A Possible Cause for the Differential Expression of a Subset of miRNAs in Mesenchymal Stem Cells Derived from Myometrium and Leiomyoma
Abstract
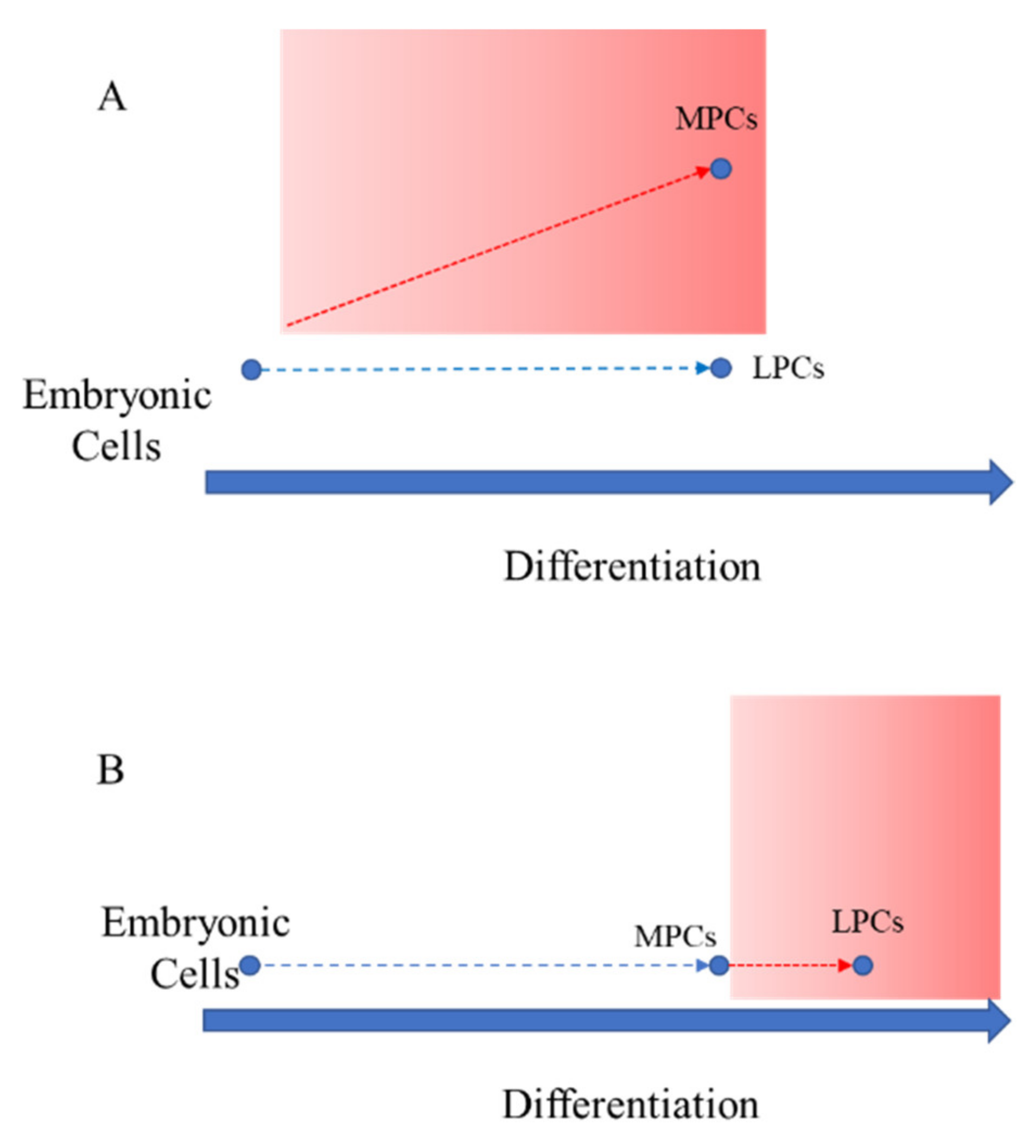
:1. Introduction
2. Materials and Methods
2.1. Tissue Collection
2.2. miRNA Profiling
2.3. Bioinformatics Analysis
2.4. miRNAs Targets Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciavattini, A.; Di Giuseppe, J.; Stortoni, P.; Montik, N.; Giannubilo, S.R.; Litta, P.; Islam, M.; Tranquilli, A.L.; Reis, F.M.; Ciarmela, P. Uterine fibroids: Pathogenesis and interactions with endometrium and endomyometrial junction. Obstet. Gynecol. Int. 2013, 2013, 173184. [Google Scholar] [CrossRef] [PubMed]
- Flake, G.; Andersen, J.; Dixon, D. Etiology and pathogenesis of uterine leiomyomas: A review. Environ. Health Perspect. 2003, 111, 1037–1054. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Qiang, W.; Serna, V.A.; Yin, P.; Coon, J.S.; Navarro, A.; Monsivais, D.; Kakinuma, T.; Dyson, M.; Druschitz, S.; et al. Role of stem cells in human uterine leiomyoma growth. PLoS ONE 2012, 7, e36935. [Google Scholar] [CrossRef]
- Orciani, M.; Caffarini, M.; Biagini, A.; Lucarini, G.; Delli Carpini, G.; Berretta, A.; Di Primio, R.; Ciavattini, A. Cronic Inflammation May Enhance Leiomyoma Development by the Involvement of Progenitor Cells. Stem Cells Int. 2018, 2018, 1716246. [Google Scholar] [CrossRef]
- Lazzarini, R.; Caffarini, M.; Delli Carpini, G.; Ciavattini, A.; Di Primio, R.; Orciani, M. From 2646 to 15: Differentially regulated microRNAs between progenitors from normal myometrium and leiomyoma. Am. J. Obstet. Gynecol. 2020, 222, 596.e1–596.e9. [Google Scholar] [CrossRef]
- Kørbling, M.; Estrov, Z. Adult stem cells for tissue repair—A new therapeutic concept? N. Engl. J. Med. 2003, 349, 570–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues World J. Stem Cells 2019, 11, 347–374. [Google Scholar]
- Borlongan, C.V. Amniotic fluid as a source of engraftable stem cells. Brain Circ. 2017, 3, 175–179. [Google Scholar] [CrossRef]
- Choi, E.; Choi, E.; Hwang, K.C. MicroRNAs as novel regulators of stem cell fate. World J. Stem Cells 2013, 5, 172–187. [Google Scholar] [CrossRef]
