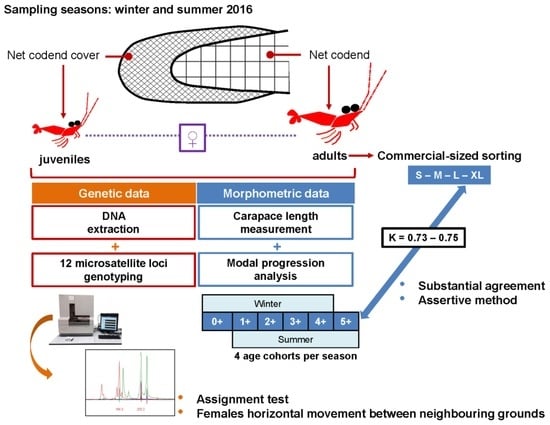
Genetic Demography of the Blue and Red Shrimp, Aristeus antennatus: A Female-Based Case Study Integrating Multilocus Genotyping and Morphometric Data
Abstract
:1. Introduction
2. Materials and Methods
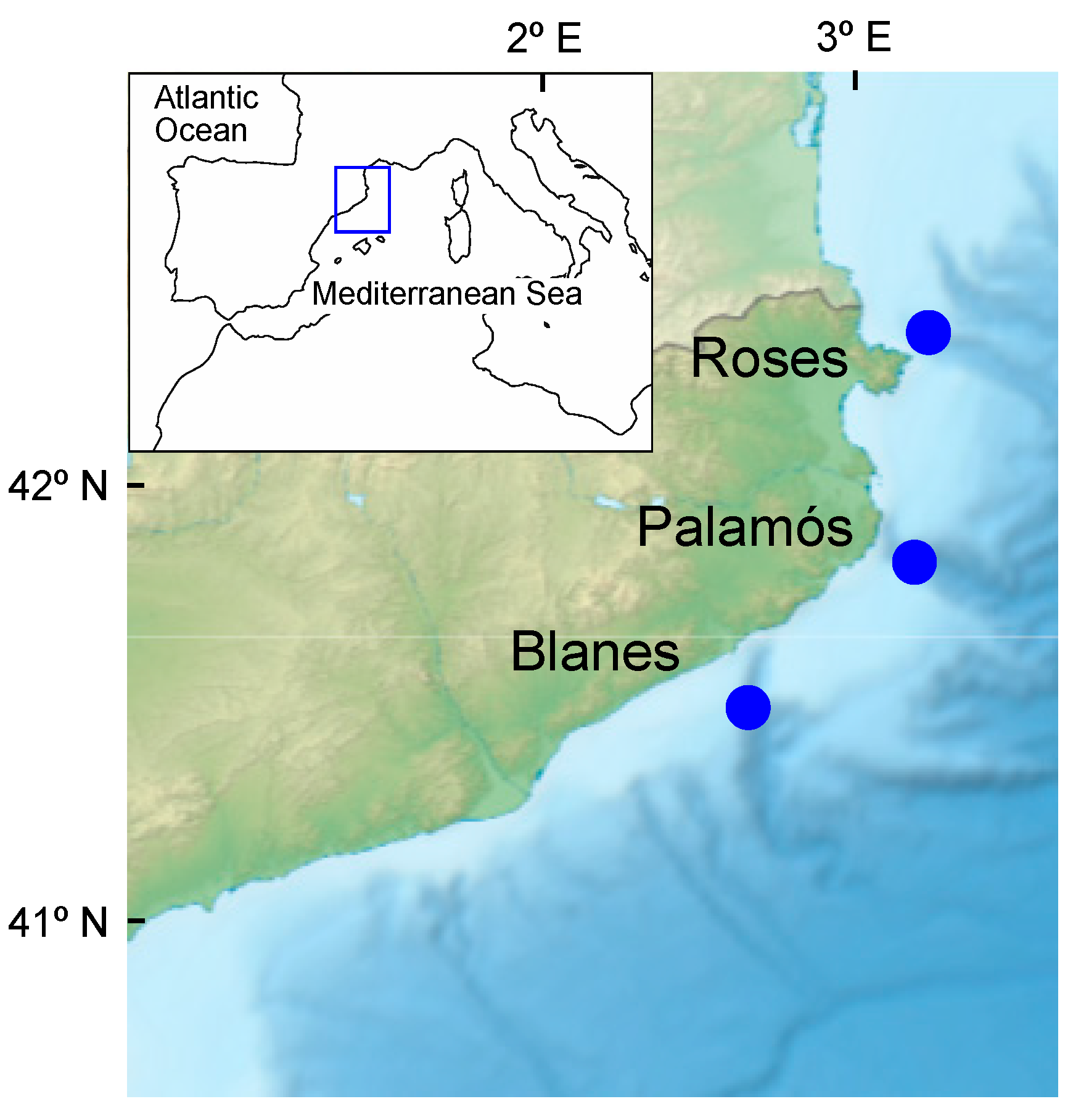
2.1. Biological Material
2.2. DNA Extraction and Molecular Markers
2.3. Size Frequency Distributions, Cohort Identification, and Comparison with Commercial Categories
2.4. Genetic Analysis
3. Results
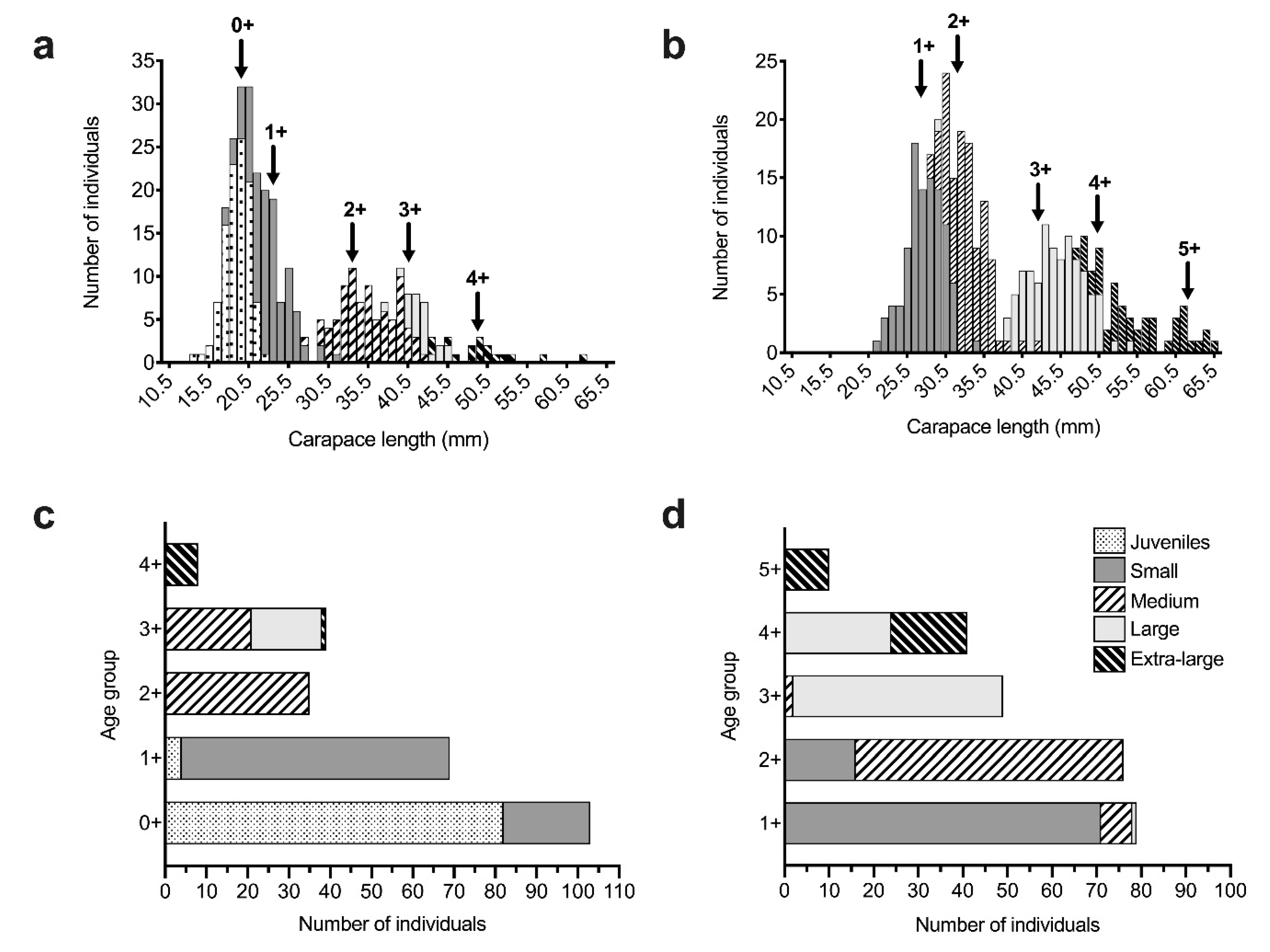
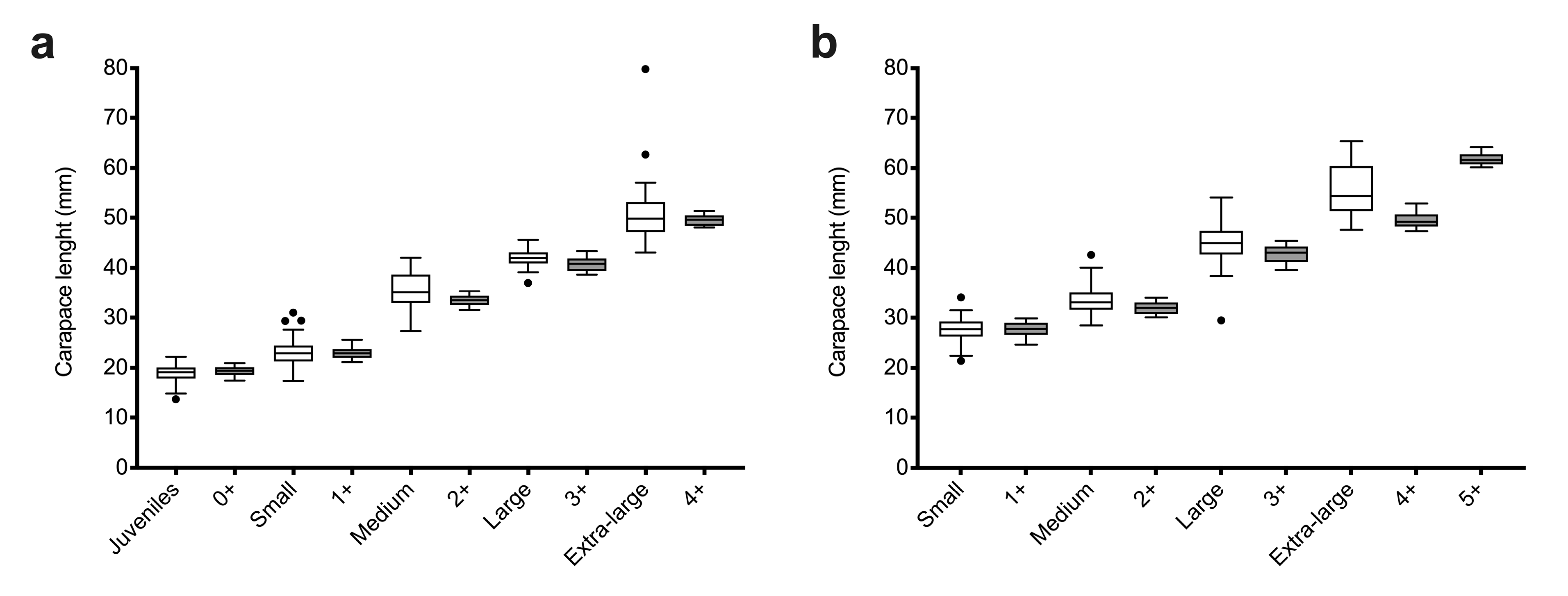
3.1. Size-Age Composition of A. antennatus Females
3.2. Genetic Diversity and Genetic Divergence of A. antennatus Females
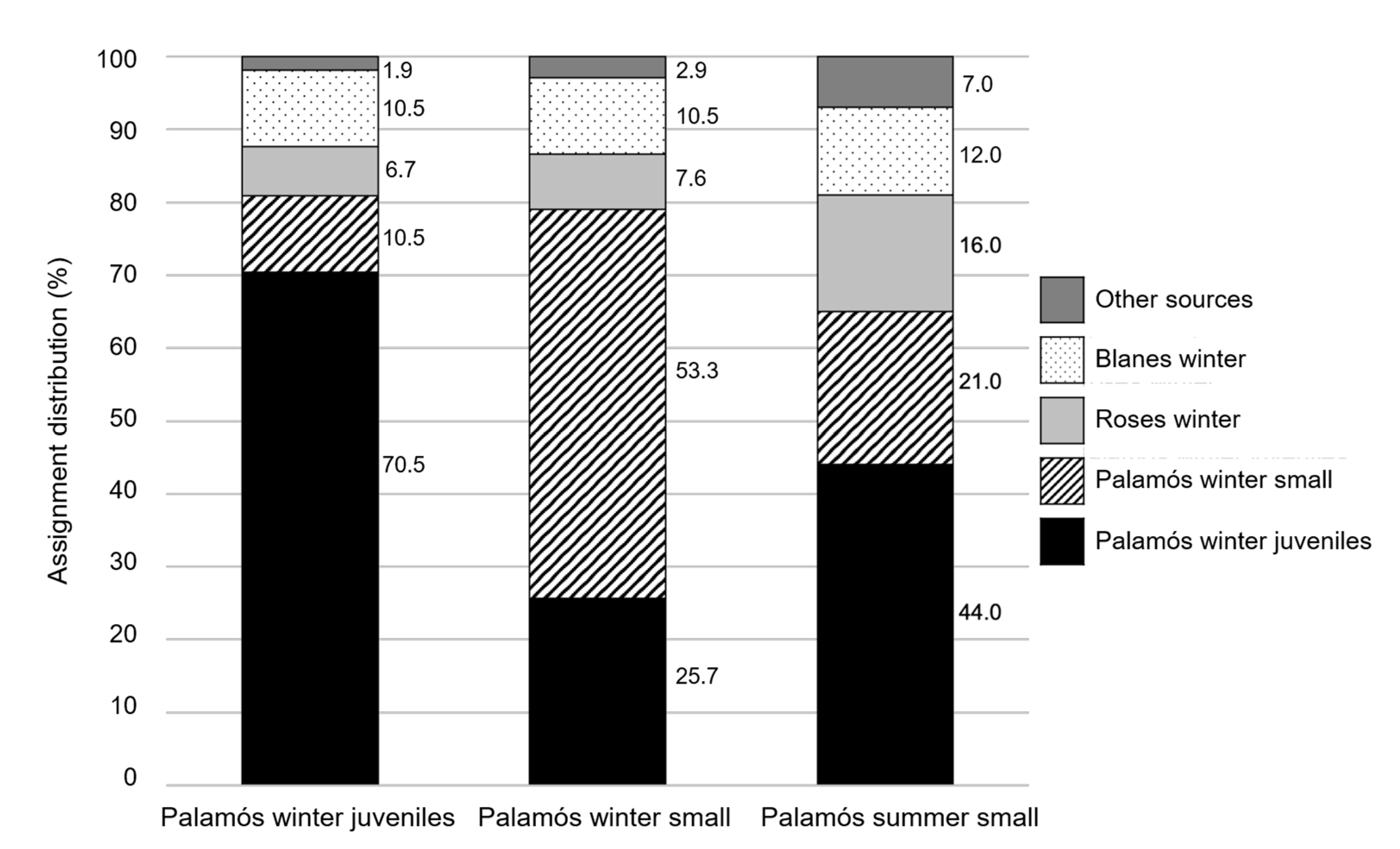
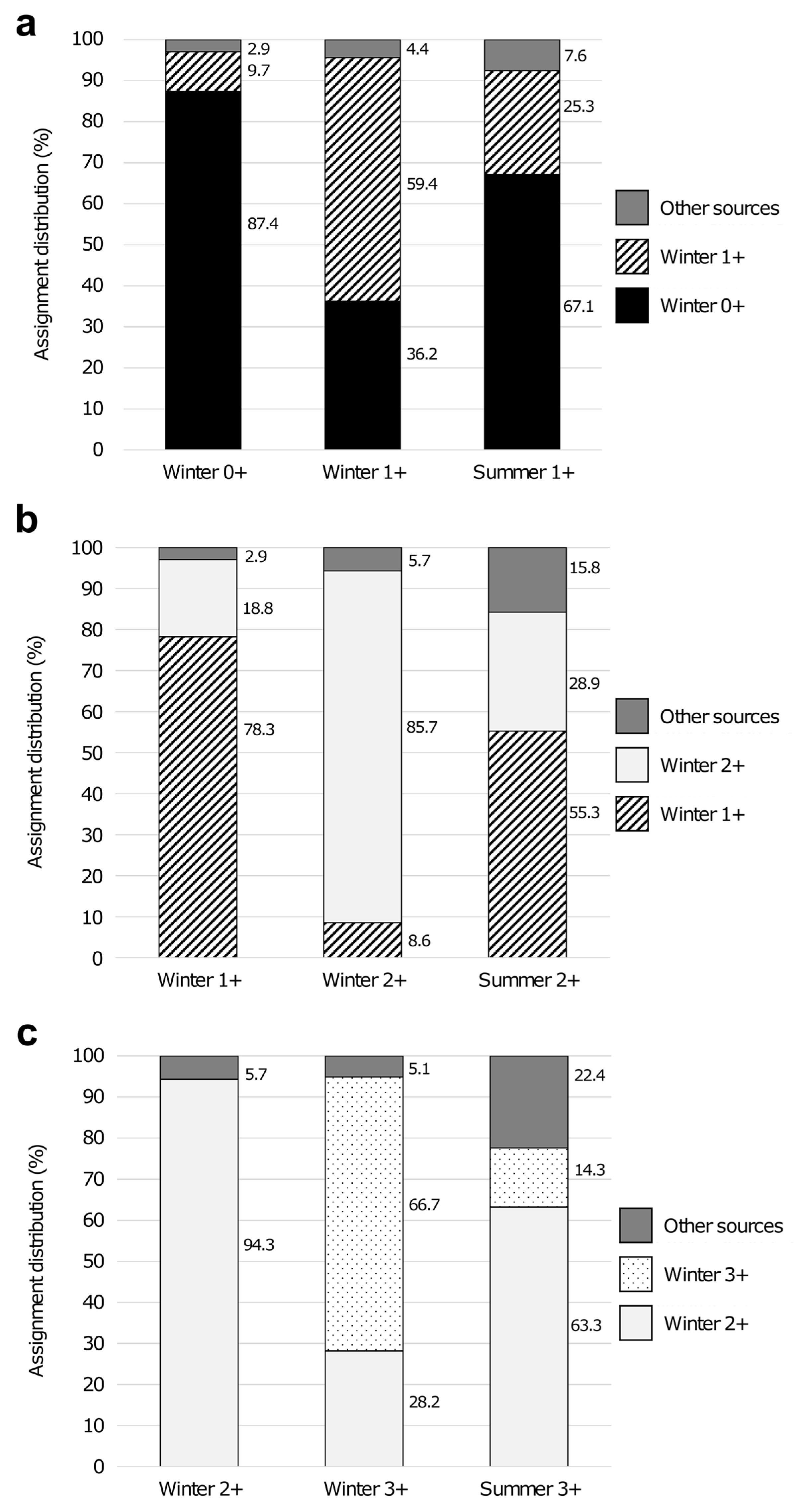
3.3. Origin of Juvenile and Small Females
4. Discussion
4.1. Size-Age Composition and Commercial Categories of A. antennatus Females
4.2. Recruitment and Geographical Origin of A. antennatus Females
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sardà, F.; D’Onghia, G.; Politou, C.Y.; Company, J.B.; Maiorano, P.; Kapiris, K. Deep-sea distribution, biological and ecological aspects of Aristeus antennatus (Risso, 1816) in the western and central Mediterranean Sea. Sci. Mar. 2004, 68, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Sardà, F.; Roldán, M.I.; Heras, S.; Maltagliati, F. Influence of the genetic structure of the red and blue shrimp, Aristeus antennatus (Risso, 1816), on the sustainability of a deep-sea population along a depth gradient in the western Mediterranean. Sci. Mar. 2010, 74, 569–575. [Google Scholar] [CrossRef] [Green Version]
- Sardà, F.; Cartes, J.E.; Norbis, W. Spatio-temporal structure of the deep-water shrimp Aristeus antennatus (Decapoda: Aristeidae) population in the Western Mediterranean. Fish. Bull. 1994, 92, 599–607. [Google Scholar]
- Sardà, F.; Maynou, F.; Talló, L. Seasonal and spatial mobility patterns of rose shrimp Aristeus antennatus in the Western Mediterranean: Results of a long-term study. Mar. Ecol. Prog. Ser. 1997, 159, 133–141. [Google Scholar] [CrossRef]
