Methionine Adenosyltransferase I/III Deficiency Detected by Newborn Screening
Abstract
1. Introduction
2. Materials and Methods
3. Results
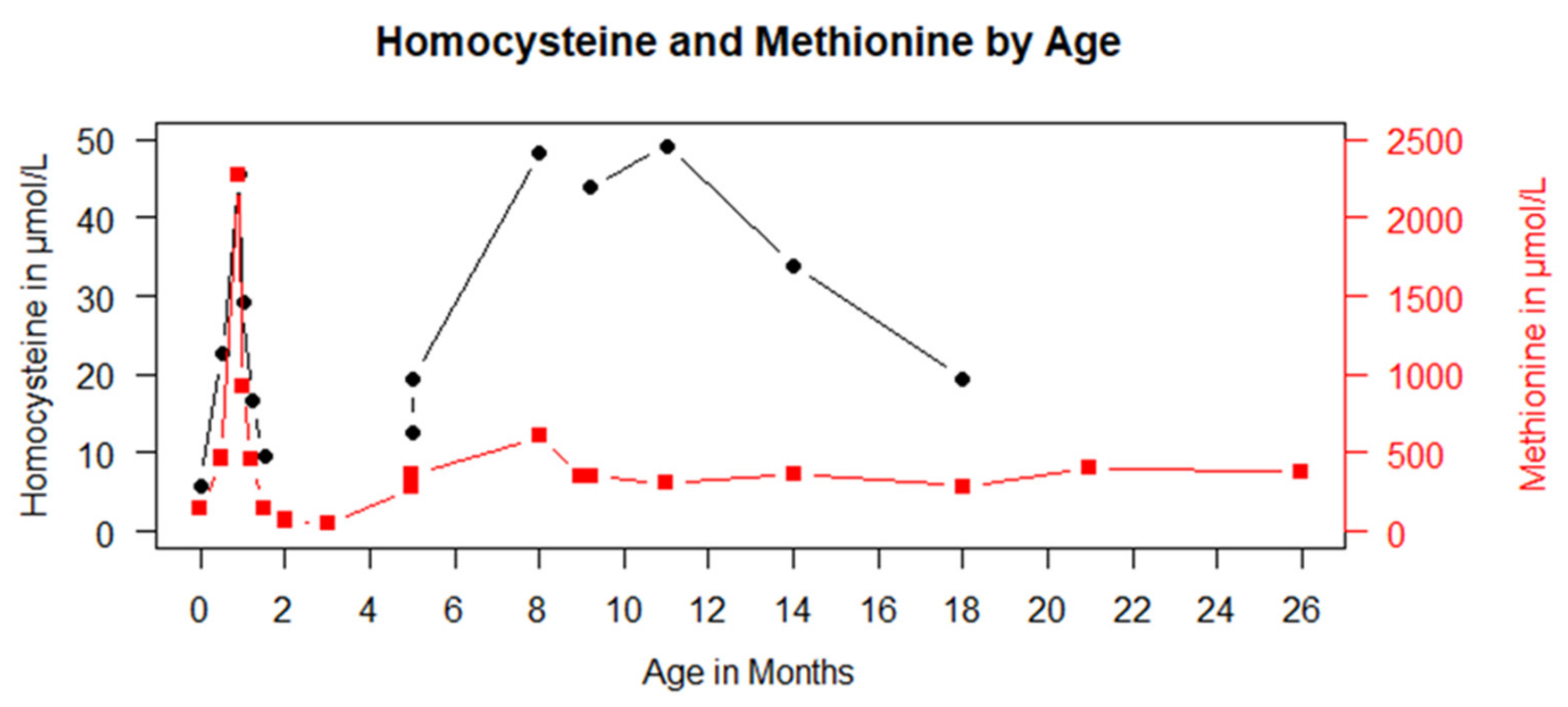
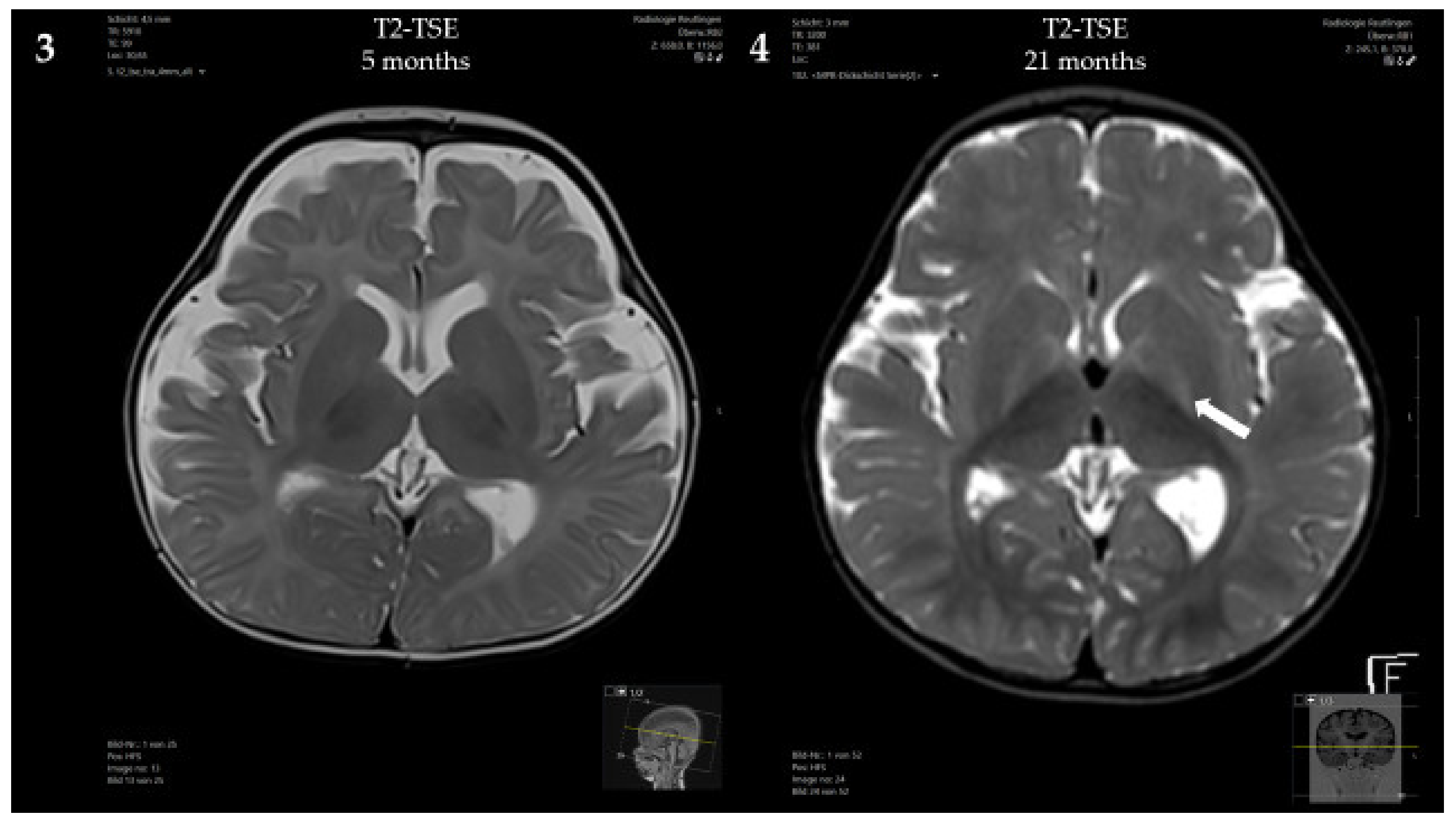
3.1. Case Presentation
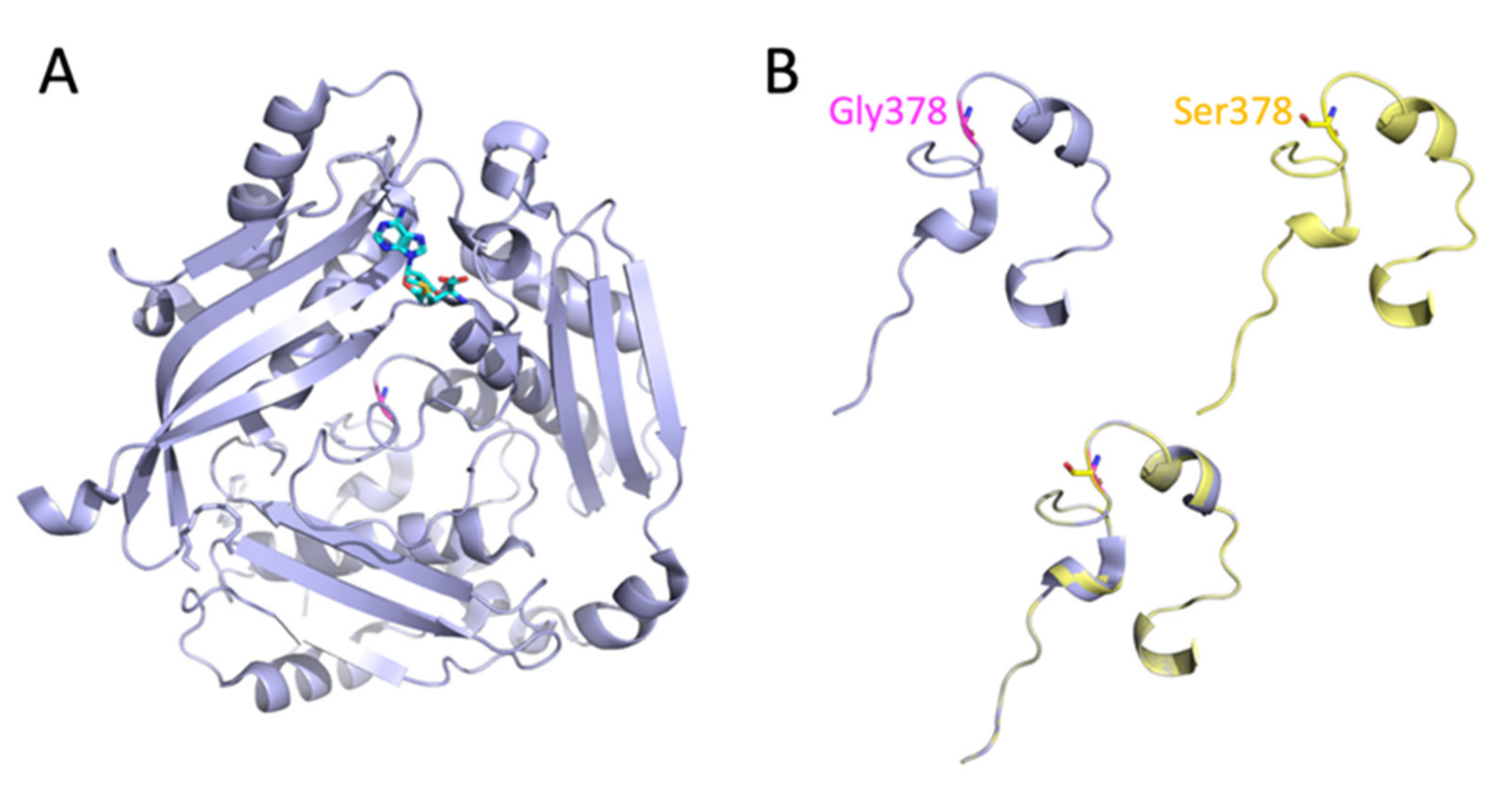
3.2. Analysis of the MAT1A Protein Structure
3.3. Predicted Effect of the Homozygous c.1132G>A; p.Gly378Ser Mutation on the Enzymatic Activity of MAT I/III
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.M.; Kim, J.H.; Choi, J.H.; Kim, G.H.; Kim, J.M.; Kang, M.; Choi, I.H.; Cheon, C.K.; Sohn, Y.B.; Maccarana, M.; et al. Determination of Autosomal Dominant or Recessive Methionine Adenosyltransferase I/III Deficiencies Based on Clinical and Molecular Studies. Mol. Med. 2016, 22, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Mudd, S.H. Hypermethioninemias of genetic and non-genetic origin: A review. Am. J. Med. Genet. C Semin. Med. Genet. 2011, 157, 3–32. [Google Scholar] [CrossRef] [PubMed]
- Lauinger, L.; Kaiser, P. Sensing and Signaling of Methionine Metabolism. Metabolites 2021, 11, 83. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-H.; Abdenur, J.E.; Baronio, F.; Bannick, A.A.; Corrales, F.; Couce, M.; Donner, M.G.; Ficicioglu, C.; Freehauf, C.; Frithiof, D.; et al. Mudd’s disease (MAT I/III deficiency): A survey of data for MAT1A homozygotes and compound heterozygotes. Orphanet J. Rare Dis. 2015, 10, 99. [Google Scholar] [CrossRef]
