Evaluation of the Effects of Different Sample Collection Strategies on DNA/RNA Co-Analysis of Forensic Stains
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Mock Stains
2.2. Collection of Mock Stains
2.3. Swab Storage
2.4. Isolation and Quantitation of Nucleic Acids
2.5. DNA Typing
2.6. mRNA Profiling and Body Fluid Classification
2.7. Statistical Analysis
3. Results
3.1. DNA and RNA Yields
3.2. DNA Profiling
3.3. mRNA Profiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roeder, A.D.; Haas, C. Body Fluid Identification Using MRNA Profiling. Methods Mol. Biol. Clifton NJ 2016, 1420, 13–31. [Google Scholar] [CrossRef]
- Sijen, T. Molecular Approaches for Forensic Cell Type Identification: On MRNA, MiRNA, DNA Methylation and Microbial Markers. Forensic Sci. Int. Genet. 2015, 18, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Salzmann, A.P.; Bamberg, M.; Courts, C.; Dørum, G.; Gosch, A.; Hadrys, T.; Hadzic, G.; Neis, M.; Schneider, P.M.; Sijen, T.; et al. MRNA Profiling of Mock Casework Samples: Results of a FoRNAP Collaborative Exercise. Forensic Sci. Int. Genet. 2021, 50, 102409. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shanthan, G.; Bouzga, M.M.; Thi Dinh, H.M.; Haas, C.; Fonneløp, A.E. Evaluating the Performance of Five Up-to-Date DNA/RNA Co-Extraction Methods for Forensic Application. Forensic Sci. Int. 2021, 328, 110996. [Google Scholar] [CrossRef]
- van Oorschot, R.A.; Ballantyne, K.N.; Mitchell, R.J. Forensic Trace DNA: A Review. Investig. Genet. 2010, 1, 14. [Google Scholar] [CrossRef]
- Thomasma, S.M.; Foran, D.R. The Influence of Swabbing Solutions on DNA Recovery from Touch Samples. J. Forensic Sci. 2013, 58, 465–469. [Google Scholar] [CrossRef]
- Bonsu, D.O.M.; Higgins, D.; Austin, J.J. Forensic Touch DNA Recovery from Metal Surfaces—A Review. Sci. Justice J. Forensic Sci. Soc. 2020, 60, 206–215. [Google Scholar] [CrossRef]
- Lacerenza, D.; Aneli, S.; Omedei, M.; Gino, S.; Pasino, S.; Berchialla, P.; Robino, C. A Molecular Exploration of Human DNA/RNA Co-Extracted from the Palmar Surface of the Hands and Fingers. Forensic Sci. Int. Genet. 2016, 22, 44–53. [Google Scholar] [CrossRef]
- van Oorschot, P.; Futlong, R.; Scarfo, G.; Holding, N.; Cummins, M. Are You Collecting All the Available DNA from Touched Objects? Int. Congr. Ser. 2003, 1239, 803–807. [Google Scholar] [CrossRef]
- Butler, J.M. U.S. Initiatives to Strengthen Forensic Science & International Standards in Forensic DNA. Forensic Sci. Int. Genet. 2015, 18, 4–20. [Google Scholar] [CrossRef][Green Version]
