Tooth Formation as Experimental Model to Study Chemotherapy on Tissue Development: Effect of a Specific Dose of Temozolomide/Veliparib
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Tissues
2.2. Processing of Tissues and Histology
2.3. Immunohistochemistry
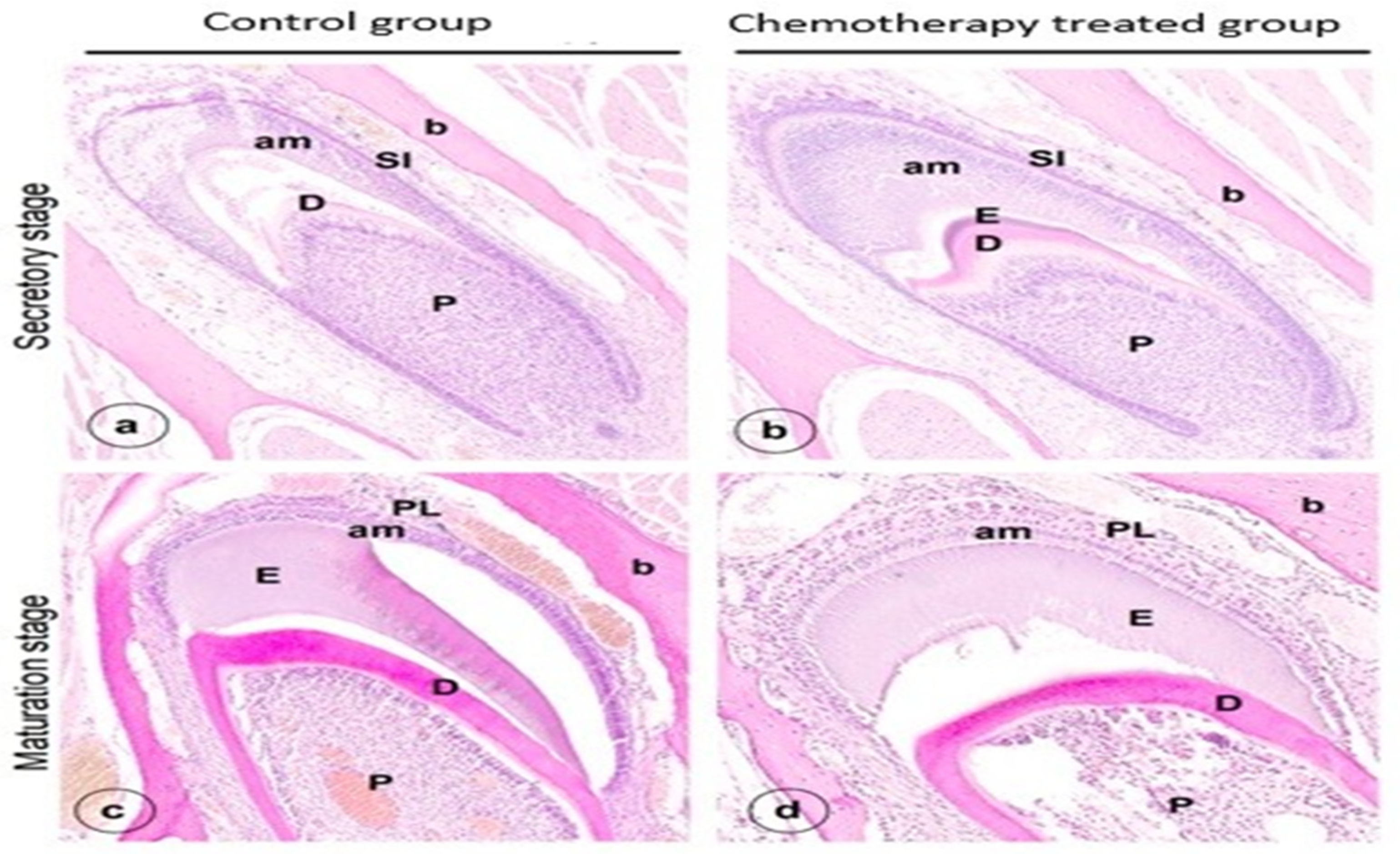
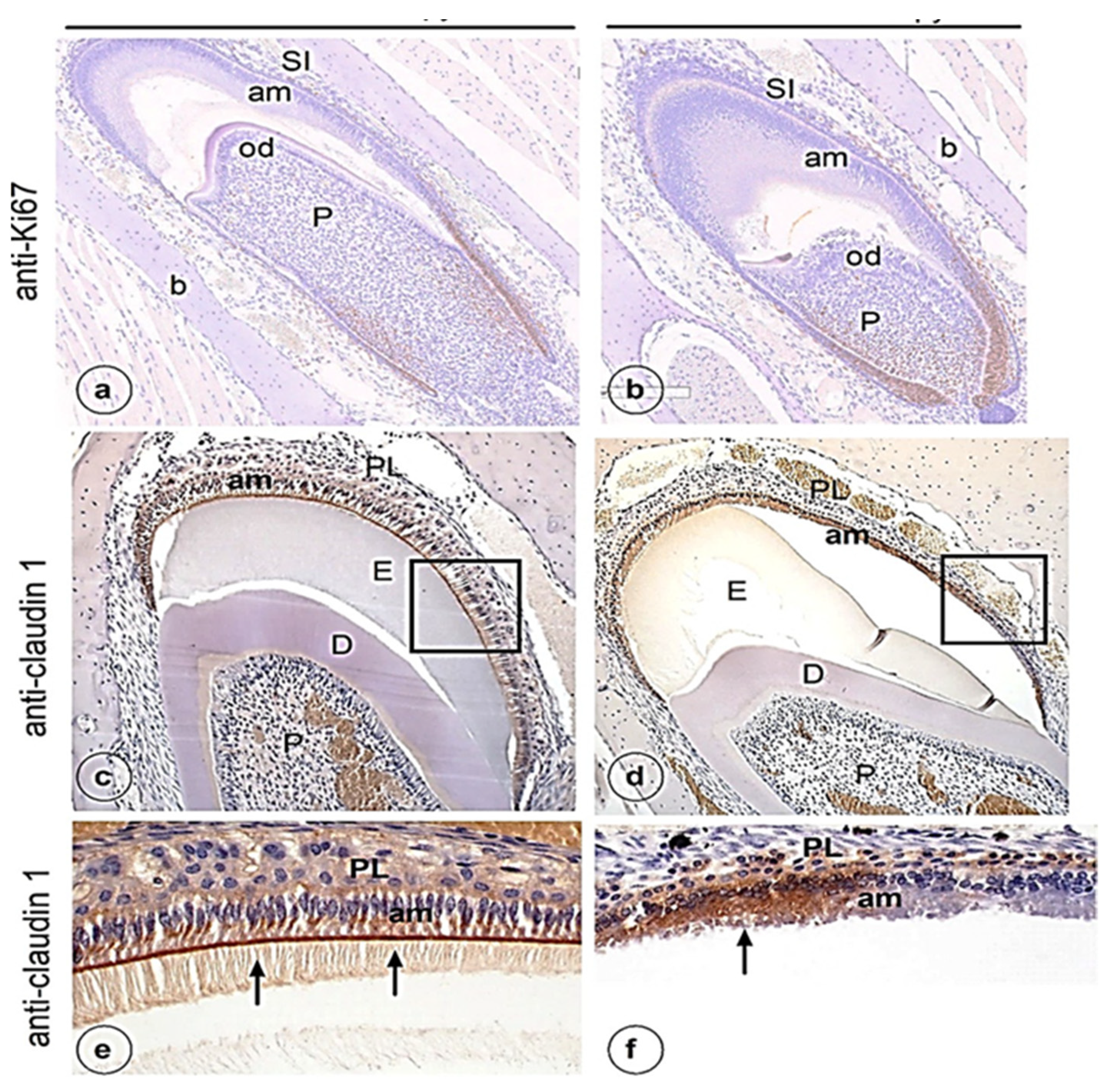
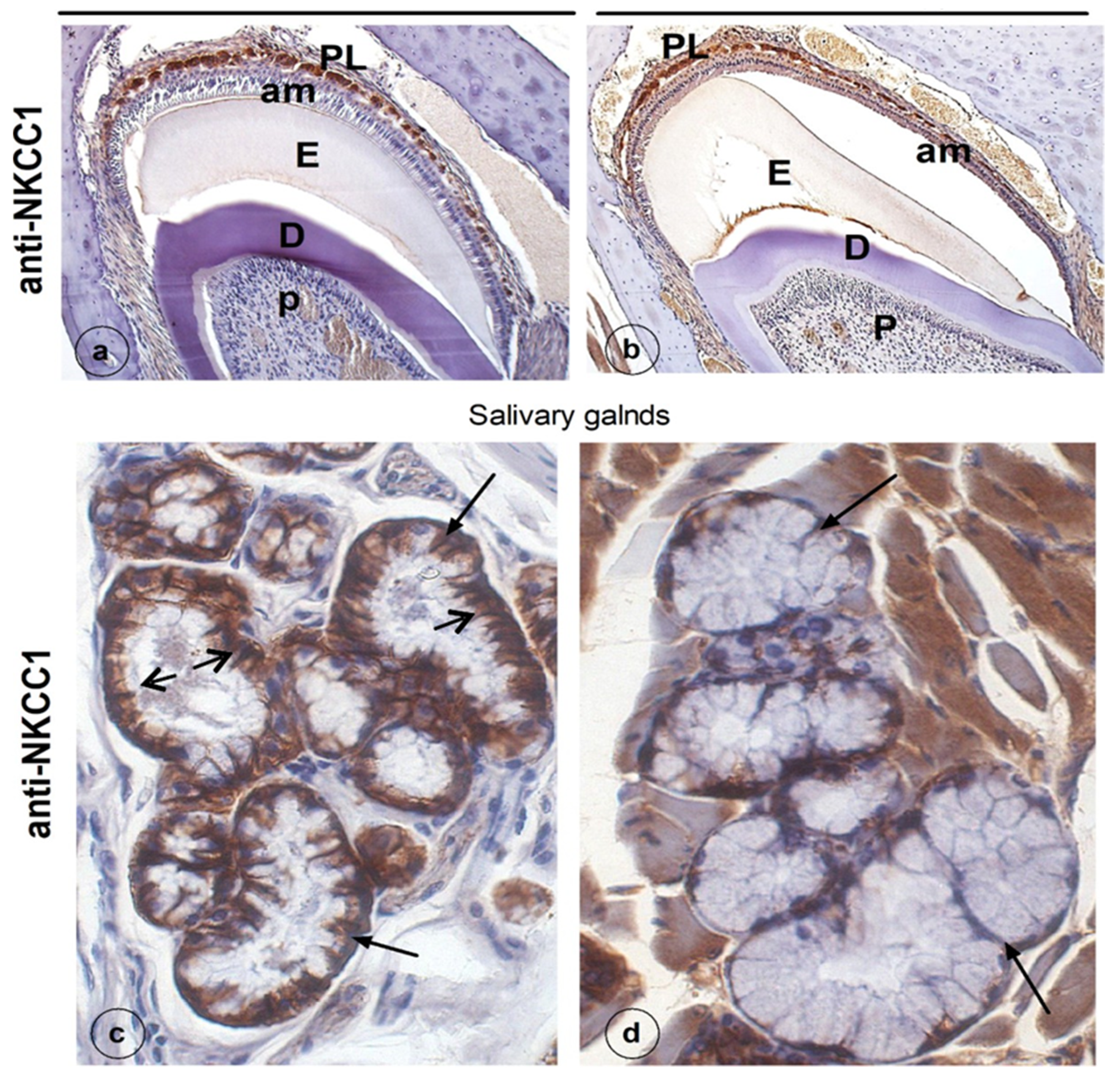
3. Results
4. Discussion
4.1. Mice as a Study Model
4.2. Chemotherapeutic Agents and Dental Development
4.3. Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toth, B.; Martin, J.W.; Fleming, T.J. Oral and dental care associated with cancer therapy. Cancer Bull. 1991, 43, 397–402. [Google Scholar]
- Vasconcelos, N.P.S.; Caran, E.M.M.; Lee, M.L.; Lopes, N.N.F.; Weiler, R.M.J. Dental maturity assessment in children with acute lymphoblastic leukemia after cancer therapy. Forensic Sci. Int. 2009, 184, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Köstler, W.J.; Hejna, M.; Wenzel, C.; Zielinsk, C. Oral Mucositis Complicating Chemotherapy and/or Radiotherapy: Options for Prevention and Treatment. CA Cancer J. Clin. 2001, 51, 290–315. [Google Scholar] [CrossRef] [PubMed]
- Kaste, S.C.; Hopkins, K.P.; Jones, D.; Crom, D.; Greenwald, C.A.; Santana, V.M. Dental abnormalities in children treated for acute lymphoblastic leukemia. Leukemia 1997, 11, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Goho, C. Chemoradiation therapy: Effect on dental development. Pediatric. Dent. 1993, 15, 6–11. [Google Scholar]
- Dahllof, G.; Rozell, B.; Forsberg, C.M.; Borgstrom, B. Histologic changes in dental morphology induced by high dose chemotherapy and total body irradiation, Oral Surg. Oral Med. Oral. Pathol. 1994, 77, 56–60. [Google Scholar] [CrossRef]
- Sonis, A.L.; Tarbell, N.; Valachovic, R.W.; Gelber, R.; Schwenn, M.; Sallan, S. Dentofacial development in long-term survivors of acute lymphoblastic leukaemia: A comparison of three treatment modalities. Cancer 1990, 66, 2645–2652. [Google Scholar] [CrossRef]
