, J.; Chen, J.; Li, J.; Yin, L.; Zhu, H.; Zhou, Z.; Sha, J. Identification and characterization of a novel human testis-specific Golgi protein, NYD-SP12. Mol. Hum. Reprod. 2003, 9, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Dam, A.H.; Koscinski, I.; Kremer, J.A.; Moutou, C.; Jaeger, A.S.; Oudakker, A.R.; Tournaye, H.; Charlet, N.; Lagier-Tourenne, C.; van Bokhoven, H.; et al. Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia. Am. J. Hum. Genet. 2007, 81, 813–820. [Google Scholar] [CrossRef] [Green Version]
- ElInati, E.; Fossard, C.; Okutman, O.; Ghedir, H.; Ibala-Romdhane, S.; Ray, P.F.; Saad, A.; Hennebicq, S.; Viville, S. A new mutation identified in SPATA16 in two globozoospermic patients. J. Assist. Reprod. Genet. 2016, 33, 815–820. [Google Scholar] [CrossRef] [Green Version]
- Behvarz, M.; Rahmani, S.A.; Siasi Torbati, E.; Danaei Mehrabad, S.; Bikhof Torbati, M. Association of CATSPER1, SPATA16 and TEX11 genes polymorphism with idiopathic azoospermia and oligospermia risk in Iranian population. BMC Med. Genom. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.S.; Shamu, C.E.; Shen, M.M.; Seifert, K.J.; Hirsch, B.; Hodgkin, J.; Zarkower, D. Evidence for evolutionary conservation of sex-determining genes. Nature 1998, 391, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.A.; McClive, P.J.; Western, P.S.; Reed, K.J.; Sinclair, A.H. Conservation of a sex-determining gene. Nature 1999, 402, 601–602. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.S.; Kettlewell, J.R.; Hirsch, B.; Bardwell, V.J.; Zarkower, D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev. Biol. 1999, 215, 208–220. [Google Scholar] [CrossRef]
- Moniot, B.; Berta, P.; Scherer, G.; Sudbeck, P.; Poulat, F. Male specific expression suggests role of DMRT1 in human sex determination. Mech. Dev. 2000, 91, 323–325. [Google Scholar] [CrossRef]
- Zhang, T.; Zarkower, D. DMRT proteins and coordination of mammalian spermatogenesis. Stem. Cell Res. 2017, 24, 195–202. [Google Scholar] [CrossRef]
- Ottolenghi, C.; McElreavey, K. Deletions of 9p and the quest for a conserved mechanism of sex determination. Mol. Genet. Metab. 2000, 71, 397–404. [Google Scholar] [CrossRef]
- Raymond, C.S.; Parker, E.D.; Kettlewell, J.R.; Brown, L.G.; Page, D.C.; Kusz, K.; Jaruzelska, J.; Reinberg, Y.; Flejter, W.L.; Bardwell, V.J.; et al. A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators. Hum. Mol. Genet. 1999, 8, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Muroya, K.; Okuyama, T.; Goishi, K.; Ogiso, Y.; Fukuda, S.; Kameyama, J.; Sato, H.; Suzuki, Y.; Terasaki, H.; Gomyo, H.; et al. Sex-determining gene(s) on distal 9p: Clinical and molecular studies in six cases. J. Clin. Endocrinol Metab. 2000, 85, 3094–3100. [Google Scholar] [CrossRef]
- Kim, S.; Bardwell, V.J.; Zarkower, D. Cell type-autonomous and non-autonomous requirements for Dmrt1 in postnatal testis differentiation. Dev. Biol. 2007, 307, 314–327. [Google Scholar] [CrossRef] [Green Version]