- Verdon, T.J.; Mitchell, R.J.; van Oorschot, R.A.H. Swabs as DNA Collection Devices for Sampling Different Biological Materials from Different Substrates. J. Forensic Sci. 2014, 59, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Setzer, M.; Juusola, J.; Ballantyne, J. Recovery and Stability of RNA in Vaginal Swabs and Blood, Semen, and Saliva Stains. J. Forensic Sci. 2008, 53, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Zubakov, D.; Hanekamp, E.; Kokshoorn, M.; van Ijcken, W.; Kayser, M. Stable RNA Markers for Identification of Blood and Saliva Stains Revealed from Whole Genome Expression Analysis of Time-Wise Degraded Samples. Int. J. Legal Med. 2008, 122, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.H.; Fanning, T.G.; Hultin, J.V.; Taubenberger, J.K. Origin and Evolution of the 1918 “Spanish” Influenza Virus Hemagglutinin Gene. Proc. Natl. Acad. Sci. USA 1999, 96, 1651–1656. [Google Scholar] [CrossRef]
- Fattorini, P.; Bonin, S.; Marrubini, G.; Bertoglio, B.; Grignani, P.; Recchia, E.; Pitacco, P.; Zupanič Pajnič, I.; Sorçaburu-Ciglieri, S.; Previderè, C. Highly Degraded RNA Can Still Provide Molecular Information: An in Vitro Approach. Electrophoresis 2020, 41, 386–393. [Google Scholar] [CrossRef]
- Sirker, M.; Schneider, P.M.; Gomes, I. A 17-Month Time Course Study of Human RNA and DNA Degradation in Body Fluids under Dry and Humid Environmental Conditions. Int. J. Legal Med. 2016, 130, 1431–1438. [Google Scholar] [CrossRef]
- Menke, S.; Gillingham, M.A.F.; Wilhelm, K.; Sommer, S. Home-Made Cost Effective Preservation Buffer Is a Better Alternative to Commercial Preservation Methods for Microbiome Research. Front. Microbiol. 2017, 8, 102. [Google Scholar] [CrossRef]
- Camacho-Sanchez, M.; Burraco, P.; Gomez-Mestre, I.; Leonard, J.A. Preservation of RNA and DNA from Mammal Samples under Field Conditions. Mol. Ecol. Resour. 2013, 13, 663–673. [Google Scholar] [CrossRef]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA Integrity Number for Assigning Integrity Values to RNA Measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef]
- van den Berge, M.; Bhoelai, B.; Harteveld, J.; Matai, A.; Sijen, T. Advancing Forensic RNA Typing: On Non-Target Secretions, a Nasal Mucosa Marker, a Differential Co-Extraction Protocol and the Sensitivity of DNA and RNA Profiling. Forensic Sci. Int. Genet. 2016, 20, 119–129. [Google Scholar] [CrossRef]
- Carnevali, E.; Lacerenza, D.; Severini, S.; Alessandrini, F.; Bini, C.; Di Nunzio, C.; Di Nunzio, M.; Fabbri, M.; Fattorini, P.; Piccinini, A.; et al. A GEFI Collaborative Exercise on DNA/RNA Co-Analysis and MRNA Profiling Interpretation. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e18–e20. [Google Scholar] [CrossRef]
