Watercore Pear Fruit Respiration Changed and Accumulated γ-Aminobutyric Acid (GABA) in Response to Inner Hypoxia Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Measurement of GABA Content and GAD Activity
2.3. RNA Extraction and Sequencing
2.4. qPCR Analysis
2.5. Statistical Analysis
3. Results
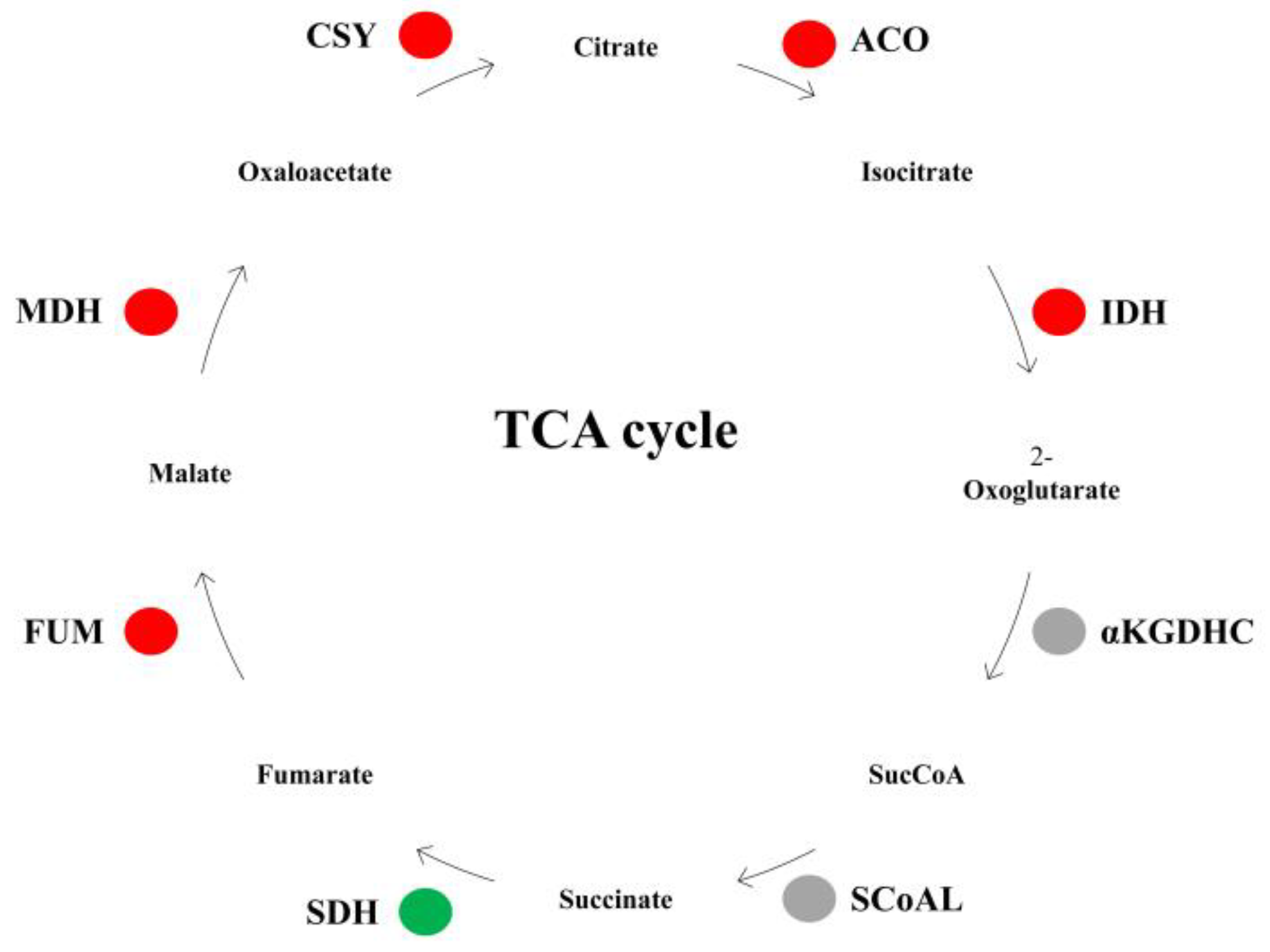
3.1. Analysis of TCA Cycle-Related Gene Expression in Watercore Fruit
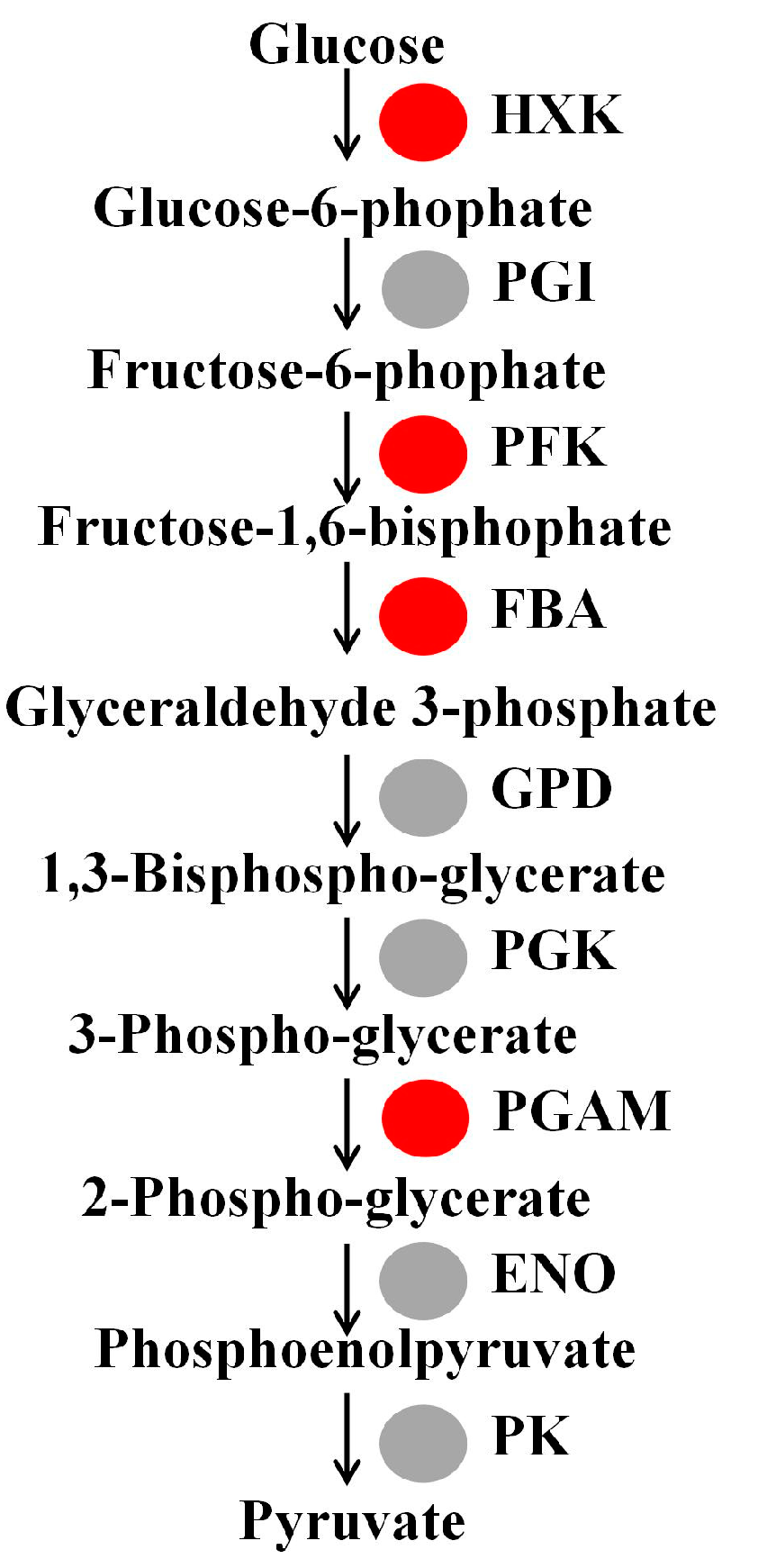
3.2. Analysis of Glycolysis Metabolism-Related Gene in Watercore Fruit
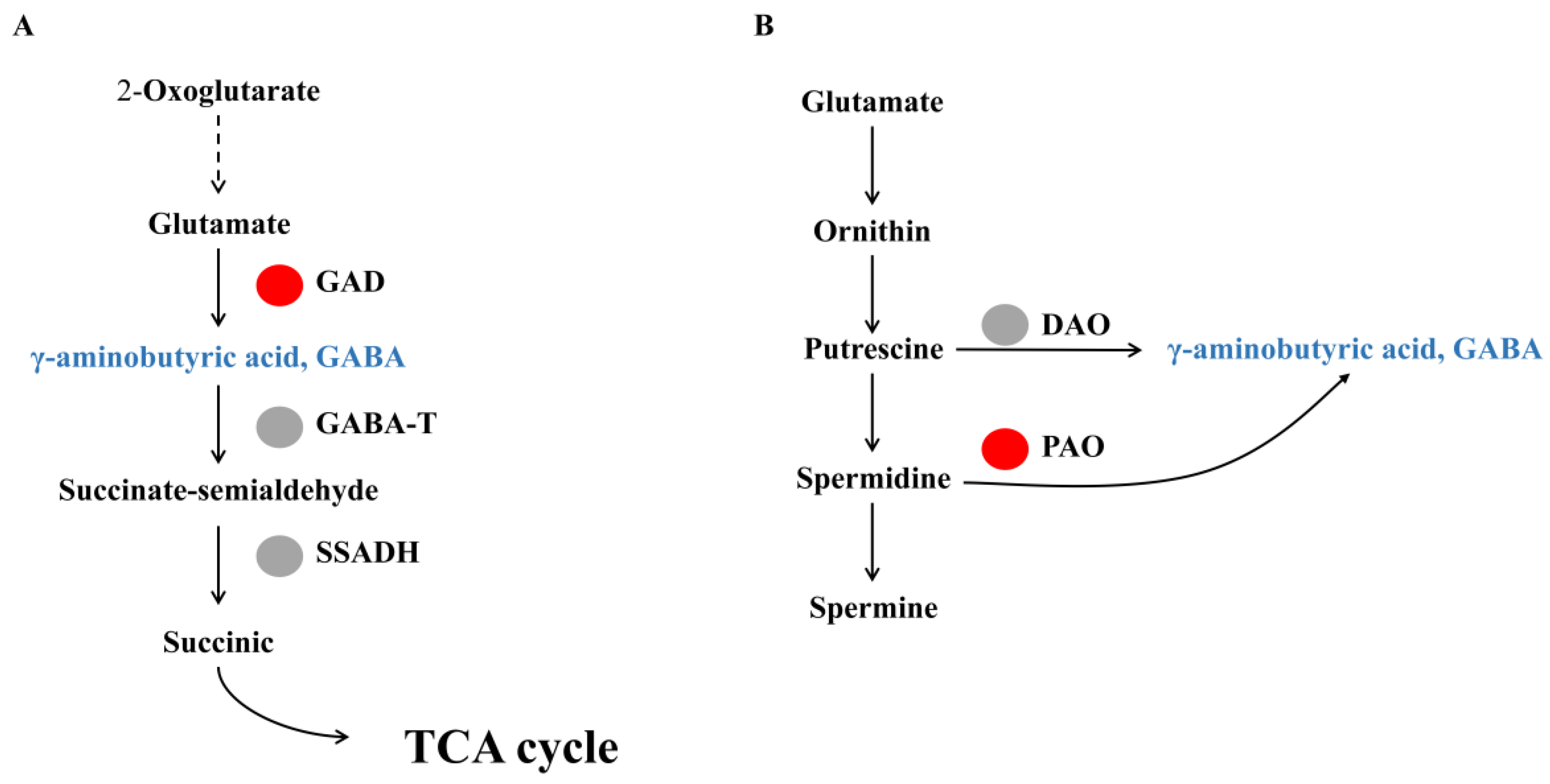
3.3. Analysis of Genes to GABA Produced Pathway by GABA Shunt and Polyamine Degradation Related Gene Expression in Watercore Fruit
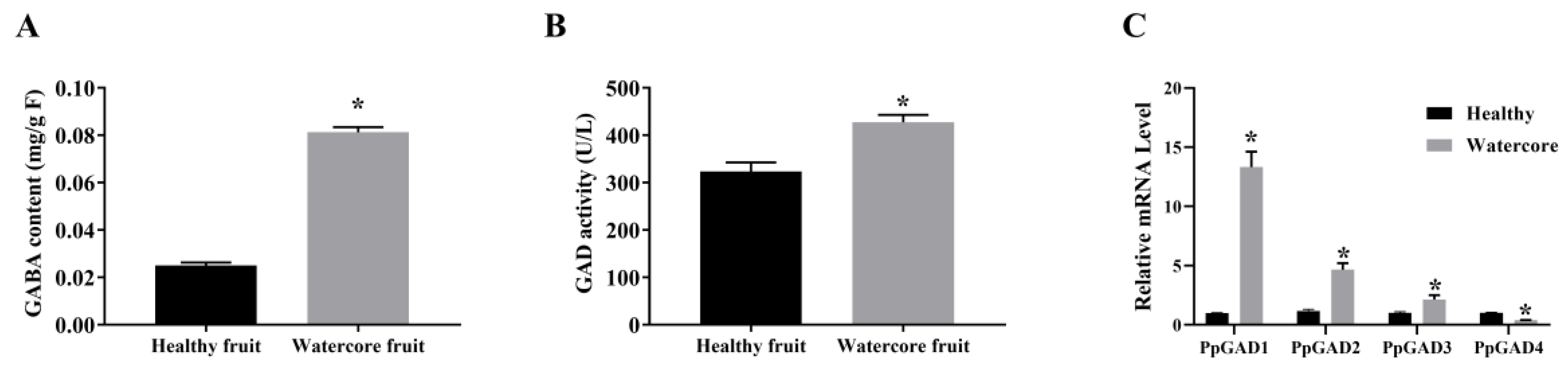
3.4. GABA Content, GAD Activity and qRT-PCR Analysis of PpGAD Genes in Fruits at Different Developmental Stages or Watercore Fruit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Itai, A. Abiotic Stress Biology in Horticultural Plants. In Watercore in Fruits; Springer: Berlin/Heidelberg, Germany, 2015; pp. 127–145. [Google Scholar]
- Cebulj, A.; Mikulic-Petkovsek, M.; Lucaciu, C.R.; Veberic, R.; Marinovic, S.; Kolarek, M.; Hutabarat, O.S.; Faramarzi, S.; Rattei, T.; Molitor, C.; et al. Alteration of the phenylpropanoid pathway by watercore disorder in apple (Malus x domestica). Sci. Hortic. 2021, 289, 110438. [Google Scholar] [CrossRef]
- Gao, Z.F.; Jayanty, S.; Beaudry, R.; Loescher, W. Sorbitol transporter expression in apple sink tissues: Implications for fruit sugar accumulation and watercore development. J. Am. Soc. Hortic. Sci. 2005, 130, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Shen, Y.; Liu, D.H.; Liu, J.; Zhang, J.; Wei, J.; Wang, C.L. A sorbitol transporter gene plays specific role in the occurrence of watercore by modulating the level of intercellular sorbitol in pear. Plant Sci. 2022, 371, 111179. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, H.M.; Liu, D.H.; Liu, J.; Shen, Y.; Zhang, J.; Wei, J.; Wang, C.L. Transcriptome and metabolome analyses provide insights into the watercore disorder on “akibae” pear fruit. Int. J. Mol. Sci. 2021, 22, 4911. [Google Scholar] [CrossRef]
- Zheng, J.; Ying, Q.; Fang, C.; Sun, N.; Si, M.; Yang, J.; Zhu, B.; Ruan, Y.L.; Zhu, Z.; He, Y. Alternative oxidase pathway is likely involved in waterlogging tolerance of watermelon. Sci. Hortic. 2021, 278, 109831. [Google Scholar] [CrossRef]
- Gupta, K.J.; Zabalza, A.; Dongen, J. Regulation of respiration when the oxygen availability changes. Physiol Plant. 2009, 137, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, W.; Beckett, P.M.; Colmer, T.D.; Setter, T.L.; Greenway, H. Tolerance of roots to low oxygen: ‘Anoxic’ cores, the phytoglobin-nitric oxide cycle, and energy or oxygen sensing. J. Plant Physiol. 2019, 239, 92–108. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, L.; Lv, J.; Zhang, Y.Z.; Sun, M.Y.; Chen, J.X.; Ge, Y.H. Effects of 1-methylcyclopropene (1-MCP) treatment on ethanol fermentation of Nanguo pear fruit during ripening. J. Food Biochem. 2022, 45, e14035. [Google Scholar] [CrossRef]
- Xu, J.J.; Liu, T.; Yang, S.C.; Jin, X.Q.; Qu, F.; Huang, N.; Hu, X.X. Polyamines are involved in GABA-regulated salinity-alkalinity stress tolerance in muskmelon. Environ. Exp. Bot. 2019, 164, 181–189. [Google Scholar] [CrossRef]
- Miyashita, Y.; Allen, G.G. Contribution of the GABA shunt to hypoxia-induced alanine accumulation in roots of Arabidopsis thaliana. Plant Cell Physiol. 2008, 49, 92–102. [Google Scholar] [CrossRef]