- Minicucci, E.M.; Lopes, L.F.; Crocci, A.J. Dental abnormalities in children after chemotherapy treatment for acute lymphoid leukemia. Leukemia Res. 2003, 27, 45–50. [Google Scholar] [CrossRef]
- Lopes, N.N.F.; Petrilli, A.S.; Caran, E.M.M.; Franc, C.M.; Chilvarquer, I.; Lederman, H. Dental abnormalities in children submitted to antineoplastic therapy. J. Dent. Child. 2006, 73, 140–146. [Google Scholar]
- World Health Organization. Handbook for Reporting Results of Cancer Treatment; World Health Organization: Geneva, Switzerland, 1979. [Google Scholar]
- Kenny, S.A. Effect of two oral care protocols on the incidence of stomatitis in hematology patients. Cancer Nurs. 1990, 13, 345–353. [Google Scholar] [CrossRef]
- Petrelli, N.J.; Rustum, Y.M.; Bruckner, H.; Stablein, D. The Roswell Park Memorial Institute and Gastrointestinal Tumor Study Group phase III experience with the modulation of 5-fluorouracil by leucovorin in metastatic colorectal adenocarcinoma. Adv. Exp. Med. Biol. 1988, 244, 143–155. [Google Scholar] [PubMed]
- Rosenberg, S.W.; Kolodney, H.; Wong, G.Y.; Murphy, M.L. Altered dental root development in long term survivors of pediatric acute lymphoblastic leukemia: A review of 17 cases. Cancer 1987, 59, 1640–1648. [Google Scholar] [CrossRef]
- Purdell-Lewis, D.J.; Stalman, M.S.; Leeuw, J.A.; Humphrey, G.B.; Kalsbeck, H. Long term results of chemotherapy on the developing dentition: Caries risk and developmental aspects. Community Dent. Oral Epidemiol. 1988, 16, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Adatia, A.K. Response of dental elements to chemotherapy of Burkitt’s tumour. Int. Dent. J. 1968, 18, 646–654. [Google Scholar]