- Takashima, S.; Hirose, M.; Ogonuki, N.; Ebisuya, M.; Inoue, K.; Kanatsu-Shinohara, M.; Tanaka, T.; Nishida, E.; Ogura, A.; Shinohara, T. Regulation of pluripotency in male germline stem cells by Dmrt1. Genes Dev. 2013, 27, 1949–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, N.; Hao, G.; Zhao, Z.; Wang, F.; Cao, J.; Yang, A. MicroRNA-224 regulates self-renewal of mouse spermatogonial stem cells via targeting DMRT1. J. Cell Mol. Med. 2016, 20, 1503–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Oatley, J.; Bardwell, V.J.; Zarkower, D. DMRT1 Is Required for Mouse Spermatogonial Stem Cell Maintenance and Replenishment. PLoS Genet. 2016, 12, e1006293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Yang, H.; Zhao, J.; Fang, L.; Shi, H.; Li, M.; Sun, Y.; Zhang, X.; Jiang, D.; Zhou, L.; et al. Efficient and heritable gene targeting in tilapia by CRISPR/Cas9. Genetics 2014, 197, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Agbor, V.A.; Tao, S.; Lei, N.; Heckert, L.L. A Wt1-Dmrt1 transgene restores DMRT1 to sertoli cells of Dmrt1(-/-) testes: A novel model of DMRT1-deficient germ cells. Biol. Reprod. 2013, 88, 51. [Google Scholar] [CrossRef] [Green Version]
- Koscinski, I.; Elinati, E.; Fossard, C.; Redin, C.; Muller, J.; Velez de la Calle, J.; Schmitt, F.; Ben Khelifa, M.; Ray, P.F.; Kilani, Z.; et al. DPY19L2 deletion as a major cause of globozoospermia. Am. J. Hum. Genet. 2011, 88, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Yassine, S.; Escoffier, J.; Abi Nahed, R.; Pierre, V.; Karaouzene, T.; Ray, P.F.; Arnoult, C. Dynamics of Sun5 localization during spermatogenesis in wild type and Dpy19l2 knock-out mice indicates that Sun5 is not involved in acrosome attachment to the nuclear envelope. PLoS ONE 2015, 10, e0118698. [Google Scholar] [CrossRef]
- Pereira, C.D.; Serrano, J.B.; Martins, F.; da Cruz, E.S.O.A.B.; Rebelo, S. Nuclear envelope dynamics during mammalian spermatogenesis: New insights on male fertility. Biol. Rev. Camb. Philos. Soc. 2019, 94, 1195–1219. [Google Scholar] [CrossRef]
- Harbuz, R.; Zouari, R.; Pierre, V.; Ben Khelifa, M.; Kharouf, M.; Coutton, C.; Merdassi, G.; Abada, F.; Escoffier, J.; Nikas, Y.; et al. A recurrent deletion of DPY19L2 causes infertility in man by blocking sperm head elongation and acrosome formation. Am. J. Hum. Genet. 2011, 88, 351–361. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Gong, F.; Lin, G.; Lu, G. DPY19L2 gene mutations are a major cause of globozoospermia: Identification of three novel point mutations. Mol. Hum. Reprod. 2013, 19, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Coutton, C.; Zouari, R.; Abada, F.; Ben Khelifa, M.; Merdassi, G.; Triki, C.; Escalier, D.; Hesters, L.; Mitchell, V.; Levy, R.; et al. MLPA and sequence analysis of DPY19L2 reveals point mutations causing globozoospermia. Hum. Reprod. 2012, 27, 2549–2558. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Z.; Wu, R.F.; Zhu, X.S.; Liu, W.S.; Ye, Y.Y.; Lu, Z.X.; Li, N. Identification of a novel deletion mutation in DPY19L2 from an infertile patient with globozoospermia: A case report. Mol. Cytogenet. 2020, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Jiang, J.; Zhang, H.; Wen, Y.; Zhang, H.; Cui, Y.; Tian, J.; Jiang, M.; Liu, X.; Wang, G.; et al. Proteomic Analysis of Dpy19l2-Deficient Human Globozoospermia Reveals Multiple Molecular Defects. Proteomics Clin. Appl. 2019, 13, e1900007. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, J.M.; Shimada, K.; Satouh, Y.; Yu, Z.; Devlin, D.J.; Ikawa, M.; Matzuk, M.M. FAM209 associates with DPY19L2, and is required for sperm acrosome biogenesis and fertility in mice. J. Cell Sci. 2021, 134, jcs259206. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.L.; Zhu, F.X.; Yan, J.; Chen, L.; Tang, W.H.; Xiao, S.; Mo, W.K.; Zhang, Z.G.; He, X.J.; Qiao, J.; et al. Novel DPY19L2 variants in globozoospermic patients and the overcoming this male infertility. Asian J. Androl. 2019, 21, 183–189. [Google Scholar] [CrossRef]