- Kaspal, M.; Kanapaddalagamage, M.H.; Ramesh, S.A. Emerging roles of γ-aminobutyric acid (GABA) gated channels in plant stress tolerance. Plant 2021, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yang, R.; Chen, H.; Song, Y.; Gu, Z. Accumulation of γ-aminobutyric acid in germinated soybean (Glycine max L.) in relation to glutamate decarboxylase and diamine oxidase activity induced by additives under hypoxia. Eur. Food Res. Technol. 2012, 4, 679–687. [Google Scholar] [CrossRef]
- Vergara, R.; Parada, F.; Regulation, F.J. Is GABA-shunt functional in endodormant grapevine buds under respiratory stress? Plant Growth Regul. 2013, 71, 253–260. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Naderi, R.; Jannatizadeh, A.; Sarcheshmeh, A.M.; Babalara, M. Enhancement of postharvest chilling tolerance of anthurium cut flowers by gamma-aminobutyric acid (GABA) treatments. Sci. Hortic. 2016, 198, 52–60. [Google Scholar] [CrossRef]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193, 130–135. [Google Scholar] [CrossRef]
- Podleáková, K.; Ugena, L.; Spíchal, L.; Doleal, K.; Diego, N.D. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2018, 48, 53–56. [Google Scholar] [CrossRef]
- Chi, Z.; Dai, Y.; Cao, S.; Wei, Y.; Shao, X.; Huang, X.; Xui, F.; Wang, H. Exogenous Calcium chloride (CaCl2) promotes γ-aminobutyric acid (GABA) accumulation in fresh-cut pears. Postharvest Biol. Tec. 2021, 174, 111446. [Google Scholar] [CrossRef]
- Dong, X.; Wang, Z.; Tian, L.; Zhang, Y.; Cao, Y.J. De novo assembly of a wild pear (Pyrus betuleafolia) genome. Plant Biotechnol. J. 2020, 18, 581–595. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yamada, H.; Morita, T.; Amano, S. Water relations in fruit, leaves and stems of two apple cultivars that differ in susceptibility to watercore. J. Hortic. Sci. Biotech. 2015, 80, 70–74. [Google Scholar] [CrossRef]