- Nashabat, M.; Al-Khenaizan, S.; Alfadhel, M. Methionine adenosyltransferase I/III deficiency: Beyond the central nervous system manifestations. Ther. Clin. Risk Manag. 2018, 14, 225–229. [Google Scholar] [CrossRef]
- Surtees, R.; Leonard, J.; Austin, S. Association of demyelination with deficiency of cerebrospinal-fluid S-adenosylmethionine in inborn errors of methyl-transfer pathway. Lancet 1991, 338, 1550–1554. [Google Scholar] [CrossRef]
- Couce, M.L.; Bóveda, M.D.; Castiñeiras, D.E.; Corrales, F.J.; Mora, M.I.; Fraga, J.M.; Mudd, S.H. Hypermethioninaemia due to methionine adenosyltransferase I/III (MAT I/III) deficiency: Diagnosis in an expanded neonatal screening programme. J. Inherit. Metab. Dis. 2008, 31 (Suppl. S2), S233–S239. [Google Scholar] [CrossRef][Green Version]
- Couce, M.L.; Bóveda, M.D.; García-Jimémez, C.; Balmaseda, E.; Vives, I.; Castiñeiras, D.E.; Fernández-Marmiesse, A.; Fraga, J.M.; Mudd, S.H.; Corrales, F.J. Clinical and metabolic findings in patients with methionine adenosyltransferase I/III deficiency detected by newborn screening. Mol. Genet. Metab. 2013, 110, 218–221. [Google Scholar] [CrossRef]
- Martins, E.; Marcão, A.; Bandeira, A.; Fonseca, H.; Nogueira, C.; Vilarinho, L. Methionine Adenosyltransferase I/III Deficiency in Portugal: High Frequency of a Dominantly Inherited Form in a Small Area of Douro High Lands. JIMD Rep. 2012, 6, 107–112. [Google Scholar]