- Kapiris, K.; Thessalou-Legaki, M. Comparative reproduction aspects of the deep-water shrimps Aristaeomorpha foliacea and Aristeus antennatus (Decapoda, Aristeidae) in the Greek Ionian Sea (Eastern Mediterranean). Int. J. Zool. 2009, 2009, 979512. [Google Scholar] [CrossRef] [Green Version]
- Demestre, M.; Fortuño, J.M. Reproduction of the deep-water shrimp Aristeus antennatus (Decapoda: Dendrobranchiata). Mar. Ecol. Prog. Ser. 1992, 84, 41–51. [Google Scholar] [CrossRef]
- Gorelli, G.; Company, J.B.; Sardà, F. Management strategies for the fishery of the red shrimp Aristeus antennatus in Catalonia (NE Spain). Mar. Steward. Counc. Sci. Ser. 2014, 2, 116–127. [Google Scholar]
- Sardà, F.; Company, J.B.; Castellón, A. Intraespecific aggregation structure of a shoal of a Western Mediterranean (Catalan Coast) deep-sea shrimp, Aristeus antennatus (Risso, 1816), during the reproductive period. J. Shellfish Res. 2003, 22, 569–579. [Google Scholar]
- Cartes, J.E.; López-Pérez, C.; Carbonell, A. Condition and recruitment of Aristeus antennatus at great depths (to 2300 m) in the Mediterranean: Relationship with environmental factors. Fish. Oceanogr. 2018, 27, 114–126. [Google Scholar] [CrossRef]
- Roldán, M.I.; Heras, S.; Patellani, R.; Maltagliati, F. Analysis of genetic structure of the red shrimp Aristeus antennatus from the Western Mediterranean employing two mitochondrial regions. Genetica 2009, 136, 1–4. [Google Scholar] [CrossRef]
- Maggio, T.; Lo Brutto, S.; Cannas, R.; Deiana, A.M.; Arculeo, M. Environmental features of deep-sea habitats linked to the genetic population structure of a crustacean species in the Mediterranean Sea. Mar. Ecol. 2009, 30, 354–365. [Google Scholar] [CrossRef]
- Fernández, M.V.; Heras, S.; Maltagliati, F.; Turco, A.; Roldán, M.I. Genetic structure in the blue and red shrimp Aristeus antennatus and the role played by hydrographical and oceanographical barriers. Mar. Ecol. Prog. Ser. 2011, 421, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Cannas, R.; Sacco, F.; Follesa, M.C.; Sabatini, A.; Arculeo, M.; Lo Brutto, S.; Maggio, T.; Deiana, A.M.; Cau, A. Genetic variability of the blue and red shrimp Aristeus antennatus in the Western Mediterranean Sea inferred by DNA microsatellite loci. Mar. Ecol. 2012, 33, 350–363. [Google Scholar] [CrossRef]