- Brown, L.R.; Dreizen, S.; Handler, S.; Johnston, D.A. Effect of radiation- inducedxerostomia on human oral microflora. J. Dent. Res. 1975, 54, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Roesink, J.M.; Moerland, M.A.; Battermann, J.J.; Hordijk, G.J.; Terhaard, C.H. Quantitative dose-volume response analysis of changes in parotid glandfunction after radiotherapy in the head-and-neck region. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 938–946. [Google Scholar] [CrossRef]
- Kielbassa, A.M.; Hinkelbein, W.; Hellwig, E.; Meyer-Lückel, H. Radiation- related damage to dentition. Lancet Oncol. 2006, 7, 326–335. [Google Scholar] [CrossRef]
- Villano, J.L.; Seery, T.E.; Bressler, L.R. Temozolomide in malignant gliomas: Current use and future targets. Cancer Chemother Pharmacol. 2009, 64, 647–655. [Google Scholar] [CrossRef]
- Wesolowski, J.R.; Rajdev, P.; Mukherji, S.K. Temozolomide (Temodar). AJNR Am. J. Neuroradiol. 2010, 31, 1383–1384. [Google Scholar] [CrossRef]
- Agarwala, S.S.; Kirkwood, J.M. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist 2000, 5, 144–151. [Google Scholar] [CrossRef]
- Friedman, H.S.; Kerby, T.; Calvert, H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 2000, 6, 2585–2597. [Google Scholar] [PubMed]
- Marchesi, F.; Turriziani, M.; Tortorelli, G.; Avvisati, G.; Torino, F.; De Vecchis, L. Triazene compounds: Mechanism of action and related DNA repair systems. Pharmacol. Res. 2007, 56, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Thon, N.; Kreth, S.; Kreth, F.W. Personalized treatment strategies in glioblastoma: MGMT promoter methylation status. OncoTargets Ther. 2013, 6, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Parker, N.R.; Khong, P.; Parkinson, J.F.; Howell, V.M.; Wheeler, H.R. Molecular heterogeneity in glioblastoma: Potential clinical implications. Front. Oncol. 2015, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Felsberg, J.; Thon, N.; Eigenbrod, S.; Hentschel, B.; Sabel, M.C.; Westphal, M.; Schackert, G.; Kreth, F.W.; Pietsch, T.; Löffler, M.; et al. German Glioma Network. Promoter methylation and expression of MGMT and the DNA mismatch repair genes MLH1, MSH2, MSH6 and PMS2 in paired primary and recurrent glioblastomas. Int. J. Cancer 2011, 129, 659–670. [Google Scholar] [CrossRef]