- Vitor, S.C.; Sodek, L.J. Products of anaerobic metabolism in waterlogged roots of soybean are exported in the xylem. Plant Sci. 2019, 284, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Borella, J.; Oliveira, H.C.; Oliveira, D.S.C.; Braga, E.B.; Oliveira, A.C.B.; Sodek, L.; Amarante, L.D. Hypoxia-driven changes in glycolytic and tricarboxylic acid cycle metabolites of two nodulated soybean genotypes. Environ. Exp. Bot. 2017, 133, 118–127. [Google Scholar] [CrossRef]
- Ruperti, B.; Botton, A.; Populin, F.; Eccher, G.; Brilli, M.; Quaggiotti, S.; Trevisan, S.; Cainelli, N.; Guarracino, P.; Schievano, E.; et al. Flooding Responses on Grapevine: A physiological, transcriptional, and metabolic perspective. Front. Plant Sci. 2019, 10, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Hu, X.M.; Jin, L.F.; Shi, C.Y.; Liu, Y.Z. Identification and transcript analysis of two glutamate decarboxylase genes, CsGAD1 and CsGAD2, reveal the strong relationship between CsGAD1 and citrate utilization in citrus fruit. Mole. Biol. Rep. 2014, 41, 6253–6262. [Google Scholar] [CrossRef]
- Michaeli, S.; Fait, A.; Lagor, K.; Nunes-Nesi, A.; Fromm, H.J.; Biology, M. A mitochondrial GABA permease connects the GABA shunt and the TCA cycle, and is essential for normal carbon metabolism. Plant J. 2011, 67, 485–498. [Google Scholar] [CrossRef]
- Shelp, B.J.; Mullen, R.T.; Waller, J.C. Compartmentation of GABA metabolism raises intriguing questions. Trends Plant Sci. 2012, 17, 57–59. [Google Scholar] [CrossRef]