- Lindenbergh, A.; Maaskant, P.; Sijen, T. Implementation of RNA Profiling in Forensic Casework. Forensic Sci. Int. Genet. 2013, 7, 159–166. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 16 March 2021).
- Bonsu, D.O.M.; Rodie, M.; Higgins, D.; Henry, J.; Austin, J.J. Comparison of IsohelixTM and Rayon Swabbing Systems for Touch DNA Recovery from Metal Surfaces. Forensic Sci. Med. Pathol. 2021, 17, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Bruijns, B.B.; Tiggelaar, R.M.; Gardeniers, H. The Extraction and Recovery Efficiency of Pure DNA for Different Types of Swabs. J. Forensic Sci. 2018, 63, 1492–1499. [Google Scholar] [CrossRef]
- Tang, J.; Ostrander, J.; Wickenheiser, R.; Hall, A. Touch DNA in Forensic Science: The Use of Laboratory-Created Eccrine Fingerprints to Quantify DNA Loss. Forensic Sci. Int. Synergy 2020, 2, 1–16. [Google Scholar] [CrossRef]
- Kanokwongnuwut, P.; Kirkbride, K.P.; Linacre, A. Detection of Latent DNA. Forensic Sci. Int. Genet. 2018, 37, 95–101. [Google Scholar] [CrossRef]
- Goray, M.; Mitchell, R.J.; van Oorschot, R.A.H. Investigation of Secondary DNA Transfer of Skin Cells under Controlled Test Conditions. Leg. Med. Tokyo Jpn. 2010, 12, 117–120. [Google Scholar] [CrossRef]
- Phetpeng, S.; Kitpipit, T.; Thanakiatkrai, P. Systematic Study for DNA Recovery and Profiling from Common IED Substrates: From Laboratory to Casework. Forensic Sci. Int. Genet. 2015, 17, 53–60. [Google Scholar] [CrossRef]
- Athanasio, C.G.; Chipman, J.K.; Viant, M.R.; Mirbahai, L. Optimisation of DNA Extraction from the Crustacean Daphnia. PeerJ 2016, 4, e2004. [Google Scholar] [CrossRef]
- Alaeddini, R.; Walsh, S.J.; Abbas, A. Forensic Implications of Genetic Analyses from Degraded DNA—A Review. Forensic Sci. Int. Genet. 2010, 4, 148–157. [Google Scholar] [CrossRef]
- Bonsu, D.O.M.; Higgins, D.; Henry, J.; Austin, J.J. Evaluation of the Efficiency of IsohelixTM and Rayon Swabs for Recovery of DNA from Metal Surfaces. Forensic Sci. Med. Pathol. 2021, 17, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Garvin, A.M.; Holzinger, R.; Berner, F.; Krebs, W.; Hostettler, B.; Lardi, E.; Hertli, C.; Quartermaine, R.; Stamm, C. The ForensiX Evidence Collection Tube and Its Impact on DNA Preservation and Recovery. BioMed Res. Int. 2013, 2013, 105797. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; Hanson, E.; Anjos, M.J.; Banemann, R.; Berti, A.; Borges, E.; Carracedo, A.; Carvalho, M.; Courts, C.; De Cock, G.; et al. RNA/DNA Co-Analysis from Human Saliva and Semen Stains--Results of a Third Collaborative EDNAP Exercise. Forensic Sci. Int. Genet. 2013, 7, 230–239. [Google Scholar] [CrossRef]