- Xiao, S.; Yang, Z.; Qiu, X.; Lv, R.; Liu, J.; Wu, M.; Liao, Y.; Liu, Q. miR-29c contribute to glioma cells temozolomide sensitivity by targeting O6-methylguanine-DNA methyltransferases indirectly. Oncotarget 2016, 7, 50229–50237. [Google Scholar] [CrossRef][Green Version]
- Marton, E.; Giordan, E.; Siddi, F.; Curzi, C.; Canova, G.; Scarpa, B.; Guerriero, A.; Rossi, S.; D’ Avella, D.; Longatti, P.; et al. Over ten years overall survival in glioblastoma: A different disease? J. Neurol. Sci. 2020, 408, 116518. [Google Scholar] [CrossRef]
- Dantzer, F.; Amé, J.C.; Schreiber, V.; Nakamura, J.; Ménissier-de Murcia, J.; de Murcia, G. Poly(ADP-ribose) polymerase-1 activation during DNA damage and repair. Methods Enzymol. 2006, 409, 493–510. [Google Scholar] [CrossRef]
- Kinsella, T.J. Coordination of DNA mismatch repair and base excision repair processing of chemotherapy and radiation damage for targeting resistant cancers. Clin. Cancer Res. 2009, 15, 1853–1859. [Google Scholar] [CrossRef]
- Abou-Antoun, T.J.; Hale, J.S.; Lathia, J.D.; Dombrowski, S.M. Brain Cancer Stem Cells in Adults and Children: Cell Biology and Therapeutic Implications. Neurotherapeutics 2017, 14, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Fritah, S.; Muller, A.; Jiang, W.; Mitra, R.; Sarmini, M.; Dieterle, M.; Golebiewska, A.; Ye, T.; Van Dyck, E.; Herold-Mende, C.; et al. Temozolomide-Induced RNA Interactome Uncovers Novel LncRNA Regulatory Loops in Glioblastoma. Cancers 2020, 12, 2583. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Mitra, R.; Zhao, M.-M.; Fan, W.; Eischen, C.M.; Yin, F.; Zhao, Z. The Potential Roles of Long Noncoding RNAs (lncRNA) in Glioblastoma Development. Mol. Cancer Ther. 2016, 15, 2977–2986. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.-Q.; Liu, A.-S.; Zhang, M.-J.; Liu, H.-J.; Meng, X.-L.; Qian, H.-P.; Wan, J.