- Marra, A.; Mona, S.; Sà, R.M.; D’Onghia, G.; Maiorano, P. Population genetic history of Aristeus antennatus (Crustacea: Decapoda) in the Western and Central Mediterranean Sea. PLoS ONE 2009, 10, e0117272. [Google Scholar] [CrossRef] [Green Version]
- Heras, S.; Planella, L.; García-Marín, J.L.; Vera, M.; Roldán, M.I. Genetic structure and population connectivity of the blue and red shrimp Aristeus antennatus. Sci. Rep. 2019, 9, 13531. [Google Scholar] [CrossRef]
- Agulló, M.; Heras, S.; García-Marín, J.L.; Vera, M.; Planella, L.; Roldán, M.I. Genetic analyses reveal temporal stability and connectivity pattern in blue and red shrimp Aristeus antennatus populations. Sci. Rep. 2020, 10, 21505. [Google Scholar] [CrossRef]
- Agulló, M.; Heras, S.; García-Marín, J.L.; Vera, M.; Abras, A.; Planella, L.; Roldán, M.I. An evaluation of the genetic connectivity and temporal stability of the blue and red shrimp Aristeus antennatus: A case study of spawning females’ grounds in the Western Mediterranean Sea. Hydrobiologia 2022, 849, 2043–2055. [Google Scholar] [CrossRef]
- Guijarro, B.; Massutí, E.; Moranta, J.; Díaz, P. Population dynamics of the red shrimp Aristeus antennatus in the Balearic Islands (western Mediterranean): Short spatio-temporal differences and influence of environmental factors. J. Mar. Syst. 2008, 71, 385–402. [Google Scholar] [CrossRef]
- Planella, L.; Vera, M.; García-Marín, J.L.; Heras, S.; Roldán, M.I. Mating structure of the blue and red shrimp, Aristeus antennatus (Risso, 1816) characterized by relatedness analysis. Sci. Rep. 2019, 9, 7227. [Google Scholar] [CrossRef]
- Abras, A.; García-Marín, J.L.; Heras, S.; Vera, M.; Agulló, M.; Planella, L.; Roldán, M.I. Male Deep-sea shrimps Aristeus antennatus at fishing grounds: Growth and first evaluation of recruitment by multilocus genotyping. Life 2021, 11, 116. [Google Scholar] [CrossRef]