- Kuentz, P.; Vanden Meerschaut, F.; Elinati, E.; Nasr-Esfahani, M.H.; Gurgan, T.; Iqbal, N.; Carre-Pigeon, F.; Brugnon, F.; Gitlin, S.A.; Velez de la Calle, J.; et al. Assisted oocyte activation overcomes fertilization failure in globozoospermic patients regardless of the DPY19L2 status. Hum. Reprod. 2013, 28, 1054–1061. [Google Scholar] [CrossRef] [Green Version]
- Modarressi, M.H.; Cameron, J.; Taylor, K.E.; Wolfe, J. Identification and characterisation of a novel gene, TSGA10, expressed in testis. Gene 2001, 262, 249–255. [Google Scholar] [CrossRef]
- Modarressi, M.H.; Behnam, B.; Cheng, M.; Taylor, K.E.; Wolfe, J.; van der Hoorn, F.A. Tsga10 encodes a 65-kilodalton protein that is processed to the 27-kilodalton fibrous sheath protein. Biol. Reprod. 2004, 70, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Sha, Y.W.; Sha, Y.K.; Ji, Z.Y.; Mei, L.B.; Ding, L.; Zhang, Q.; Qiu, P.P.; Lin, S.B.; Wang, X.; Li, P.; et al. TSGA10 is a novel candidate gene associated with acephalic spermatozoa. Clin. Genet. 2018, 93, 776–783. [Google Scholar] [CrossRef]
- Xiang, M.; Wang, Y.; Xu, W.; Zheng, N.; Zhang, J.; Duan, Z.; Zha, X.; Shi, X.; Wang, F.; Cao, Y.; et al. Pathogenesis of acephalic spermatozoa syndrome caused by splicing mutation and de novo deletion in TSGA10. J. Assist. Reprod. Genet. 2021, 38, 2791–2799. [Google Scholar] [CrossRef]
- Liu, G.; Wang, N.; Zhang, H.; Yin, S.; Dai, H.; Lin, G.; Li, W. Novel mutations in PMFBP1, TSGA10 and SUN5: Expanding the spectrum of mutations that may cause acephalic spermatozoa. Clin. Genet. 2020, 97, 938–939. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wei, X.; Sha, Y.; Li, N.; Yan, X.; Cheng, L.; Qiao, D.; Zhou, W.; Wu, R.; Liu, Q.; et al. Loss-of-function mutation in TSGA10 causes acephalic spermatozoa phenotype in human. Mol. Genet. Genomic Med. 2020, 8, e1284. [Google Scholar] [CrossRef]
- Asgari, R.; Bakhtiari, M.; Rezazadeh, D.; Yarani, R.; Esmaeili, F.; Mansouri, K. TSGA10 as a Potential Key Factor in the Process of Spermatid Differentiation/Maturation: Deciphering Its Association with Autophagy Pathway. Reprod. Sci. 2021, 28, 3228–3240. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liang, D.; Wang, Y.; Bai, M.; Tang, W.; Bao, S.; Yan, Z.; Li, D.; Li, J. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem. Cell 2013, 13, 659–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Zhou, H.; Fan, X.; Zhang, Y.; Zhang, M.; Wang, Y.; Xie, Z.; Bai, M.; Yin, Q.; Liang, D.; et al. Correction of a genetic disease by CRISPR-Cas9-mediated gene editing in mouse spermatogonial stem cells. Cell Res. 2015, 25, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sun, T.; Wang, X.; Tang, J.; Liu, Y. Restore natural fertility of Kit(w)/Kit(wv) mouse with nonobstructive azoospermia through gene editing on SSCs mediated by CRISPR-Cas9. Stem. Cell Res. Ther. 2019, 10, 271. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Li, G.; Zhang, M.; Xing, P.; Li, Z.; Chen, B.; Yang, H.; Wu, Z. Establishment of Etv5 gene knockout mice as a recipient model for spermatogonial stem cell transplantation. Biol. Open 2021, 10, bio056804. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, Y.; Gao, Y.; Lin, Z.; Yang, S.; Wang, T.; Wang, Q.; Xie, N.; Hua, R.; Liu, M.; et al. Single-cell RNA-seq uncovers dynamic processes and critical regulators in mouse spermatogenesis. Cell Res. 2018, 28, 879–896. [Google Scholar] [CrossRef] [Green Version]
