1. Introduction
Autoimmune regulator (AIRE) is a multi-potent protein [
1,
2] that plays a major role in the induction of self-tolerance through the expression of tissue specific antigens (TSAs) [
3] and their presentation to T-cells by medullary thymic epithelial cells [
4] and indirectly by dendritic cells [
5] in the thymus. Cortical thymic epithelial cells also express
AIRE but Nishijima et al. [
6] did not observe TSA gene expression in these cells. AIRE-expressing cells were detected outside the thymus and were named extra-thymic
AIRE-expressing cells (eTACs). Such eTACs were found in secondary murine lymphoid organs [
7], where antigen-presenting cells (APCs) [
8] and eTACs absence was shown to restrict intrauterine development in mice models [
9]. Finding of new eTACs and eTACs in new locations can give us further understanding of the developmental biology and the maintenance of self-tolerance at various stages of ontogenesis at different tissues.
In this work, we analyzed publicly available CD71+ cells’ gene expression data set (GSE199228), found that there is detectable
AIRE gene expression in human fetal liver CD71+ cells, and decided to validate
AIRE gene expression as well as check for the induction of selected TSAs (
GCG,
INS and
TFF3) [
10] gene expression by
AIRE by a less noisy pipeline than NanoString, i.e., touchdown PCR, melt curve analysis and DNA gel electrophoresis.
2. Materials and Methods
2.1. NanoString Data Analysis
We analyzed previously available NanoString data of CD71+ cells from human adult bone marrow and fetal liver parenchyma (GSE199228). We performed background subtraction of the data using the mean of negative controls +2 Standard Deviations in order to filter out noise from the data and considered genes with >2 detected probe count numbers as noise-free.
2.2. Study Population
Human fetal liver parenchyma samples (20–22 weeks of pregnancy) were obtained from the “Bank Stvolovih Kletok” LLC (Tomsk, Russia) cell bank; sex is unknown (n = 6).
2.3. Cell Isolation
We thawed fetal liver parenchyma samples stored in 10% DMSO and 90% FBS (up to 1.5 mL in volume) in a water bath at 37 °C and then washed them with the 6 mL mixture containing 5 mL full RPMI 1640 cell culture medium and 1 mL FBS. We isolated fetal liver parenchyma mononuclear cells using density gradient centrifugation (Ficoll-Paque™ (Sigma-Aldrich, St. Louis, MO, USA) with the density of 1.077 g/mL) at 266 RCF for 30 min.
2.4. Cell Sorting
We performed magnetic sorting of fetal liver parenchyma mononuclear cells using a magnetic stand and a magnet (Miltenyi Biotec, 130-042-102, Bergisch Gladbach, Germany) and anti-CD71 MicroBeads (Miltenyi Biotec, 130-046-201) according to the manufacturer’s protocols.
2.5. Viability Staining
We measured the magnetically sorted cells’ viability on a Countess 3 Automated Cell Counter (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocols using Trypan Blue. Trypan Blue staining showed >95% viability of all samples.
2.6. Flow Cytometry
We washed magnetically sorted cells in PBS. We used anti-CD71-PE (BioLegend, 334106, San Diego, CA, USA) and anti-CD235a-FITC (BioLegend, 349104) antibody for staining according to the manufacturer’s protocols. Flow cytometry showed >94% purity of the cells. A gating strategy was to isolate singlets from cells, measure CD71 in the singlets and measure CD235a in the CD71+ singlets fetal liver CECs. CD71
+ erythroid cells (CECs) comprised >90% of the cells (
Figure 1).
2.7. Total RNA Extraction
We isolated total RNA from cells using the Total RNA Purification Plus Kit (Norgen Biotek, 48400), and measured concentration of the RNA on the NanoDrop 2000c. We froze the total RNA at −80 °C until the reverse transcription of the RNA.
2.8. Reverse Transcription
We performed reverse transcription of the total RNA samples (n = 6) using RNAscribe RT and oligo-dT primers (Biolabmix, R04-50). We used an input of 100 ng of the RNA. Reverse transcription was conducted as following: 55 °C for 50 min, 80 °C for 10 min.