- Furujo, M.; Kinoshita, M.; Nagao, M.; Kubo, T. Methionine adenosyltransferase I/III deficiency: Neurological manifestations and relevance of S-adenosylmethionine. Mol. Genet. Metab. 2012, 107, 253–256. [Google Scholar] [CrossRef]
- Barić, I.; Staufner, C.; Augoustides-Savvopoulou, P.; Chien, Y.-H.; Dobbelaere, D.; Grünert, S.C.; Opladen, T.; Ramadža, D.P.; Rakić, B.; Wedell, A.; et al. Consensus recommendations for the diagnosis, treatment and follow-up of inherited methylation disorders. J. Inherit. Metab. Dis. 2017, 40, 5–20. [Google Scholar] [CrossRef]
- Shafqat, N.; Muniz, J.R.C.; Pilka, E.S.; Papagrigoriou, E.; von Delft, F.; Oppermann, U.; Yue, W.W. Insight into S-adenosylmethionine biosynthesis from the crystal structures of the human methionine adenosyltransferase catalytic and regulatory subunits. Biochem. J. 2013, 452, 27–36. [Google Scholar] [CrossRef]
- Markham, G.D.; Pajares, M.A. Structure-function relationships in methionine adenosyltransferases. Cell. Mol. Life Sci. 2009, 66, 636–648. [Google Scholar] [CrossRef]
- Houry, W.A.; Frishman, D.; Eckerskorn, C.; Lottspeich, F.; Hartl, F.U. Identification of in vivo substrates of the chaperonin GroEL. Nature 1999, 402, 147–154. [Google Scholar] [CrossRef]
- Chamberlin, M.E.; Ubagai, T.; Mudd, S.H.; Wilson, W.G.; Leonard, J.V.; Chou, J.Y. Demyelination of the brain is associated with methionine adenosyltransferase I/III deficiency. J. Clin. Investig. 1996, 98, 1021–1027. [Google Scholar] [CrossRef]