- Bowden, A.; Fleming, R.; Harbison, S. A Method for DNA and RNA Co-Extraction for Use on Forensic Samples Using the Promega DNA IQTM System. Forensic Sci. Int. Genet. 2011, 5, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Grabmüller, M.; Madea, B.; Courts, C. Comparative Evaluation of Different Extraction and Quantification Methods for Forensic RNA Analysis. Forensic Sci. Int. Genet. 2015, 16, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.; Muheim, C.; Kratzer, A.; Bär, W.; Maake, C. MRNA Profiling for the Identification of Sperm and Seminal Plasma. Forensic Sci. Int. Genet. Suppl. Ser. 2009, 2, 534–535. [Google Scholar] [CrossRef]
- Fleige, S.; Walf, V.; Huch, S.; Prgomet, C.; Sehm, J.; Pfaffl, M.W. Comparison of Relative MRNA Quantification Models and the Impact of RNA Integrity in Quantitative Real-Time RT-PCR. Biotechnol. Lett. 2006, 28, 1601–1613. [Google Scholar] [CrossRef]
- Lin, M.-H.; Albani, P.P.; Fleming, R. Degraded RNA Transcript Stable Regions (StaRs) as Targets for Enhanced Forensic RNA Body Fluid Identification. Forensic Sci. Int. Genet. 2016, 20, 61–70. [Google Scholar] [CrossRef]
- Allewell, N.M.; Sama, A. The Effect of Ammonium Sulfate on the Activity of Ribonuclease A. Biochim. Biophys. Acta 1974, 341, 484–488. [Google Scholar] [CrossRef]
- Fordyce, S.L.; Kampmann, M.-L.; van Doorn, N.L.; Gilbert, M.T.P. Long-Term RNA Persistence in Postmortem Contexts. Investig. Genet. 2013, 4, 7. [Google Scholar] [CrossRef]
- Bauer, M.; Polzin, S.; Patzelt, D. Quantification of RNA Degradation by Semi-Quantitative Duplex and Competitive RT-PCR: A Possible Indicator of the Age of Bloodstains? Forensic Sci. Int. 2003, 138, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Budowle, B.; Leggitt, J.L.; Defenbaugh, D.A.; Keys, K.M.; Malkiewicz, S.F. The Presumptive Reagent Fluorescein for Detection of Dilute Bloodstains and Subsequent STR Typing of Recovered DNA. J. Forensic Sci. 2000, 45, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Khan, P.; Idrees, D.; Moxley, M.A.; Corbett, J.A.; Ahmad, F.; von Figura, G.; Sly, W.S.; Waheed, A.; Hassan, M.I. Luminol-Based Chemiluminescent Signals: Clinical and Non-Clinical Application and Future Uses. Appl. Biochem. Biotechnol. 2014, 173, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Todd, A.R. Nucleotides. Part X. Some Observations on the Structure and Chemical Behaviour of the Nucleic Acids. J. Chem. Soc. 1952, 52–58. [Google Scholar] [CrossRef]