2.9. PCR and Melt Curve Analysis
We performed touchdown PCR with melt curve analysis using UDG HS-qPCR Lo-ROX SYBR (×2) Mix (Biolabmix, MHR033-2040), 1 μL out of the 20 μL of RT product,
AIRE,
INS,
GCG and
TFF3 gene primers at a final concentration of 500 μM. Used primers and predicted amplicon characteristics are presented in the
Table 1. All reaction were conducted using 4 technical replicates. Touchdown PCR and melt curve analysis were conducted as following: 55 °C for 2 min; 95 °C for 5 min; 11 cycles of: 95 °C for 20 s, 65 °C -> 55 °C for 30 s (1 °C/cycle decrement), 72 °C for 1 min; 29 cycles of 95 °C for 15 s, 55 °C for 20 s, 72 °C for 30 s; 72 °C for 5 min; and melt curve 95 °C -> 65 °C (1 °C/step decrement). Melt curve plots are shown in
Figure 2a–d.
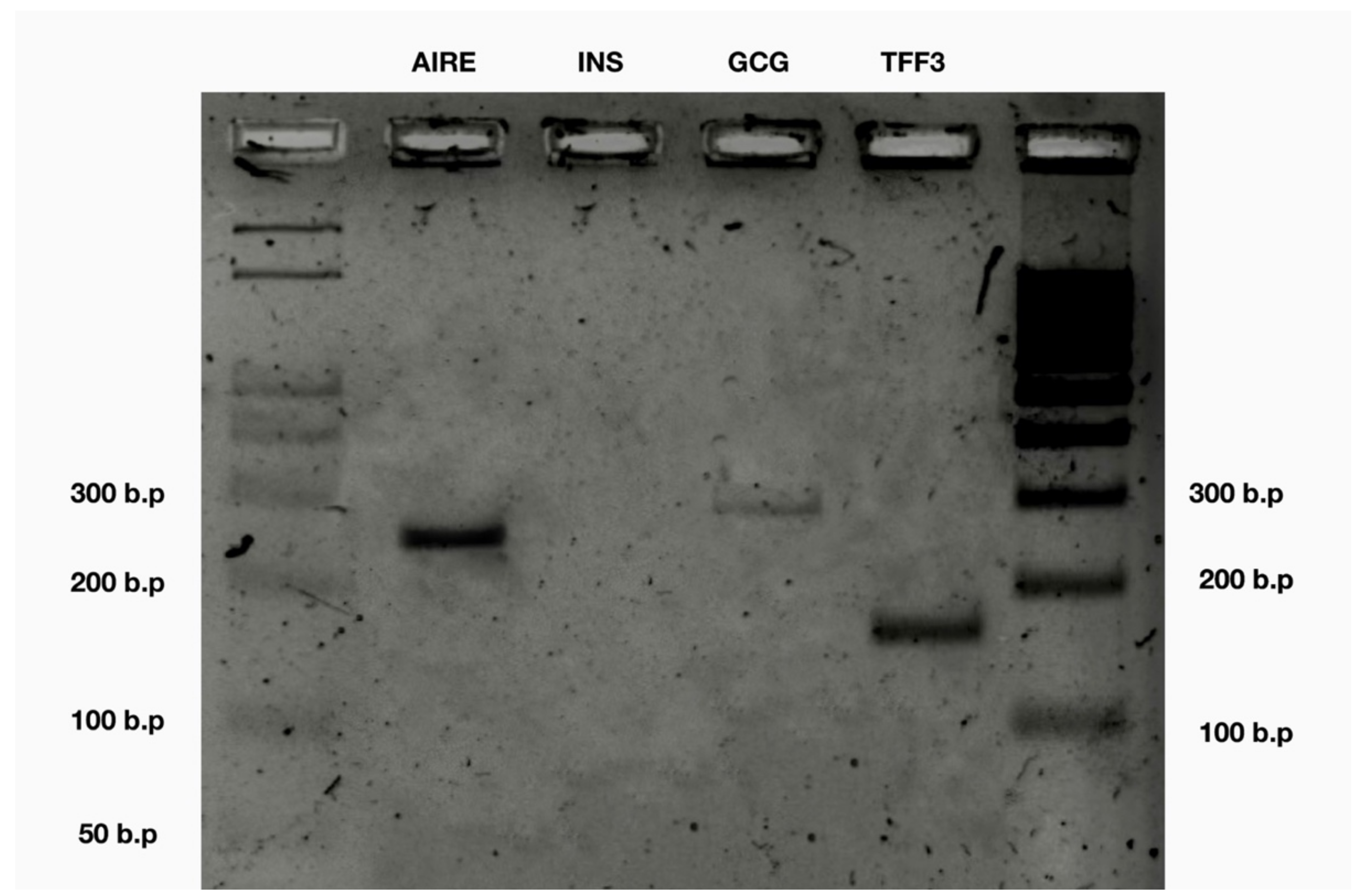
2.10. Agarose Gel-Electrophoresis of PCR Products
We took 1 μL of the PCR product for each gene of interest, combined it with 8 μL of nuclease-free water and 1 μL of 10× gel loading buffer. We used a 15 cm wide 2% agarose gel, Bio-Rad power supply and the Bio-Rad gel tray. We ran the gel electrophoresis for 30 min with the power supply set to 100 V (
Figure 3).