- Braverman, N.E.; Mudd, S.H.; Barker, P.B.; Pomper, M.G. Characteristic MR imaging changes in severe hypermethioninemic states. AJNR Am. J. Neuroradiol. 2005, 26, 2705–2706. [Google Scholar]
- Tada, H.; Takanashi, J.; Barkovich, A.J.; Yamamoto, S.; Kohno, Y. Reversible white matter lesion in methionine adenosyltransferase I/III deficiency. AJNR Am. J. Neuroradiol. 2004, 25, 1843–1845. [Google Scholar]
- Mudd, S.H.; Braverman, N.; Pomper, M.; Tezcan, K.; Kronick, J.; Jayakar, P.; Garganta, C.; Ampola, M.G.; Levy, H.L.; McCandless, S.E.; et al. Infantile hypermethioninemia and hyperhomocysteinemia due to high methionine intake: A diagnostic trap. Mol. Genet. Metab. 2003, 79, 6–16. [Google Scholar] [CrossRef]
- Young, S.N.; Shalchi, M. The effect of methionine and S-adenosylmethionine on S-adenosylmethionine levels in the rat brain. J. Psychiatry Neurosci. 2005, 30, 44–48. [Google Scholar]
- Chamberlin, M.E.; Ubagai, T.; Mudd, S.H.; Thomas, J.; Pao, V.Y.; Nguyen, T.K.; Levy, H.L.; Greene, C.; Freehauf, C.; Chou, J.Y. Methionine Adenosyltransferase I/III Deficiency: Novel Mutations and Clinical Variations. Am. J. Hum. Genet. 2000, 66, 347–355. [Google Scholar] [CrossRef]
- Inoue, K.; Khajavi, M.; Ohyama, T.; Hirabayashi, S.-I.; Wilson, J.H.; Reggin, J.D.; Mancias, P.; Butler, I.J.; Wilkinson, M.F.; Wegner, M.; et al. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat. Genet. 2004, 36, 361–369. [Google Scholar] [CrossRef]