- Relini, M.; Maiorano, P.; D’Onghia, G.; Orsi Relini, L.; Tursi, A.; Panza, M. A pilot experiment of tagging the deep shrimp Aristeus antennatus (Risso, 1816). Sci. Mar. 2000, 64, 357–361. [Google Scholar] [CrossRef] [Green Version]
- Carbonell, A.; Carbonell, M.; Demestre, M.; Grau, A.; Monserrat, S. The red shrimp Aristeus antennatus (Risso, 1816) fishery and biology in the Balearic Island, Western Mediterranean. Fish. Res. 1999, 44, 1–13. [Google Scholar] [CrossRef]
- D’Onghia, G.; Maiorano, P.; Capezzuto, F.; Carlucci, R.; Battista, D.; Giove, A.; Sion, L.; Tursi, A. Further evidences of deep-sea recruitment of Aristeus antennatus (Crustacea: Decapoda) and its role in the population renewal on the exploited bottoms of the Mediterranean. Fish. Res. 2009, 95, 236–245. [Google Scholar] [CrossRef]
- Sardà, F.; Cartes, J.E. Morphological features and ecological aspects of early juvenile specimens of the aristeid shrimp Aristeus antennatus (Risso, 1816). Mar. Freshw. Res. 1997, 48, 73–77. [Google Scholar] [CrossRef]
- Demestre, M.; Lleonart, J. Population dynamics of Aristeus antennatus (Decapoda: Dendrobranchiata) in the northwestern Mediterranean. Sci. Mar. 1993, 57, 183–189. [Google Scholar]
- Company, J.B.; Puig, P.; Sardà, F.; Palanques, A.; Latasa, M.; Scharek, R. Climate influence on deep sea populations. PLoS ONE 2008, 3, e1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Confraria de Pescadors de Palamós. Estadístiques 2018. Available online: https://confraria.cat/estadistiques/ (accessed on 23 May 2022).
- Gorelli, G.; Company, J.B.; Bahamón, N.; Sardà, F. Improving codend selectivity in the fishery of the deep-sea red shrimp Aristeus antennatus in the northwestern Mediterranean Sea. Sci. Mar. 2017, 81, 381–386. [Google Scholar] [CrossRef] [Green Version]
- Ragonese, S.; Bianchini, M.L. Growth, mortality and yield-per-recruit of the deep-water shrimp Aristeus antennatus (Crustacea-Aristeidae) of the Strait of Sicily (Mediterranean Sea). Fish. Res. 1996, 26, 125–137. [Google Scholar] [CrossRef]
- Papaconstantinou, C.; Kapiris, K. Distribution and population structure of the red shrimp (Aristeus antennatus) on an unexploited fishing ground in the Greek Ionian Sea. Aquat. Living Resour. 2001, 14, 303–312. [Google Scholar] [CrossRef]