-H. Identifying Predictive Gene Expression and Signature Related to Temozolomide Sensitivity of Glioblastomas. Front. Oncol. 2020, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Gainer, J.L.; Sheehan, J.P.; Larner, J.M.; Jones, D.R. Trans sodium crocetinate with temozolomide and radiation therapy for glioblastoma multiforme. J. Neurosurg. 2017, 126, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Lin, F.; De Gooijer, M.C.; Moreno Roig, E.; Buil, L.C.M.; Christner, S.M.; Beumer, J.H.; Wurdinger, T.; Beijnen, J.H.; Van Tellingen, O. ABCB1, ABCG2, and PTEN Determine the Response of Glioblastoma to Temozolomide and ABT-888 Therapy. Clin. Cancer Res. 2014, 20, 2703–2713. [Google Scholar] [CrossRef]
- Pietanza, M.C.; Waqar, S.N.; Krug, L.M.; Dowlati, A.; Hann, C.L.; Chiappori, A.; Owonikoko, T.K.; Woo, K.M.; Cardnell, R.J.; Fujimoto, J.; et al. Randomized, double blind, phase ii study of temozolomide in combination with either veliparib or placebo in patients with relapsed sensitive or refractory small-cell lung cancer. J. Clin. Oncol. 2018, 36, 2386–2394. [Google Scholar] [CrossRef]
- Gupta, S.K.; Mladek, A.C.; Carlson, B.L.; Boakye-Agyeman, F.; Bakken, K.K.; Kizilbash, S.H.; Schroeder, M.A.; Reid, J.; Sarkaria, J.N. Discordant in vitro and in vivo chemopotentiating effects of the PARP inhibitor veliparib in temozolomide sensitive versus -resistant glioblastoma multiforme xenografts. Clin. Cancer Res. 2014, 20, 3730–3741. [Google Scholar] [CrossRef]
- Plummer, R.; Jones, C.; Middleton, M.; Wilson, R.; Evans, J.; Olsen, A.; Curtin, N.; Boddy, A.; McHugh, P.; Newell, D.; et al. Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 7917–7923. [Google Scholar] [CrossRef]
- Daniel, R.A.; Rozanska, A.L.; Mulligan, E.A.; Drew, Y.; Thomas, H.D.; Castelbuono, D.J.; Hostomsky, Z.; Plummer, E.R.; Tweddle, D.A.; Boddy, A.V.; et al. Central nervous system penetration and enhancement of temozolomide activity in childhood medulloblastoma models by poly(ADP-ribose) polymerase inhibitor AG-014699. Br. J. Cancer 2010, 103, 1588–1596. [Google Scholar] [PubMed]