- Kim, S.Z.; Santamaria, E.; Jeong, T.E.; Levy, H.L.; Mato, J.M.; Corrales, F.J.; Mudd, S.H. Methionine adenosyltransferase I/III deficiency: Two Korean compound heterozygous siblings with a novel mutation. J. Inherit. Metab. Dis. 2003, 25, 661–671. [Google Scholar] [CrossRef]
- Cohen, B.M.; Renshaw, P.F.; Stoll, A.L.; Wurtman, R.J.; Yurgelun-Todd, D.; Babb, S.M. Decreased brain choline uptake in older adults. An in vivo proton magnetic resonance spectroscopy study. JAMA 1995, 274, 902–907. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hübner, V.; Hannibal, L.; Janzen, N.; Grünert, S.C.; Freisinger, P. Methionine Adenosyltransferase I/III Deficiency Detected by Newborn Screening. Genes 2022, 13, 1163. https://doi.org/10.3390/genes13071163
Hübner V, Hannibal L, Janzen N, Grünert SC, Freisinger P. Methionine Adenosyltransferase I/III Deficiency Detected by Newborn Screening. Genes. 2022; 13(7):1163. https://doi.org/10.3390/genes13071163
Chicago/Turabian StyleHübner, Vanessa, Luciana Hannibal, Nils Janzen, Sarah Catharina Grünert, and Peter Freisinger. 2022. "Methionine Adenosyltransferase I/III Deficiency Detected by Newborn Screening" Genes 13, no. 7: 1163. https://doi.org/10.3390/genes13071163
APA StyleHübner, V., Hannibal, L., Janzen, N., Grünert, S. C., & Freisinger, P. (2022). Methionine Adenosyltransferase I/III Deficiency Detected by Newborn Screening. Genes, 13(7), 1163. https://doi.org/10.3390/genes13071163