- Carbonell, T.; Gomes, A.V. MicroRNAs in the regulation of cellular redox status and its implications in myocardial ischemia-reperfusion injury. Redox Biol. 2020, 36, 101607. [Google Scholar] [CrossRef] [PubMed]
- Orciani, M.; Morabito, C.; Emanuelli, M.; Guarnieri, S.; Sartini, D.; Giannubilo, S.R.; Di Primio, R.; Tranquilli, A.L.; Mariggiò, M.A. Neurogenic potential of mesenchymal-like stem cells from human amniotic fluid: The influence of extracellular growth factors. J. Biol. Regul. Homeost. Agents 2011, 25, 115–130. [Google Scholar] [PubMed]
- Orciani, M.; Emanuelli, M.; Martino, C.; Pugnaloni, A.; Tranquilli, A.L.; Di Primio, R. Potential role of culture mediums for successful isolation and neuronal differentiation of amniotic fluid stem cells. Int. J. Immunopath. Pharmacol. 2008, 21, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Orciani, M.; Sorgentoni, G.; Consales, V.; Offidani, A.; Di Primio, R. Pathogenetic characteristics of mesenchymal stem cells in hidradenitis suppurativa. JAMA Dermatol. 2018, 154, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, M.; Di Vincenzo, M.; Caffarini, M.; Mei, F.; Salati, M.; Zuccatosta, L.; Refai, M.; Mattioli-Belmonte, M.; Gasparini, S.; Orciani, M. How the Pathological Microenvironment Affects the Behavior of Mesenchymal Stem Cells in the Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 8140. [Google Scholar] [CrossRef]
- Orciani, M.; Caffarini, M.; Lazzarini, R.; Delli Carpini, G.; Tsiroglou, D.; Di Primio, R.; Ciavattini, A. Mesenchymal Stem Cells from Cervix and Age: New Insights into CIN Regression Rate. Oxid. Med. Cell. Longev. 2018, 2018, 1545784. [Google Scholar] [CrossRef] [Green Version]
- Halfon, S.; Abramov, N.; Grinblat, B.; Ginis, I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011, 20, 53–66. [Google Scholar] [CrossRef]
- FastQC Software. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 20 April 2020).
- MiRbase. Available online: https://www.mirbase.org/ (accessed on 27 April 2020).
- DIANA-mirPath. Available online: http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=site/page&view=software (accessed on 11 May 2020).
- mirTarBase. Available online: https://mirtarbase.cuhk.edu.cn/ (accessed on 18 May 2020).
- STRING: Functional Protein Association Networks. Available online: https://string-db.org/ (accessed on 8 June 2020).