- Wen, L.; Liu, Q.; Xu, J.; Liu, X.; Shi, C.; Yang, Z.; Zhang, Y.; Xu, H.; Liu, J.; Yang, H.; et al. Recent advances in mammalian reproductive biology. Sci. China Life Sci. 2020, 63, 18–58. [Google Scholar] [CrossRef]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol 2014, 30, 2–13. [Google Scholar] [CrossRef] [Green Version]
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chocu, S.; Calvel, P.; Rolland, A.D.; Pineau, C. Spermatogenesis in mammals: Proteomic insights. Syst. Biol. Reprod. Med. 2012, 58, 179–190. [Google Scholar] [CrossRef] [PubMed]
| Gene | Species | Techniques Used for Function Analysis | Fertility | Phenotype/Clinical Symptoms | References |
|---|---|---|---|---|---|
| Akap4 | Mus musculus | KO 1 | Male infertility | Abnormal sperm morphology and reduced motility | [32] |
| Amh | Danio rerio | KO | - | Dysregulation of germ cell development and the over-proliferation of spermatogonia | [33] |
| Armc2 | M. musculus | KO | Male infertility | Multiple morphological abnormalities of the flagella | [34] |
| Asb17 | M. musculus | KO | Fertile | Oligospermia and a disorganized ES junction | [35] |
| Bcorl1 | M. musculus | KO | Male infertility | Impaired sperm viability and abnormal mitochondrial structure of sperm cells | [36] |
| Cabs1 | M. musculus | KO | Significantly impaired fertility | Defective sperm flagellum differentiation and abnormal sperm tail structure | [37] |
| Ccdc63 | M. musculus | KO | Male infertility | Shortened flagella | [38] |
| Cct6b | M. musculus | KO | - | No differences in development, fertility, appearance, testis weight, or sperm counts. Nuclear base bending abnormality | [39] |
| Cdc14a | M. musculus | KO | Significantly impaired fertility | Low sperm count, impaired sperm motility and high percentage of morphologically abnormal sperm | [40] |
| Cib4 | M. musculus | KO | Male infertility | Impaired haploid differentiation and absence of elongated spermatozoa in the epididymal tail | [41] |
| Cmtm4 | M. musculus | KO | Significantly impaired fertility | Decreased sperm count, decreased epididymal sperm motility, increased percentage of abnormal backward bending of sperm head and bending of sperm mid-section | [42] |
| CSR-1a | Caenorhabditis elegans | KI 2/KO | - | A transgenerational loss of sperm-based fertility in hermaphrodites | [43] |
| Cyp11c1 | Danio rerio | KO | - | Exhibits female secondary sexual characteristics, severe deficiency of androgens and cortisol, impaired spermatogenesis and characteristic reproductive behavior, disturbed arrangement of spermatogenic tubules, and abnormal differentiation of spermatogonia. | [44] |
| Ddx4 | M. musculus | cKO 3(Cre-loxP) | - | Spermatogonia developed and became arrested at the round spermatid stage | [45] |
| Defb23/26/42 | R. norvegicus | KO | No clear phenotype for single knockout, but 23/26 or 23/26/42 combined knockout is infertile. | Impaired sperm motility, the sperm showed precocious capacitation and increased spontaneous acrosome reaction. | [46] |
| Dmrt1 | Danio rerio | KO | - | Severe testicular developmental defects and gradual loss of all Vasa-positive germ cells | [33] |
| Dmrt6 | Oreochromis mossambicus | KO | - | Fewer spermatocytes | [47] |