- Fabbri, M.; Frisoni, P.; Marini, C.; Gaudio, R.; Marti, M.; Meri, M. MRNA Profiling in Casework Analyses. J. Integr. OMICS 2020, 10, 19–21. [Google Scholar] [CrossRef]
- Creamer, J.I.; Quickenden, T.I.; Apanah, M.V.; Kerr, K.A.; Robertson, P. A Comprehensive Experimental Study of Industrial, Domestic and Environmental Interferences with the Forensic Luminol Test for Blood. Lumin. J. Biol. Chem. Lumin. 2003, 18, 193–198. [Google Scholar] [CrossRef]
- Hochmeister, M.N.; Budowle, B.; Sparkes, R.; Rudin, O.; Gehrig, C.; Thali, M.; Schmidt, L.; Cordier, A.; Dirnhofer, R. Validation Studies of an Immunochromatographic 1-Step Test for the Forensic Identification of Human Blood. J. Forensic Sci. 1999, 44, 597–602. [Google Scholar] [CrossRef]
- Plaza, D.T.; Mealy, J.L.; Lane, J.N.; Parsons, M.N.; Bathrick, A.S.; Slack, D.P. Nondestructive Biological Evidence Collection with Alternative Swabs and Adhesive Lifters. J. Forensic Sci. 2016, 61, 485–488. [Google Scholar] [CrossRef]
- Bleay, S.M.; Croxton, R.S.; De Puit, M. Introduction: Fingerprint Development Techniques: Theory and Application. In Fingerprint Development Techniques: Theory and Application; John Wiley & Sons Ltd.: Chichester, UK, 2018; pp. 1–500. ISBN 978-1-119-99261-5. [Google Scholar]
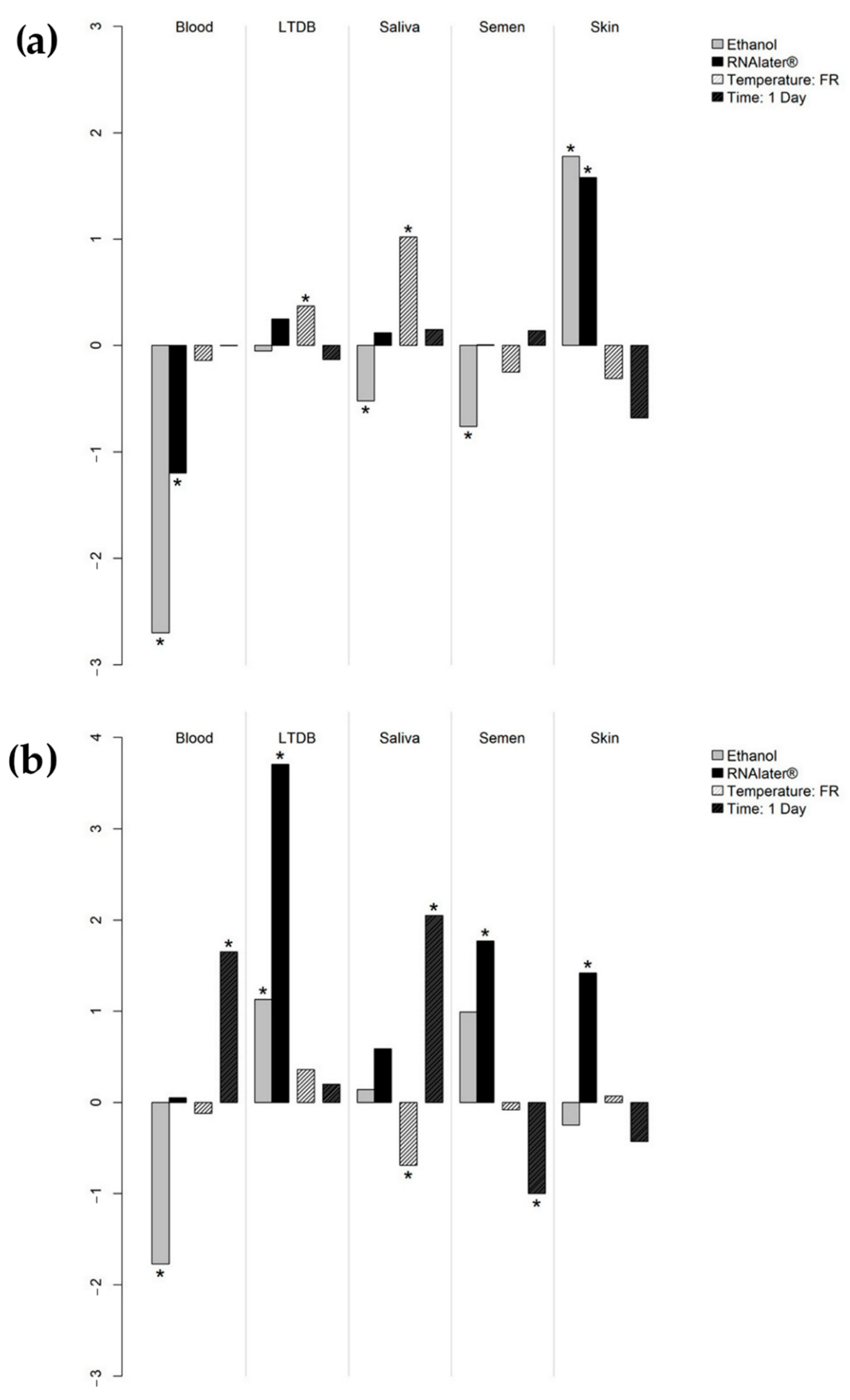
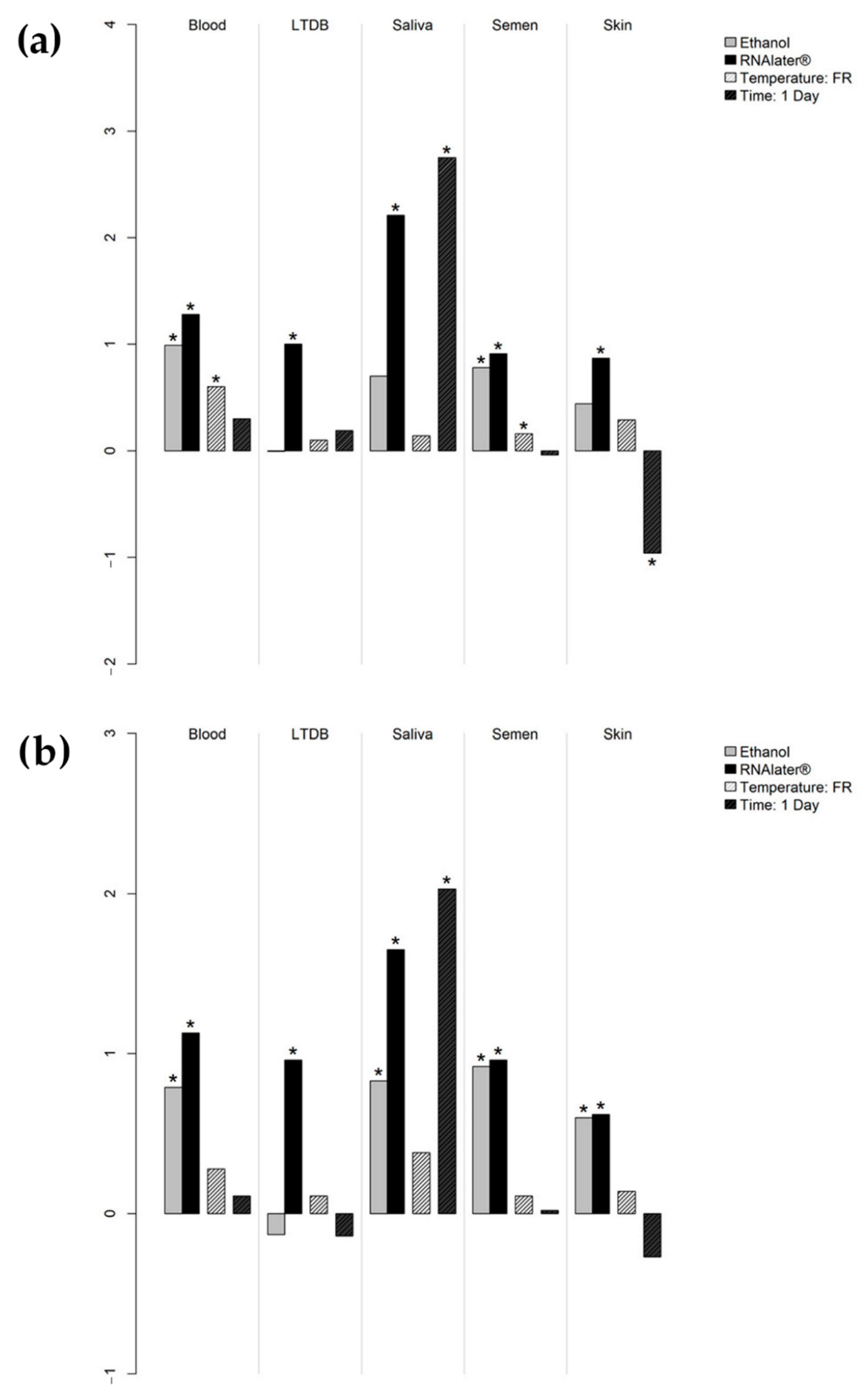
| Median DNA Concentration (ng/μL) | Post Hoc Test (Nemenyi Test) | ||
|---|---|---|---|
| Blood (n = 24) | |||
| Moistening Agent | |||
| Water (n = 8) | 4.727 [3.680–5.339] | Water vs. Ethanol | p < 0.001 |
| Ethanol (n = 8) | 0.166 [0.120–0.302] | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 1.320 [0.969–1.506] | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 1.106 [0.607–3.180] | RT vs. Frozen | ns |
| Frozen (n = 12) | 1.245 [0.308–4.113] | ||
| Storage time | |||
| 1 day (n = 12) | 1.105 [0.287–4.025] | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 1.115 [0.737–1.922] | ||
| Luminol-treated diluted blood (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 0.117 [0.063–0.131] | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 0.084 [0.065–0.137] | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 0.133 [0.122–0.149] | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 0.095 [0.063–0.128] | RT vs. Frozen | p = 0.019 |
| Frozen (n = 12) | 0.137 [0.103–0.169] | ||
| Storage time | |||
| 1 day (n = 12) | 0.103 [0.066–0.141] | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 0.129 [0.103–0.137] | ||