- Wu, Q.; Su, N.; Huang, X.; Cui, J. Hypoxia-induced increase in GABA content is essential for restoration of membrane potential and preventing ROS-induced disturbance to ion homeostasis. Plant Commun. 2021, 2, 100188. [Google Scholar] [CrossRef]
- Yang, R.; Yin, Y.; Gu, Z. Polyamine degradation pathway regulating growth and GABA accumulation in germinating fava bean under hypoxia-NaCl Stress. J. Agric. Sci. Technol. 2015, 17, 311–320. [Google Scholar]
- Yang, R.Q.; Guo, Q.H.; Gu, Z.X. GABA shunt and polyamine degradation pathway on γ-aminobutyric acid accumulation in germinating fava bean (Vicia faba L.) under hypoxia. Food Chem. 2013, 136, 152–159. [Google Scholar] [CrossRef]
- Lü, G.; Liang, Y.; Wu, X.; Li, J.; Ma, W.; Zhang, Y.; Gao, H. Molecular cloning and functional characterization of mitochondrial malate dehydrogenase (mMDH) is involved in exogenous GABA increasing root hypoxia tolerance in muskmelon plants. Sci. Hortic. 2019, 258, 108741. [Google Scholar] [CrossRef]
- Hijaz, F.; Killiny, N. Exogenous GABA is quickly metabolized to succinic acid and fed into the plant TCA cycle. Plant Signal Behav. 2019, 14, e1573096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Gene ID | log2Fold Change | Gene Description |
|---|---|---|
| Chr16.g30574 | 1.10 | Citrate synthase, CS |
| Chr4.g39633 | 2.37 | Citrate synthase, CS |
| Chr9.g46434 | 1.25 | Aconitase, ACO |
| Chr15.g02728 | 8.70 | Aconitase, ACO |
| Chr15.g01127 | 1.02 | Isocitrate dehydrogenase, IDH |
| Chr15.g02763 | 1.46 | Isocitrate dehydrogenase, IDH |
| Chr3.g17593 | 1.65 | Fumarase, FUM |
| Chr10.g17307 | −1.10 | Succinate dehydrogenase, SDH |
| Chr2.g43436 | 2.07 | Malate dehydrogenase, MDH |
| Gene ID | log2Fold Change | Gene Description |
|---|---|---|
| Chr9.g45423 | 1.04 | Hexokinase, HXK |