- Jue, T.R.; Nozue, K.; Lester, A.J.; Joshi, S.; Schroder, L.B.; Whittaker, S.P.; Nixdorf, S.; Rapkins, R.W.; Khasraw, M.; McDonald, K.L. Veliparib in combination with radiotherapy for the treatment of MGMT unmethylated glioblastoma. J. Transl Med. 2017, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Jalali, R.; Lodder, J.C.; Zandieh-Doulabi, B.; Micha, D.; Melvin, J.E.; Catalan, M.A.; Mansvelder, H.D.; DenBesten, P.; Bronckers, A. The Role of Na:K:2Cl Cotransporter 1 (NKCC1/SLC12A2) in Dental Epithelium during Enamel Formation in Mice. Front. Physiol. 2017, 8, 924. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hou, J. Claudins in barrier and transport function. The kidney. Pflugers Arch. 2017, 469, 105–113. [Google Scholar] [CrossRef]
- Milatza, S.; Krug, M.; Rosenthal, R.; Günzel, D.; Müller, D.; Schulzke, J.D.; Amasheh, S.; Fromm, M. Claudin-3 acts as a sealing component of the tight junction for ions of either charge and uncharged solutes. Biochimica et Biophysica Acta (BBA)-Biomembranes 2010, 1798, 2048–2057. [Google Scholar]
- Milatza, S.; Breiderhoff, T. One gene, two paracellular ion channels claudin-1 in the kidney. Pflugers. Arch. 2017, 469, 115–121. [Google Scholar] [CrossRef]
- Nishikawa, S.; Abe, M. Immunocytochemical localization of claudin-1 in the maturation ameloblasts of rat incisors. Front. Physiol. 2010, 1, 150. [Google Scholar] [CrossRef]
- Inai, T.; Sengoku, A.; Hirose, E.; Iida, H.; Shibata, Y. Differential expression of the tight junction proteins, claudin-1, claudin-4, occludin, ZO-1, and PAR3, in the ameloblasts of rat upper incisors. Anat. Rec. Adv. Integr. Anat. Evol. Biol. Adv. Integr. Anat. Evol. Biol. 2008, 291, 577–585. [Google Scholar] [CrossRef]
- Bardet, C.; Courson, F.; Wu, Y.; Khaddam, M.; Salmo, B.; Ribes, S.; Thumfart, J.; Yamaguti, P.M.; Rochefort, G.Y.; Figueres, M.-L.; et al. Claudin-16 deficiency impairs tight junction function in ameloblasts, leading to abnormal enamel formation. J. Bone Min. Res. 2016, 31, 498–513. [Google Scholar] [CrossRef]