- Arculeo, M.; Vitale, S.; Cannizaro, L.; Lo Brutto, S. Growth parameters and population structure of Aristeus antennatus (Decapoda, Penaeidae) in the South Tyrrheninan Sea (Southern coast of Italy). Crustaceana 2011, 84, 1099–1109. [Google Scholar] [CrossRef] [Green Version]
- Orsi-Relini, L.; Mannini, A.; Relini, G. Updating knowledge on growth, population dynamics, and ecology of the blue and red shrimp, Aristeus antennatus (Risso, 1816), on the basis of the study of its instars. Mar. Ecol. 2013, 34, 90–102. [Google Scholar] [CrossRef]
- Sardà, F.; Demestre, M. Estudio biológico de la gamba Aristeus antennatus (Risso, 1816) en el Mar Catalán (NE de España). Investigación Pesquera 1987, 51 (Suppl. S1), 213–232. [Google Scholar]
- Carbonell, A. Evaluación de la Gamba Rosada, Aristeus antennatus (Risso 1816), en el Mar Balear. Ph.D. Thesis, Universitat de les Illes Balears, Mallorca, Spain, December 2005. [Google Scholar]
- Sardà, F.; Company, J.B. The deep-sea recruitment of Aristeus antennatus (Risso, 1816) (Crustacea: Decapoda) in the Mediterranean Sea. J. Mar. Syst. 2012, 105–108, 145–151. [Google Scholar] [CrossRef]
- Boletín Oficial del Estado. Orden AAA/923/2013, de 16 de mayo, por la que se regula la pesca de gamba rosada (Aristeus antennatus) con arte de arrastre de fondo en determinadas zonas marítimas próximas a Palamós. BOE 126 (Sec. III). 2013, pp. 40016–40022. Available online: https://www.boe.es/eli/es/o/2013/05/16/aaa923/dof/spa/pdf (accessed on 22 May 2022).
- Boletín Oficial del Estado. Orden APM/532/2018, de 25 de mayo de 2018, por la que se regula la pesca de gamba rosada (Aristeus antennatus) con arte de arrastre de fondo en determinadas zonas marítimas próximas a Palamós. BOE 128 (Sec. III). 2018, pp. 55045–55051. Available online: https://www.boe.es/boe/dias/2018/05/26/pdfs/BOE-A-2018-7015.pdf (accessed on 22 May 2022).
- Confaria de Pescadors de Palamós. Marca de Garantia. 2018. Available online: https://confraria.cat/quees/ (accessed on 23 May 2022).