| Saliva (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 0.328 [0.220–0.578] | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 0.230 [0.135–0.320] | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 0.373 [0.198–0.542] | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 0.151 [0.127–0.245] | RT vs. Frozen | p < 0.001 |
| Frozen (n = 12) | 0.473 [0.336–0.578] | ||
| Storage time | |||
| 1 day (n = 12) | 0.296 [0.203–0.416] | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 0.305 [0.127–0.547] | ||
| Semen (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 23.020 [14.706–31.575] | Water vs. Ethanol | p = 0.029 |
| Ethanol (n = 8) | 9.114 [8.492–12.148] | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 22.770 [17.380–25.110] | Ethanol vs. RNAlater® | p = 0.022 |
| Storage temperature | |||
| RT (n = 12) | 19.545 [13.635–26.527] | RT vs. Frozen | ns |
| Frozen (n = 12) | 14.375 [9.045–20.015] | ||
| Storage time | |||
| 1 day (n = 12) | 19.990 [13.164–26.398] | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 14.330 [9.045–19.100] | ||
| Skin (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 0.007 [0.003–0.010] | Water vs. Ethanol | p = 0.014 |
| Ethanol (n = 8) | 0.038 [0.014–0.091] | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 0.020 [0.011–0.059] | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 0.011 [0.008–0.041] | RT vs. Frozen | ns |
| Frozen (n = 12) | 0.020 [0.011–0.048] | ||
| Storage time | |||
| 1 day (n = 12) | 0.012 [0.008–0.020] | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 0.028 [0.010–0.057] | ||
| Median RNA Concentration (ng/μL) | Post Hoc Test (Nemenyi Test) | ||
|---|---|---|---|
| Blood (n = 24) | |||
| Moistening Agent | |||
| Water (n = 8) | 0.023 [0.014–0.210] | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 0.013 [0.009–0.024] | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 0.127 [0.056–0.165] | Ethanol vs. RNAlater® | p = 0.020 |
| Storage temperature | |||
| RT (n = 12) | 0.029 [0.012–0.120] | RT vs. Frozen | ns |
| Frozen (n = 12) | 0.026 [0.015–0.073] | ||
| Storage time | |||
| 1 day (n = 12) | 0.078 [0.012–0.032] | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 0.023 [0.013–0.032] | ||
| Luminol-treated diluted Blood (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 0.027 [0.024–0.061] | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 0.096 [0.086–0.152] | Water vs. RNAlater® | p < 0.001 |
| RNAlater® (n = 8) | 1.669 [0.882–2.268] | Ethanol vs. RNAlater® | p = 0.047 |
| Storage temperature | |||
| RT (n = 12) | 0.095 [0.042–0.882] | RT vs. Frozen | ns |
| Frozen (n = 12) | 0.152 [0.066–0.267] | ||