| Dnah17 | M. musculus | KO | - | Asthenozoospermia, abnormal sperm flagellar morphology and low sperm activity. | [48,49] |
| Dpy19l2 | M. musculus | KO (NA) 9 | Male infertility | The NDL facing the acrosome, the acro-plaxome, caudal descent and acrosome spreading are defective. | [50] |
| Ephb2 | M. musculus | KO (SSCs) 7 | - | Proliferation and stem cell activity are impaired. | [51] |
| Fam170a | M. musculus | KO | Significantly impaired fertility | Abnormal spermiation, abnormal head morphology, and reduced progressive sperm motility. | [52] |
| Fto | M. musculus | KO (spermatogonia) | - | Chromosome instability and G2/M arrest | [53] |
| Gh1 | Danio rerio | Point mutation | - | Delayed spermatogenesis | [54] |
| HIF-1α | R. norvegicus | KD 4 | - | The distribution of germ cells was disordered and apoptosis of spermatogenic cells increased significantly. | [55,56] |
| Hsf5 | Danio rerio | KO | Male infertility | Reduced sperm count, increased sperm head size, and abnormal tail architecture | [57] |
| Hydin | M. musculus | Biallelic mutations (ESCs) | - | Hydin-disrupted sperm obtained from the chimeric mice possessed short tails and were immotile, but it can produce viable pups. | [58] |
| KO (NA) | - | Die within 3 weeks before sexual maturation due to hydrocephaly. | [58] | ||
| Igf3 | Oreochromis niloticus | KO | Male infertility | The proliferation and differentiation of spermatogonia are severely inhibited at the beginning of meiosis, and semen volume and sperm count are drastically reduced. | [59] |
| Lipocalin8 | M. musculus | KO | Normal fertility | There was no significant effect on the morphological appearance of the testes but epididymal sperm maturation defects. | [60] |
| cKI 5 | Normal fertility | - | [61] | ||
| Mct8 | R. norvegicus | KO | Fertile, lower fertilization rate | Serum THs (T3 and T4) level were significantly increased, growth delay along with thyroid dysfunction, testis maldevelopment and impaired spermiogenesis. | [62] |
| Meig1 | M. musculus | Y68 point mutation | Male infertility | The sperm count is significantly reduced, and a few developed sperm fail to move and exhibit a variety of abnormalities. | [63] |
| Pick1 | M. musculus | KO (NA) | Male infertility | Fragmentation of acrosomes in the early stages of spermiogenesis, round-headed sperm, reduced sperm count, and severely impaired sperm motility. | [64] |
| Pmfbp1 | Bombyx mori | Point mutation | Male infertility | Defects in the development of eupyrene sperm bundles | [65] |
| Prss55 | M. musculus | KO/DKO 6 | Male infertility | Impaired migration from the uterus to the oviduct and impaired ability to bind the zona pellucida (ZP) of oocytes | [66] |
| Rln3a | Oreochromis niloticus | KO | Significantly impaired fertility | Hypogonadism, sperm deformation and a significant decrease in sperm motility. | [67] |
| Rnf216 | M. musculus | KO | Male mice are sterile and females are capable of reproduction. | Smaller testes, defective meiosis, and reduced number of germ cells. | [68,69] |
| Sox30 | Oreochromis niloticus | KO | Significantly impaired fertility | Abnormal spermiogenesis, reduction of sperm motility | [70] |
| M. musculus | cKO (Cre-loxP) | Male infertility | Stagnant germ cell development, abnormal acrosome and axon development and complete cessation of spermatogenesis. | [71] | |
| Spata16 | M. musculus | 851G→A/R284Q point mutation | Fertile | - | [72] |