- Gorelli, G.; Blanco, M.; Sardà, F.; Carretón, M.; Company, J.B. Spatio-temporal variability of discards in the fishery of the deep-sea shrimp Aristeus antennatus in the northwestern Mediterranean Sea: Implications for management. Sci. Mar. 2016, 80, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Clavel-Henry, M.; Solé, J.; Bahamon, N.; Carretón, M.; Company, J.B. Larval transport of Aristeus antennatus shrimp (Crustacea: Decapoda: Dendrobranchiata: Aristeidae) near the Palamós submarine canyon (NW Mediterranean Sea) linked to the North Balearic Front. Prog. Oceanogr. 2021, 192, 102515. [Google Scholar] [CrossRef]
- Fernández, M.V.; Heras, S.; Viñas, J.; Maltagliati, F.; Roldán, M.I. Multilocus comparative phylogeography of two Aristeid shrimps of high commercial interest (Aristeus antennatus and Aristaeomorpha foliacea) reveals different responses to past environmental changes. PLoS ONE 2013, 8, e59033. [Google Scholar] [CrossRef] [Green Version]
- Heras, S.; Planella, L.; Caldarazzo, I.; Vera, M.; García-Marín, J.L.; Roldán, M.I. Development and characterization of novel microsatellite markers by Next Generation Sequencing for the blue and red shrimp Aristeus antennatus. Peer J. 2016, 4, e2200. [Google Scholar] [CrossRef] [Green Version]
- Gayanilo, F.C.; Sparre, P.; Pauly, D. FAO-ICLARM Strock Assessment Tools II (FiSAT II) Revised version—User’s Guide. FAO Comput. Inf. Ser. 2005, 8, 1–168. [Google Scholar]
- Bhattacharya, C.G. A simple method of resolution of a distribution into Gaussian components. Biometrics 1967, 23, 115–135. [Google Scholar] [CrossRef]
- Cohen, J. A coefficient of agreement for nominal scales. Educ. Psychol. Meas. 1960, 20, 37–46. [Google Scholar] [CrossRef]
- Goudet, J. FSTAT, A Program to Estimate and Test Gene Diversities and Fixation Indices (Version 2.9.3). 2001. Available online: https://www2.unil.ch/popgen/softwares/fstat.htm (accessed on 23 May 2022).
- Rousset, F. GENEPOP’007: A complete re-implementation of the GENEPOP software for Windows and Linux. Mol. Ecol. Resour. 2008, 8, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W.; Thompson, E.A. Performing the exact test of Hardy-Weinberg proportion for multiple alleles. Biometrics 1992, 48, 361–372. [Google Scholar] [CrossRef]
- Van Oosterhout, C.; Hutchinson, W.F.; Wills, D.P.M.; Shipley, P. Micro-Checker: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Nielsen, E.E.; Bach, L.A.; Kotlicki, P. HYBRIDLAB (version 1.0): A program for generating simulated hybrids from population samples. Mol. Ecol. Notes 2006, 6, 971–973. [Google Scholar] [CrossRef]
- Rannala, B.; Mountain, J.L. Detecting immigration by using multilocus genotypes. Proc. Natl. Acad. Sci. USA 1997, 94, 9197–9201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piry, S.; Alapetite, A.; Cornuet, J.M.; Paetkau, D.; Baudouin, L.; Estoup, A. GeneClass2: A software for genetic assignment and first-generation migrant detection. J. Hered. 2004, 95, 536–539. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, M. La gamba roja Aristeus antennatus (Risso, 1816) (Crustacea, Decapoda): Distribución, Demografía, Crecimiento, Reproducción y explotación en el Golfo de Alicante, Canal de Ibiza y Golfo de Vera. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, February 2003. [Google Scholar]
- Vila-Gordillo, Y. Estudio cuantitativo de la lipofuscina mediante microscopía de fluorescencia en cerebros de crustáceos peneidos: Aplicación a la determinación de la edad en animales salvajes Parapenaeus longirostris (Lucas, 1846) y Aristeus antennatus (Risso, 1946) y cultivados Marsupenaeus japonicus (Bate, 1888). Ph.D. Thesis, Universidad de Cádiz, Cadiz, Spain, November 2001. [Google Scholar]
- Carbonell, A.; Lloret, J.; Demestre, M. Relationship between condition and recruitment success of red shrimp (Aristeus antennatus) in the Balearic Sea (Northwestern Mediterranean). J. Mar. Syst. 2008, 71, 403–412. [Google Scholar] [CrossRef]