| Storage time | |||
| 1 day (n = 12) | 0.077 [0.041–1.849] | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 0.103 [0.073–0.267] | ||
| Saliva (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 0.155 [0.073–0.230] | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 0.103 [0.039–0.178] | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 0.062 [0.041–0.553] | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 0.110 [0.044–0.383] | RT vs. Frozen | ns |
| Frozen (n = 12) | 0.094 [0.043–0.187] | ||
| Storage time | |||
| 1 day (n = 12) | 0.223 [0.142–0.334] | 1 day vs. 7 days | p < 0.001 |
| 7 days (n = 12) | 0.040 [0.024–0.047] | ||
| Semen (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 0.555 [0.353–2.788] | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 3.278 [1.942–4.146] | Water vs. RNAlater® | p = 0.039 |
| RNAlater® (n = 8) | 5.574 [4.696–6.487] | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 4.186 [0.973–6.487] | RT vs. Frozen | ns |
| Frozen (n = 12) | 3.278 [1.125–4.878] | ||
| Storage time | |||
| 1 day (n = 12) | 1.696 [0.422–4.783] | 1 day vs. 7 days | p = 0.045 |
| 7 days (n = 12) | 5.005 [3.605–6.701] | ||
| Skin (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 0.111 [0.064–0.128] | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 0.109 [0.056–0.141] | Water vs. RNAlater® | p = 0.004 |
| RNAlater® (n = 8) | 0.415 [0.364–0.464] | Ethanol vs. RNAlater® | p = 0.005 |
| Storage temperature | |||
| RT (n = 12) | 0.136 [0.097–0.366] | RT vs. Frozen | ns |
| Frozen (n = 12) | 0.193 [0.087–0.364] | ||
| Storage time | |||
| 1 day (n = 12) | 0.122 [0.077–0.391] | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 0.171 [0.120–0.360] | ||
| Expected Tissue “Observed” | Fisher’s Exact Test | ||
|---|---|---|---|
| Blood (n = 24) | |||
| Moistening Agent | |||
| Water (n = 8) | 37.5% | Water vs. Ethanol | p = 0.04 |
| Ethanol (n = 8) | 100% | Water vs. RNAlater® | p = 0.04 |
| RNAlater® (n = 8) | 100% | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 75.0% | RT vs. Frozen | ns |
| Frozen (n = 12) | 83.3% | ||
| Storage time | |||
| 1 day (n = 12) | 83.3% | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 75.0% | ||
| Luminol-treated diluted Blood (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 100% | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 100% | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 87.5% | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 91.7% | RT vs. Frozen | ns |
| Frozen (n = 12) | 100% | ||
| Storage time | |||