3. Results
Human fetal liver parenchyma CD71+ cells have AIRE gene expression.
We studied a previously published CD71+ cells transcriptome dataset and found that there is AIRE gene expression in human fetal liver CECs (2/4 samples in the dataset had detectable gene expression) but not in adult bone marrow CD71+ cells (0/4 samples in the dataset had detectable gene expression).
Touchdown PCR validation confirms AIRE as well as some tissue-specific antigen gene expression in human fetal liver CECs.
We performed a touchdown PCR with a melt curve and a gel electrophoresis and found
AIRE gene expression as well as the two out of three selected tissue-specific antigens:
GCG and
TFF3, but not
INS (
Table 2).
4. Discussion
In this work, we showed AIRE and TSAs gene expression in human fetal liver parenchyma CD71+ cells. CD71+ cells were mostly represented by CD71+ erythroid cells (CECs) both in this and previous works. We propose a hypothesis that human fetal liver parenchyma CD71+ cells had
AIRE gene expression, which in turn had induced the expression of tissue-specific antigens in these cells. It can also be that CD71+ eTACs help with the maintenance of self-tolerance in the human fetal liver parenchyma by one of the known AIRE-dependent mechanisms, such as generation of T-regs or induction of apoptosis in overzealous T-cells, while leaving any non-TSA-reactive T-cell intact [
1]. Taking into consideration the fact that most of the cells in the analyses were CECs, we can also make an assumption that
AIRE and
TSAs gene expression took place in CECs. Previous works also showed that CECs can express mRNA and produce proteins from a plethora of cytokines and chemokines [
11,
12,
13,
14,
15,
16], and, therefore, are capable of immunoregulation. Absence of
INS gene expression can possibly be due to the lack thereof in the current
AIRE-induced TSA gene expression spectrum, as such events were previously reported [
17].
Experimental limitation of this study is the absence of the protein production validation of AIRE, GCG and TFF3 proteins.
5. Conclusions
We found AIRE and TSAs-expressing cells in an unexpected organ of origin that is the human fetal liver, and which requires further investigation. In the future, we plan to: (1) study AIRE’s and TSAs’ transcripts co-localization in a single cell, (2) establish the precise lineage of these CD71+ AIRE-expressing cells and (3) show AIRE’s and TSAs’ protein production.
Author Contributions
Conceptualization, S.S., R.P.-Z. and O.P.-Z.; methodology, R.P.-Z. and O.P.-Z.; software, R.P.-Z. and O.P.-Z.; validation, R.P.-Z. and O.P.-Z.; formal analysis, R.P.-Z. and O.P.-Z.; investigation, Y.S., S.A., M.V., R.P.-Z. and O.P.-Z.; resources, R.P.-Z. and O.P.-Z.; data curation, R.P.-Z. and O.P.-Z.; writing—original draft preparation, R.P.-Z. and O.P.-Z.; writing—review and editing, R.P.-Z. and O.P.-Z.; visualization, R.P.-Z. and O.P.-Z.; supervision, K.Z. and S.S.; project administration, Y.S. and S.S.; funding acquisition, R.P.-Z., Y.S. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the support of the Russian Science Foundation, project number 21-15-00087.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The previously published publicly available dataset used in this study is available at the Gene Expression Omnibus (GEO) with the accession code GSE199228.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shevyrev, D.; Tereshchenko, V.; Kozlov, V.; Sennikov, S. Phylogeny, Structure, Functions, and Role of AIRE in the Formation of T-Cell Subsets. Cells 2022, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Perniola, R. Twenty years of AIRE. Front. Immunol. 2018, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Venanzi, E.S.; Klein, L.; Chen, Z.; Berzins, S.P.; Turley, S.J.; von Boehmer, H.; Bronson, R.; Dierich, A.; Benoist, C.; et al. Projection of an immunological self shadow within the thymus by the aire protein. Science 2002, 298, 1395–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derbinski, J.; Schulte, A.; Kyewski, B.; Klein, L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2001, 2, 1032–1039. [Google Scholar] [CrossRef] [PubMed]