- García-Rodríguez, M.; Esteban, A. On the biology and fishery of Aristeus antennatus (Risso, 1816), (Decapoda: Dendrobranchiata) in the Ibiza Channel (Balearic Islands, Spain). Sci. Mar. 1999, 63, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Demestre, M.; Martín, P. Optimum exploitation of a demersal resource in the western Mediterranean: The fishery of the deep-water shrimp Aristeus antennatus (Risso, 1816). Sci. Mar. 1993, 57, 175–182. [Google Scholar]
- Deval, M.C.; Kapiris, K. A review of biological patterns of the blue-red shrimp Aristeus antennatus in the Mediterranean Sea: A case study of the population of Antalya Bay, eastern Mediterranean Sea. Sci. Mar. 2016, 80, 330–348. [Google Scholar] [CrossRef] [Green Version]
- Demestre, M. Moult activity-related spawning success in the Mediterranean deep-water shrimp Aristeus antennatus (Decapoda: Dendrobranchiata). Mar. Ecol. Prog. Ser. 1995, 127, 57–64. [Google Scholar] [CrossRef]
- Corgos, A.; Sampedro, M.P.; González-Gurriarán, E.; Freire, J. Growth at moult, intermoult period, and moulting seasonality of the spider crab Maja brachydactyla: Combining information from mark-recapture and experimental studies. J. Crustac. Biol. 2007, 27, 255–262. [Google Scholar] [CrossRef] [Green Version]

| Sampling Season | Commercial Category | Mean CL ± SD (mm) | N | Females with Spermatophore (Double) | NA | AR | HO | HE | FIS |
|---|---|---|---|---|---|---|---|---|---|
| Winter | Juvenile 1 | 18.98 ± 1.53 | 105 | - | 9.7 | 5.32 | 0.46 | 0.63 | 0.27 |
| Small | 22.96 ± 2.52 | 105 | - | 8.9 | 5.24 | 0.49 | 0.64 | 0.23 | |
| Medium | 35.54 ± 3.41 | 82 | - | 9.2 | 5.34 | 0.43 | 0.64 | 0.33 | |
| Large | 42.03 ± 1.97 | 22 | - | 5.7 | 4.64 | 0.43 | 0.60 | 0.28 * | |
| Extra-large | 51.86 ± 8.65 | 17 | - | 5.7 | 5.07 | 0.42 | 0.61 | 0.30 * | |
| Summer | Small | 27.65 ± 2.37 | 100 | 99 | 9.7 | 5.40 | 0.51 | 0.63 | 0.19 * |
| Medium | 33.24 ± 2.46 | 99 | 97 (3) | 9.4 | 5.39 | 0.48 | 0.62 | 0.22 | |
| Large | 44.88 ± 3.78 | 91 | 91 (14) | 9.4 | 5.36 | 0.51 | 0.63 | 0.19 * | |
| Extra-large | 55.46 ± 5.10 | 44 | 44 (9 + 1 triple) | 7.8 | 5.14 | 0.45 | 0.62 | 0.27 * |
| Sampling Season | Modal Group | Age Group | Mean CL ± SD (mm) | SI | N | NA | AR | HO | HE | FIS |
|---|---|---|---|---|---|---|---|---|---|---|
| Winter | 1 | 0+ | 19.26 ± 1.77 | - | 103 | 9.7 | 3.94 | 0.46 | 0.64 | 0.28 |
| 2 | 1+ | 23.38 ± 2.23 | 2.06 | 69 | 8.3 | 3.88 | 0.49 | 0.64 | 0.24 * | |
| 3 | 2+ | 33.51 ± 2.08 | 4.70 | 35 | 7.0 | 3.95 | 0.45 | 0.65 | 0.31 | |
| 4 | 3+ | 40.89 ± 2.54 | 3.19 | 39 | 7.2 | 3.71 | 0.41 | 0.63 | 0.34 | |
| 5 | 4+ | 49.12 ± 2.41 | 3.33 | 8 | 4.3 | 3.75 | 0.43 | 0.64 | 0.33 * | |
| Summer | 1 | 1+ | 27.26 ± 2.72 | - | 79 | 9.3 | 3.88 | 0.51 | 0.63 | 0.19 |
| 2 | 2+ | 32.06 ± 1.97 | 2.05 | 76 | 9.2 | 3.95 | 0.51 | 0.63 | 0.20 | |
| 3 | 3+ | 42.53 ± 2.94 | 4.26 | 49 | 7.9 | 3.90 | 0.52 | 0.64 | 0.19 * | |
| 4 | 4+ | 50.22 ± 2.97 | 2.60 | 41 | 7.7 | 3.83 | 0.47 | 0.62 | 0.24 * | |
| 5 | 5+ | 62.09 ± 2.26 | 4.54 | 10 | 5.2 | 3.98 | 0.45 | 0.64 | 0.30 |
| Reference | Geographic Region | Sampling Period | Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Present study | Palamós (Spain, north-western Mediterranean Sea) | Winter 2016 | 19.26 ± 1.77 | 23.38 ± 2.23 | 33.51 ± 2.08 | 40.89 ± 2.54 | 49.12 ± 2.41 | - | - |
| Summer 2016 | - | 27.26 ± 2.72 | 32.06 ± 1.97 | 42.53 ± 2.94 | 50.22 ± 2.97 | 62.09 ± 2.26 | |||
| García-Rodríguez (2003) [53] | Gulf of Alicante (Spain, western Mediterranean Sea) | January 1995–December 1998 | - | 25.65 | 33.66 | 43.19 | 51.16 | - | - |
| Ibiza Channel (Spain, western Mediterranean Sea) | January 1992–December 1994 | - | 29.18 | 37.56 | 45.75 | 54.1 | - | - | |
| Gulf of Vera (Spain, western Mediterranean Sea) | January 1992–December 1994 | - | 29.1 | - | 43.8 | 52.6 | - | - | |
| Carbonell (2005) [34] | Balearic Islands and other Mediterranean Sea regions and Atlantic Ocean data compilation | 12 years | 14–16 | 20–25 | 30 | 38–40 | 48–50 | 55–58 | >60 |