- Malik, M.; Segars, J.; Catherino, W.H. Integrin β1 regulates leiomyoma cytoskeletal integrity and growth. Matrix Biol. 2012, 31, 389–397. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.S.; Ciavattini, A.; Petraglia, F.; Castellucci, M.; Ciarmela, P. Extracellular matrix in uterine leiomyoma pathogenesis: A potential target for future therapeutics. Hum. Reprod. Update 2018, 24, 59–85. [Google Scholar] [CrossRef] [Green Version]
- Leppert, P.C.; Baginski, T.; Prupas, C.; Catherino, W.H.; Pletcher, S.; Segars, J.H. Comparative ultrastructure of collagen fibrils in uterine leiomyoma and normal myometrium. Fertil. Steril. 2004, 82, 1182–1187. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.Y.; Shin, J.H.; Kim, H.; Ku, S.Y.; Suh, C.S. Variation in MicroRNA Expression Profile of Uterine Leiomyoma with Endometrial Cavity Distortion and Endometrial Cavity Non-Distortion. Int. J. Mol. Sci. 2018, 19, 2524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, T.D.; Khorram, O. Expression profiling of lncRNAs, miRNAs, and mRNAs and their differential expression in leiomyoma using next-generation RNA sequencing. Reprod. Sci. 2018, 25, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Luo, X.; Chegini, N. MicroRNA 21: Response to hormonal therapies and regulatory function in leiomyoma, transformed leiomyoma and leiomyosarcoma cells. Mol. Hum. Reprod. 2010, 16, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Prowse, A.B.J.; Chong, F.; Gray, P.P.; Munro, T.P. Stem cell integrins: Implications for ex-vivo culture and cellular therapies. Stem Cell Res. 2011, 6, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefanidis, K.; Pergialiotis, V.; Christakis, D.; Loutradis, D.; Antsaklis, A. Nodal, Nanog, DAZL and SMAD gene expression in human amniotic fluid stem cells. J. Stem Cells 2013, 8, 17–23. [Google Scholar]
- Norian, J.M.; Malik, M.; Parker, C.Y.; Joseph, D.; Leppert, P.C.; Segars, J.H.; Catherino, W.H. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod. Sci. 2009, 16, 1153–1164. [Google Scholar] [CrossRef] [Green Version]
- Ciebiera, M.; Włodarczyk, M.; Wrzosek, M.; Męczekalski, B.; Nowicka, G.; Łukaszuk, K.; Ciebiera, M.; Słabuszewska-Jóźwiak, A.; Jakiel, G. Role of Transforming Growth Factor β in Uterine Fibroid Biology. Int. J. Mol. Sci. 2017, 18, 2435. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.S.; Nowak, R.A. Human leiomyoma smooth muscle cells show increased expression of transforming growth factor-β3 (TGF-β3) and altered responses to the antiproliferative effects of TGF-β. J. Clin. Endocrinol. Metab. 2001, 86, 913–920. [Google Scholar]
- Ciebiera, M.; Wlodarczyk, M.; Slabuszewska-Jozwiak, A.; Nowicka, G.; Jakiel, G. Influence of vitamin D and transforming growth factor β3 serum concentrations, obesity, and family history on the risk for uterine fibroids. Fertil. Steril. 2016, 106, 1787–1792. [Google Scholar] [CrossRef] [Green Version]
- Joseph, D.S.; Malik, M.; Nurudeen, S.; Catherino, W.H. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor β-3. Fertil. Steril. 2010, 93, 1500–1508. [Google Scholar] [CrossRef]
- Nakao, A.; Afrakhte, M.; Moren, A.; Nakayama, T.; Christian, J.L.; Heuchel, R.; Kawabata, M.; Heldin, N.E.; Heldin, C.H.; Dijke, P. Identification of SMAD7, a TGFβ-inducible antagonist of TGF-β signalling. Nature 1997, 389, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.B.; Sporn, M.B. Physiological actions and clinical applications of transforming growth factor-β (TGF-β). Growth Factors 1993, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Huang, S.S.; Huang, J.S. TGF-b Control of Cell Proliferation. J. Cell Biochem. 2005, 96, 447–462. [Google Scholar] [CrossRef]
- Busnelli, M.; Rimoldi, V.; Viganò, P.; Persani, L.; Di Blasio, A.M.; Chini, B. Oxytocin-induced cell growth proliferation in human myometrial cells and leiomyomas. Fertil. Steril. 2010, 94, 1869–1874. [Google Scholar] [CrossRef]