- Hubert, F.X.; Kinkel, S.A.; Davey, G.M.; Phipson, B.; Mueller, S.N.; Liston, A.; Proietto, A.I.; Cannon, P.Z.; Forehan, S.; Smyth, G.K.; et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood J. Am. Soc. Hematol. 2011, 118, 2462–2472. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, H.; Kitano, S.; Miyachi, H.; Morimoto, J.; Kawano, H.; Hirota, F.; Morita, R.; Mouri, Y.; Masuda, K.; Imoto, I.; et al. Ectopic aire expression in the thymic cortex reveals inherent properties of aire as a tolerogenic factor within the medulla. J. Immunol. 2015, 195, 4641–4649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gardner, J.M.; Fletcher, A.L.; Anderson, M.S.; Turley, S.J. AIRE in the thymus and beyond. Curr. Opin. Immunol. 2009, 21, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.M.; Metzger, T.C.; McMahon, E.J.; Au-Yeung, B.B.; Krawisz, A.K.; Lu, W.; Price, J.D.; Johannes, K.P.; Satpathy, A.T.; Murphy, K.M.; et al. Extrathymic Aire-expressing cells are a distinct bone marrow-derived population that induce functional inactivation of CD4+ T cells. Immunity 2013, 39, 560–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillis-Buck, E.; Miller, H.; Sirota, M.; Sanders, S.J.; Ntranos, V.; Anderson, M.S.; Gardner, J.M.; MacKenzie, T.C. Extrathymic Aire-expressing cells support maternal-fetal tolerance. Sci. Immunol. 2021, 6, eabf1968. [Google Scholar] [CrossRef] [PubMed]
- Kont, V.; Laan, M.; Kisand, K.; Merits, A.; Scott, H.S.; Peterson, P. Modulation of Aire regulates the expression of tissue-restricted antigens. Mol. Immunol. 2008, 45, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Sennikov, S.V.; Eremina, L.V.; Samarin, D.M.; Avdeev, I.V.; Kozlov, V.A. Cytokine gene expression in erythroid cells. Eur. Cytokine Netw. 1996, 7, 771–774. [Google Scholar] [PubMed]
- Sennikov, S.V.; Injelevskaya, T.V.; Krysov, S.V.; Silkov, A.N.; Kovinev, I.B.; Dyachkova, N.J.; Zenkov, A.N.; Loseva, M.I.; Kozlov, V.A. Production of hemo-and immunoregulatory cytokines by erythroblast antigen+ and glycophorin A+ cells from human bone marrow. BMC Cell Biol. 2004, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sennikov, S.V.; Krysov, S.V.; Injelevskaya, T.V.; Silkov, A.N.; Kozlov, V.A. Production of cytokines by immature erythroid cells derived from human embryonic liver. Eur. Cytokine Netw. 2001, 12, 274–279. [Google Scholar] [PubMed]
- Denisova, V.V.; Kulagin, A.; Lisukov, I.A.; Kryuchkova, I.; Sizikova, S.; Sennikov, S.V.; Kozlov, V.A. Cytokine-producing activity of bone marrow erythrokaryocytes and its regulation under normal conditions. Bull. Exp. Biol. Med. 2007, 143, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Seledtsov, V.I.; Seledtsova, G.V.; Samarin, D.M.; Taraban, V.Y.; Sennikov, S.V.; Kozlov, V.A. Characterization of erythroid cell-derived natural suppressor activity. Immunobiology 1998, 198, 361–374. [Google Scholar] [CrossRef]
- Shahbaz, S.; Bozorgmehr, N.; Koleva, P.; Namdar, A.; Jovel, J.; Fava, R.A.; Elahi, S. CD71+ VISTA+ erythroid cells promote the development and function of regulatory T cells through TGF-β. PLoS Biol. 2018, 16, e2006649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, M.M.; Zemmour, D.; Mathis, D.; Benoist, C. Aire controls gene expression in the thymic epithelium with ordered stochasticity. Nat. Immunol. 2015, 16, 942–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).