| 1 day (n = 12) | 100% | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 91.7% | ||
| Saliva (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 37.5% | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 50% | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 87.5% | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 50% | RT vs. Frozen | ns |
| Frozen (n = 12) | 66.7% | ||
| Storage time | |||
| 1 day (n = 12) | 91.7% | 1 day vs. 7 days | p = 0.003 |
| 7 days (n = 12) | 25.0% | ||
| Semen (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 87.5% | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 100% | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 100% | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 100% | RT vs. Frozen | ns |
| Frozen (n = 12) | 91.7% | ||
| Storage time | |||
| 1 day (n = 12) | 100% | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 91.7% | ||
| Skin (n = 24) | |||
| Moistening agent | |||
| Water (n = 8) | 75.0% | Water vs. Ethanol | ns |
| Ethanol (n = 8) | 75.0% | Water vs. RNAlater® | ns |
| RNAlater® (n = 8) | 100% | Ethanol vs. RNAlater® | ns |
| Storage temperature | |||
| RT (n = 12) | 75% | RT vs. Frozen | ns |
| Frozen (n = 12) | 91.7% | ||
| Storage time | |||
| 1 day (n = 12) | 75% | 1 day vs. 7 days | ns |
| 7 days (n = 12) | 91.7% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lacerenza, D.; Caudullo, G.; Chierto, E.; Aneli, S.; Di Vella, G.; Barberis, M.; Voyron, S.; Berchialla, P.; Robino, C. Evaluation of the Effects of Different Sample Collection Strategies on DNA/RNA Co-Analysis of Forensic Stains. Genes 2022, 13, 983. https://doi.org/10.3390/genes13060983
Lacerenza D, Caudullo G, Chierto E, Aneli S, Di Vella G, Barberis M, Voyron S, Berchialla P, Robino C. Evaluation of the Effects of Different Sample Collection Strategies on DNA/RNA Co-Analysis of Forensic Stains. Genes. 2022; 13(6):983. https://doi.org/10.3390/genes13060983
Chicago/Turabian StyleLacerenza, Daniela, Giorgio Caudullo, Elena Chierto, Serena Aneli, Giancarlo Di Vella, Marco Barberis, Samuele Voyron, Paola Berchialla, and Carlo Robino. 2022. "Evaluation of the Effects of Different Sample Collection Strategies on DNA/RNA Co-Analysis of Forensic Stains" Genes 13, no. 6: 983. https://doi.org/10.3390/genes13060983
APA StyleLacerenza, D., Caudullo, G., Chierto, E., Aneli, S., Di Vella, G., Barberis, M., Voyron, S., Berchialla, P., & Robino, C. (2022). Evaluation of the Effects of Different Sample Collection Strategies on DNA/RNA Co-Analysis of Forensic Stains. Genes, 13(6), 983. https://doi.org/10.3390/genes13060983