- Bardet, C.; Ribes, S.; Wu, Y.; Diallo, M.T.; Salmon, B.; Breiderhoff, T.; Houillier, P.; Müller, D.; Chaussain, C. Claudin loss-of-function disrupts tight Junctions and impairs amelogenesis. Front. Physiol. 2017, 8, 326. [Google Scholar]
- Yamaguti, P.M.; Neves, F.A.; Hotton, D.; Bardet, C.; De La Dure-Molla, M.; Castro, L.C.; do Carmo Scher, M.; Barbosa, M.E.; Ditsch, C.; Fricain, C.-M.; et al. Amelogenesis imperfecta in familial hypomagnesaemia and hypercalciuria with nephrocalcinosis caused by CLDN19 gene mutations. J. Med. Genet. 2017, 54, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.L.; Park, K.; Turner, R.J.; Watson, G.E.; Nguyen, H.V.; Dennett, M.R.; Hand, A.R.; Flagella, M.; Shull, G.E.; Melvin, J.E. Severe impairment of salivation in Na+/K+/2Cl- cotransporter (NKCC1)-deficient mice. J. Biol Chem. 2000, 275, 26720–26726. [Google Scholar] [CrossRef]
- Zhang, J.; Pu, H.; Zhang, H.; Wei, Z.; Jiang, X.; Xu, M.; Zhang, L.; Zhang, W.; Liu, J.; Meng, H.; et al. Inhibition of Na +-K +-2Cl-cotransporter attenuates blood-brain-barrier disruption in a mouse model of traumatic brain injury. Neurochem. Int. 2017, 111, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 31, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Brook, A.H.; Brook O’Donnell, M.B. The dentition: A complex system demonstrating self-principles. In Proceedings of the 2011 IEEE Fifth International Conference on Self-Adaptive and Self Organising Systems, Ann Arbor, MI, USA, 3–7 October 2011. [Google Scholar]
- Brook, A.H.; Brook O’Donnell, M.D.; Hone, A.; Hart, E.; Hughes, T.E.; Smith, R.N.; Townsend, G.C. General and craniofacial development are complex adaptive processes influenced by diversity. Aust. Dent. J. 2014, 59, 13–22. [Google Scholar] [CrossRef]