| 781-bp deletion | Male infertility | Spermio-genic arrest, with impaired differentiation of round spermatids into the mature sperm. | [72] | ||
| Spata3 | M. musculus | KO | Normal fertility with reduced in vitro fertility | Acrosome defects and excessive lipid droplet residues in the cytoplasm. | [73] |
| Spatc1l | M. musculus | KO | Male infertility | Separation of sperm head from tail | [74] |
| Ssmem1 | M. musculus | KO | Male infertility | Globozoospermia, loss of sperm motility and abnormal localization of Golgi at steps eight and nine of spermatid development. | [75] |
| Sun3 | M. musculus | KO | Male infertility | Reduced sperm counts and a globozoospermia-like phenotype. | [76] |
| Tcfl5 | M. musculus | KO | Male infertility | Sperm cells and spermatozoa of Tcfl5+/- mice (infertility) have been abnormal. | [77] |
| Tle6 | M. musculus | KO (spermatogonia, CRISPR/Cas9, Tet-on) 8 | - | Spermatogonia proliferation and cell cycle are inhibited. | [78] |
| Tmprss12 | M. musculus | KO | Male infertility | Normal spermatogenesis and sperm morphology, but ejaculated spermatozoa failed to migrate from the uterus to the oviduct. | [79] |
| Tsga10 | M. musculus | KO | Male infertility | Disordered mitochondrial sheath formation and reduced sperm motility. | [80] |
| Tssk3 | M. musculus | KO | Male infertility | Reduced sperm count and abnormal morphology. | [81] |
| Ttc21a | M. musculus | Frameshift mutation | Male infertility (78%) | The motility and progressive motility of spermatozoa were significantly reduced. Morphological abnormalities of sperm. The structural abnormalities of the connecting piece during spermiogenesis and multiple structural defects of the flagella. | [82] |
| Ythdf2 | M. musculus | KO (spermatogonia) | - | Cell proliferation, cell adhesion and cell spread were inhibited. | [83] |
| Zfp628 | M. musculus | KO | Male infertility | Post-meiotic germ cell arrest at the round spermatid stage in the seminiferous tubules of the testis. | [84] |
| Zfy1/Zfy2 | M. musculus | KO | Normal fertility | - | [85,86] |
| DKO | Infertility | Abnormal sperm morphology, fertilization failure and early embryo development failure. | |||
| Zmym3 | M. musculus | KO | Male infertility | Abnormal spindle assembly at mid-meiotic division. | [87] |
| 1700102P08Rik | M. musculus | KO | Male infertility | Smaller testes and epididymis, stagnation of spermatogenesis at the spermatocyte stage, absence of spermatozoa in the epididymis, and apoptosis of testicular cells. | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-Q.; Wang, T.; Gao, F.; Ren, W.-Z. Application of CRISPR/Cas Technology in Spermatogenesis Research and Male Infertility Treatment. Genes 2022, 13, 1000. https://doi.org/10.3390/genes13061000
Wang H-Q, Wang T, Gao F, Ren W-Z. Application of CRISPR/Cas Technology in Spermatogenesis Research and Male Infertility Treatment. Genes. 2022; 13(6):1000. https://doi.org/10.3390/genes13061000
Chicago/Turabian StyleWang, Hao-Qi, Tian Wang, Fei Gao, and Wen-Zhi Ren. 2022. "Application of CRISPR/Cas Technology in Spermatogenesis Research and Male Infertility Treatment" Genes 13, no. 6: 1000. https://doi.org/10.3390/genes13061000
APA StyleWang, H. -Q., Wang, T., Gao, F., & Ren, W. -Z. (2022). Application of CRISPR/Cas Technology in Spermatogenesis Research and Male Infertility Treatment. Genes, 13(6), 1000. https://doi.org/10.3390/genes13061000