| Chr15.g04826 | 1.84 | Hexokinase, HXK |
| Chr17.g26626 | 1.02 | 6-phosphofructokinase, PFK |
| Chr2.g41653 | 2.38 | 6-phosphofructokinase, PFK |
| Chr1.g58362 | 1.71 | 6-phosphofructokinase, PFK |
| Chr11.g09985 | 1.19 | Fructose-bisphosphate aldolase, FBA |
| Chr10.g16756 | 1.82 | Fructose-bisphosphate aldolase, FBA |
| Chr13.g23923 | 3.90 | Fructose-bisphosphate aldolase, FBA |
| Chr14.g49092 | 1.13 | Phosphoglycerate mutase, PGAM |
| Chr15.g02324 | 6.70 | Phosphoglycerate mutase, PGAM |
| Chr15.g02323 | 3.40 | Phosphoglycerate mutase, PGAM |
| Gene ID | log2Fold Change | Gene Description |
|---|---|---|
| Chr16.g31370 | 6.45 | Glutamate decarboxylase, GAD |
| Chr9.g44430 | 1.94 | Glutamate decarboxylase, GAD |
| Chr6.g50750 | 1.51 | Glutamate decarboxylase, GAD |
| Chr14.g50570 | −2.64 | Glutamate decarboxylase, GAD |
| Chr7.g34714 | 5.78 | Polyamine oxidase, PAO |
| Chr9.g44410 | 1.08 | Polyamine oxidase, PAO |
| Chr2.g41730 | −3.88 | Polyamine oxidase, PAO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, D.-H.; Chen, T.; Zhang, J.; Wang, C.-L. Watercore Pear Fruit Respiration Changed and Accumulated γ-Aminobutyric Acid (GABA) in Response to Inner Hypoxia Stress. Genes 2022, 13, 977. https://doi.org/10.3390/genes13060977
Liu X, Liu D-H, Chen T, Zhang J, Wang C-L. Watercore Pear Fruit Respiration Changed and Accumulated γ-Aminobutyric Acid (GABA) in Response to Inner Hypoxia Stress. Genes. 2022; 13(6):977. https://doi.org/10.3390/genes13060977
Chicago/Turabian StyleLiu, Xiao, Dong-He Liu, Tao Chen, Jing Zhang, and Chun-Lei Wang. 2022. "Watercore Pear Fruit Respiration Changed and Accumulated γ-Aminobutyric Acid (GABA) in Response to Inner Hypoxia Stress" Genes 13, no. 6: 977. https://doi.org/10.3390/genes13060977