- Zavadil, J.; Ye, H.; Liu, Z.; Wu, J.; Lee, P.; Hernando, E.; Soteropoulos, P.; Toruner, G.A.; Wei, J.J. Profiling and functional analyses of microRNAs and their target gene products in human uterine leiomyomas. PLoS ONE 2010, 5, e12362. [Google Scholar] [CrossRef]
- Medikare, V.; Kandukuri, L.R.; Ananthapur, V.; Deenadayal, M.; Nallari, P. The genetic bases of uterine fibroids; a review. J. Reprod. Infertil. 2011, 12, 181–191. [Google Scholar]
- Mäkinen, N.; Mehine, M.; Tolvanen, J.; Kaasinen, E.; Li, Y.; Lehtonen, H.J.; Gentile, M.; Yan, J.; Enge, M.; Taipale, M.; et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science 2011, 334, 252–255. [Google Scholar] [CrossRef]
- Ravegnini, G.; Mariño-Enriquez, A.; Slater, J.; Eilers, G.; Wang, Y.; Zhu, M.; Nucci, M.R.; George, S.; Angelini, S.; Raut, C.P.; et al. MED12 mutations in leiomyosarcoma and extrauterine leiomyoma. Mod. Pathol. 2013, 26, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Klemke, M.; Meyer, A.; Nezhad, M.H.; Bartnitzke, S.; Drieschner, N.; Frantzen, C.; Schmidt, E.H.; Belge, G.; Bullerdiek, J. Overexpression of HMGA2 in uterine leiomyomas points to its general role for the pathogenesis of the disease. Genes Chromosomes Cancer 2009, 48, 171–178. [Google Scholar] [CrossRef]
- Ligon, A.L.; Scott, J.A.; Takahara, K.; Greenspan, D.S.; Morton, C.C. PCOLCE deletion and expression analyses in uterine leiomyomata. Cancer Genet. Cytogenet. 2002, 137, 133–137. [Google Scholar] [CrossRef]





| KEGG Pathway | miRNA | TARGET | Validation Methods | ||||
|---|---|---|---|---|---|---|---|
| Strong Evidence | Less Strong Evidence | ||||||
| Reporter Assay | Western Blot | qPCR | Microarray | NGS | |||
| Adherens junction | hsa-miR-10a-5p | ACTG1 | • | • | • | ||
| YES1 | • | ||||||
| CTNND1 | • | ||||||
| MAP3K7 | • | • | • | • | |||
| hsa-miR-200a-3p | CTNNB1 | • | • | • | |||
| TCF7L1 | • | ||||||
| ECM- receptor interaction | hsa-miR-335-3p | COL4A1 | • | ||||
| hsa-miR-335-5p | CD36 | • | |||||
| COL6A1 | • | ||||||
| COL6A5 | • | ||||||
| COL6A6 | • | ||||||
| GP9 | • | ||||||
| HSPG2 | • | ||||||
| ITGA1 | • | ||||||
| ITGA10 | • | ||||||
| ITGA2 | • | ||||||
| ITGB4 | • | ||||||
| ITGB5 | • | ||||||
| ITGB6 | • | ||||||
| ITGB8 | • | ||||||
| LAMA5 | • | ||||||
| LAMB1 | • | ||||||
| TNC | • | • | • | ||||
| VTN | • | ||||||
| THBS3 | • | ||||||
| SPP1 | • | ||||||
| TGF-β | hsa-miR-122-5p | NODAL | • | ||||
| RBL1 | • | ||||||
| RPS6KB1 | • | ||||||
| SMURF2 | • | ||||||
| Cell cycle | hsa-miR-146a-5p | CDKN1A | • | ||||
| SMAD4 | • | • | • | • | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Vincenzo, M.; De Quattro, C.; Rossato, M.; Lazzarini, R.; Delli Carpini, G.; Ciavattini, A.; Orciani, M. A Possible Cause for the Differential Expression of a Subset of miRNAs in Mesenchymal Stem Cells Derived from Myometrium and Leiomyoma. Genes 2022, 13, 1106. https://doi.org/10.3390/genes13071106
Di Vincenzo M, De Quattro C, Rossato M, Lazzarini R, Delli Carpini G, Ciavattini A, Orciani M. A Possible Cause for the Differential Expression of a Subset of miRNAs in Mesenchymal Stem Cells Derived from Myometrium and Leiomyoma. Genes. 2022; 13(7):1106. https://doi.org/10.3390/genes13071106
Chicago/Turabian StyleDi Vincenzo, Mariangela, Concetta De Quattro, Marzia Rossato, Raffaella Lazzarini, Giovanni Delli Carpini, Andrea Ciavattini, and Monia Orciani. 2022. "A Possible Cause for the Differential Expression of a Subset of miRNAs in Mesenchymal Stem Cells Derived from Myometrium and Leiomyoma" Genes 13, no. 7: 1106. https://doi.org/10.3390/genes13071106
APA StyleDi Vincenzo, M., De Quattro, C., Rossato, M., Lazzarini, R., Delli Carpini, G., Ciavattini, A., & Orciani, M. (2022). A Possible Cause for the Differential Expression of a Subset of miRNAs in Mesenchymal Stem Cells Derived from Myometrium and Leiomyoma. Genes, 13(7), 1106. https://doi.org/10.3390/genes13071106