| Orsi-Relini et al. (2013) 1 [32] | Ligurian Sea (western Mediterranean Sea) | 1994–2004 | - | 19–29 | 32–38 | 40–45 | 47–50 | 52 | 54 |
| Vila-Gordillo (2001) [54] | Alborán Sea (Spain, western Mediterranean Sea) | June 1997 | - | 23.88 ± 2.38 | 29.15 ± 2.06 | 33.58 ± 2.32 | 39.20 ± 2.65 | 47.42 ± 5.04 | - |
| Arculeo et al. (2011) [31] | San Vito Lo Capo (Sicily, Italy, western Mediterranean Sea) | June 2006–May 2007 | - | 25.25 | 36.19 | 46.44 | 57 | - | - |
| Terrasini (Sicily, Italy, western Mediterranean Sea) | June 2006–May 2007 | - | 23.91 | 32.29 | 42.86 | 51.85 | - | - | |
| D’Onghia et al. (2009) [23] | North-western Ionian Sea (eastern Mediterranean Sea) | April 2006 | - | 17.00 ± 1.49 | 30.96 ± 2.28 | 35.88 ± 1.81 | 48.35 ± 2.96 | - | - |
| May 2006 | - | 18.16 ± 2.59 | 31.00 ± 2.39 | 39.71 ± 2.85 | 45.69 ± 2.56 | 52.37 ± 2.06 | - | ||
| June 2006 | - | 20.79 ± 2.63 | 30.05 ± 1.50 | - | 42.10 ± 4.45 | 51.43 ± 1.60 | - | ||
| September 2006 | - | 24.35 ± 2.71 | - | - | 38.39 ± 2.33 | 49.63 ± 2.85 | - | ||
| Papaconstantinou and Kapiris (2001) [30] | Greek Ionian Sea (eastern Mediterranean Sea) | January–December 1997 | - | 30.0 | - | 41.0 | 49.0 | 54.0 | - |
| Commercial Categories | |||||||||||
| Win Juv | Win S | Win M | Win L | Win XL | Sum S | Sum M | Sum L | Sum XL | |||
| Win Juv | - | 0.1600 | 0.8300 | 0.2100 | 0.4200 | Sum S | - | 0.7750 | 0.1167 | 0.9083 | |
| Win S | 0.0030 | - | 0.9350 | 0.0150 | 0.6650 | Sum M | 0.0000 | - | 0.2583 | 0.7083 | |
| Win M | 0.0008 | 0.0000 | - | 0.5300 | 0.9250 | Sum L | 0.0039 | 0.0015 | - | 0.4583 | |
| Win L | 0.0018 | 0.0003 | 0.0000 | - | 0.5050 | Sum XL | 0.0000 | 0.0000 | 0.0000 | - | |
| Win XL | 0.0047 | 0.0000 | 0.0000 | 0.0000 | - | ||||||
| Modal Progression Analysis Groups | |||||||||||
| Win 0+ | Win 1+ | Win 2+ | Win 3+ | Win 4+ | Sum 1+ | Sum 2+ | Sum 3+ | Sum 4+ | Sum 5+ | ||
| Win 0+ | - | 0.3250 | 0.6100 | 0.3150 | 0.3250 | Sum 1+ | - | 0.9250 | 0.0700 | 0.9650 | 0.8350 |
| Win 1+ | 0.0023 | - | 0.3250 | 0.0400 | 0.3200 | Sum 2+ | 0.0000 | - | 0.5270 | 1.0000 | 0.4250 |
| Win 2+ | 0.0000 | 0.0000 | - | 0.0850 | 0.6950 | Sum 3+ | 0.0000 | 0.0000 | - | 0.8400 | 0.5450 |
| Win 3+ | 0.0000 | 0.0017 | 0.0000 | - | 0.6800 | Sum 4+ | 0.0000 | 0.0000 | 0.0000 | - | 0.3900 |
| Win 4+ | 0.0000 | 0.0000 | 0.0000 | - | Sum 5+ | 0.0000 | 0.0000 | 0.0000 | 0.0000 | - | |
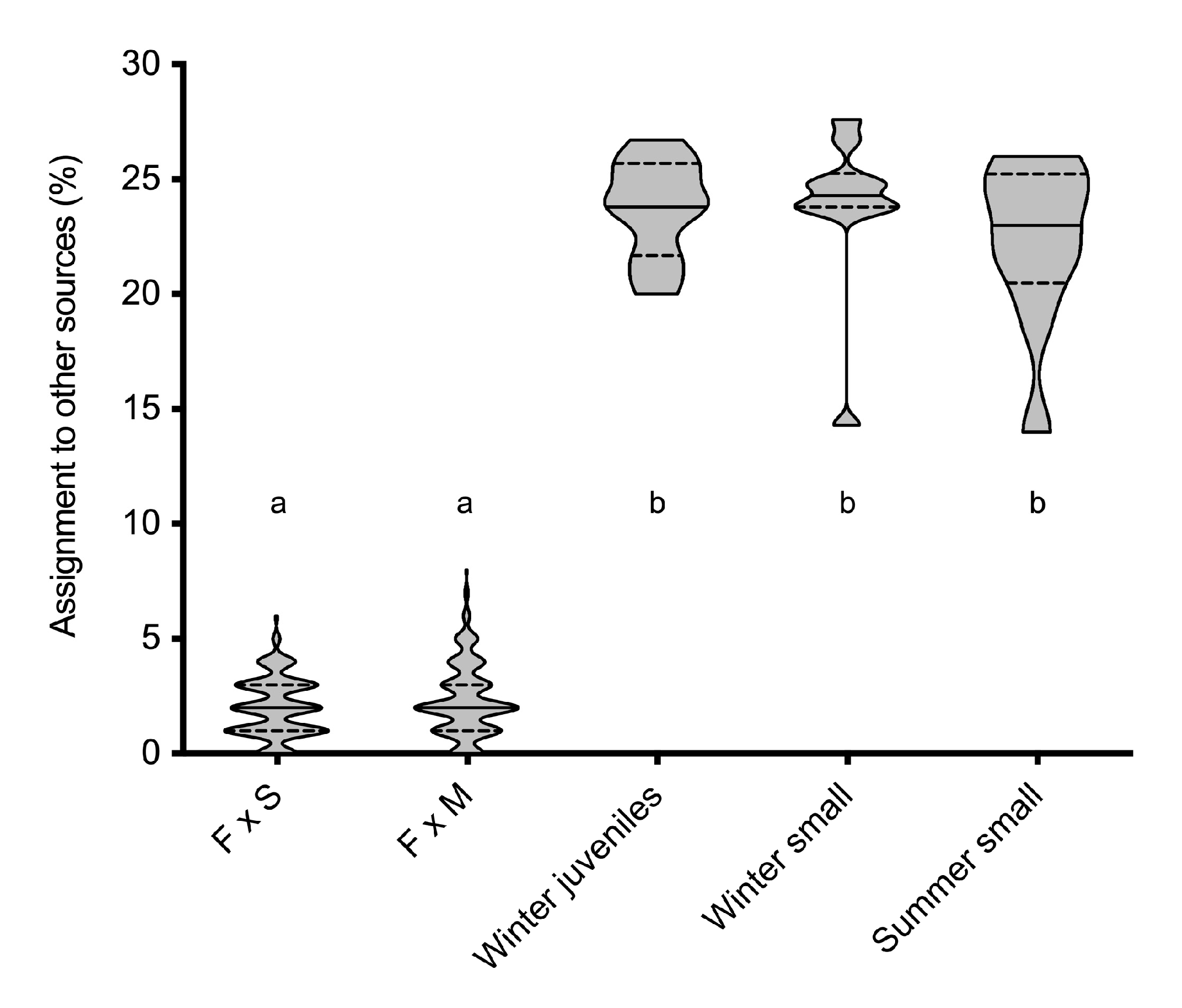
| Replicates | F × S F1 Baseline | F × M F1 Baseline | Other Sources | |
|---|---|---|---|---|
| F x S F1 | 250 | 64.5 ± 8.4 | 33.5 ± 8.3 | 2.0 ± 1.3 |
| F x M F1 | 250 | 39.1 ± 8.2 | 58.6 ± 8.4 | 2.3 ± 1.6 |
| Winter juveniles | 10 | 39.5 ± 8.2 | 36.8 ± 9.3 | 23.7 ± 4.5 |
| Winter small | 10 | 41.8 ± 10.7 | 34.4 ± 12.5 | 23.8 ± 3.6 |
| Summer small | 10 | 40.4 ± 9.0 | 37.3 ± 10.1 | 22.3 ± 3.7 |
| All categories | 10 | 40.6 ± 9.3 | 36.1 ± 10.2 | 23.3 ± 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abras, A.; García-Marín, J.-L.; Heras, S.; Agulló, M.; Vera, M.; Planella, L.; Roldán, M.I. Genetic Demography of the Blue and Red Shrimp, Aristeus antennatus: A Female-Based Case Study Integrating Multilocus Genotyping and Morphometric Data. Genes 2022, 13, 1186. https://doi.org/10.3390/genes13071186
Abras A, García-Marín J-L, Heras S, Agulló M, Vera M, Planella L, Roldán MI. Genetic Demography of the Blue and Red Shrimp, Aristeus antennatus: A Female-Based Case Study Integrating Multilocus Genotyping and Morphometric Data. Genes. 2022; 13(7):1186. https://doi.org/10.3390/genes13071186
Chicago/Turabian StyleAbras, Alba, Jose-Luis García-Marín, Sandra Heras, Melania Agulló, Manuel Vera, Laia Planella, and María Inés Roldán. 2022. "Genetic Demography of the Blue and Red Shrimp, Aristeus antennatus: A Female-Based Case Study Integrating Multilocus Genotyping and Morphometric Data" Genes 13, no. 7: 1186. https://doi.org/10.3390/genes13071186