- Brook, A.H.; Jernvall, J.; Smith, R.N.; Hughes, T.E.; Townsend, G.C. The Dentition: The outcomes of morphogenesis leading to variations of tooth number, size and shape. Aust. Dent. J. 2014, 59, 131–142. [Google Scholar] [CrossRef]
| Group | Tumor | Number Mice and Sex | Age of Mice at Start (Weeks) | Dose per Day (iv) (One Injection/Day) | Duration of Treatment (Days) |
|---|---|---|---|---|---|
| Experimental | Yes | 3 female | 8–15 weeks | 100 mg/kg TMZ + 25 mg/kg VLP | 5 days |
| Control | Yes | 2 female | 8–15 weeks | Vehicle only | 5 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ansari, S.; Jalali, R.; Bronckers, A.L.J.J.; van Tellingen, O.; Raber-Durlacher, J.; Nadjmi, N.; Brook, A.H.; de Lange, J.; Rozema, F.R. Tooth Formation as Experimental Model to Study Chemotherapy on Tissue Development: Effect of a Specific Dose of Temozolomide/Veliparib. Genes 2022, 13, 1198. https://doi.org/10.3390/genes13071198
Al-Ansari S, Jalali R, Bronckers ALJJ, van Tellingen O, Raber-Durlacher J, Nadjmi N, Brook AH, de Lange J, Rozema FR. Tooth Formation as Experimental Model to Study Chemotherapy on Tissue Development: Effect of a Specific Dose of Temozolomide/Veliparib. Genes. 2022; 13(7):1198. https://doi.org/10.3390/genes13071198
Chicago/Turabian StyleAl-Ansari, Sali, Rozita Jalali, Antonius L. J. J. Bronckers, Olaf van Tellingen, Judith Raber-Durlacher, Nasser Nadjmi, Alan Henry Brook, Jan de Lange, and Frederik R. Rozema. 2022. "Tooth Formation as Experimental Model to Study Chemotherapy on Tissue Development: Effect of a Specific Dose of Temozolomide/Veliparib" Genes 13, no. 7: 1198. https://doi.org/10.3390/genes13071198
APA StyleAl-Ansari, S., Jalali, R., Bronckers, A. L. J. J., van Tellingen, O., Raber-Durlacher, J., Nadjmi, N., Brook, A. H., de Lange, J., & Rozema, F. R. (2022). Tooth Formation as Experimental Model to Study Chemotherapy on Tissue Development: Effect of a Specific Dose of Temozolomide/Veliparib. Genes, 13(7), 1198. https://doi.org/10.3390/genes13071198







