Circular RNAs: New Players in Cardiomyopathy
Abstract
1. Background
1.1. Cardiomyopathies
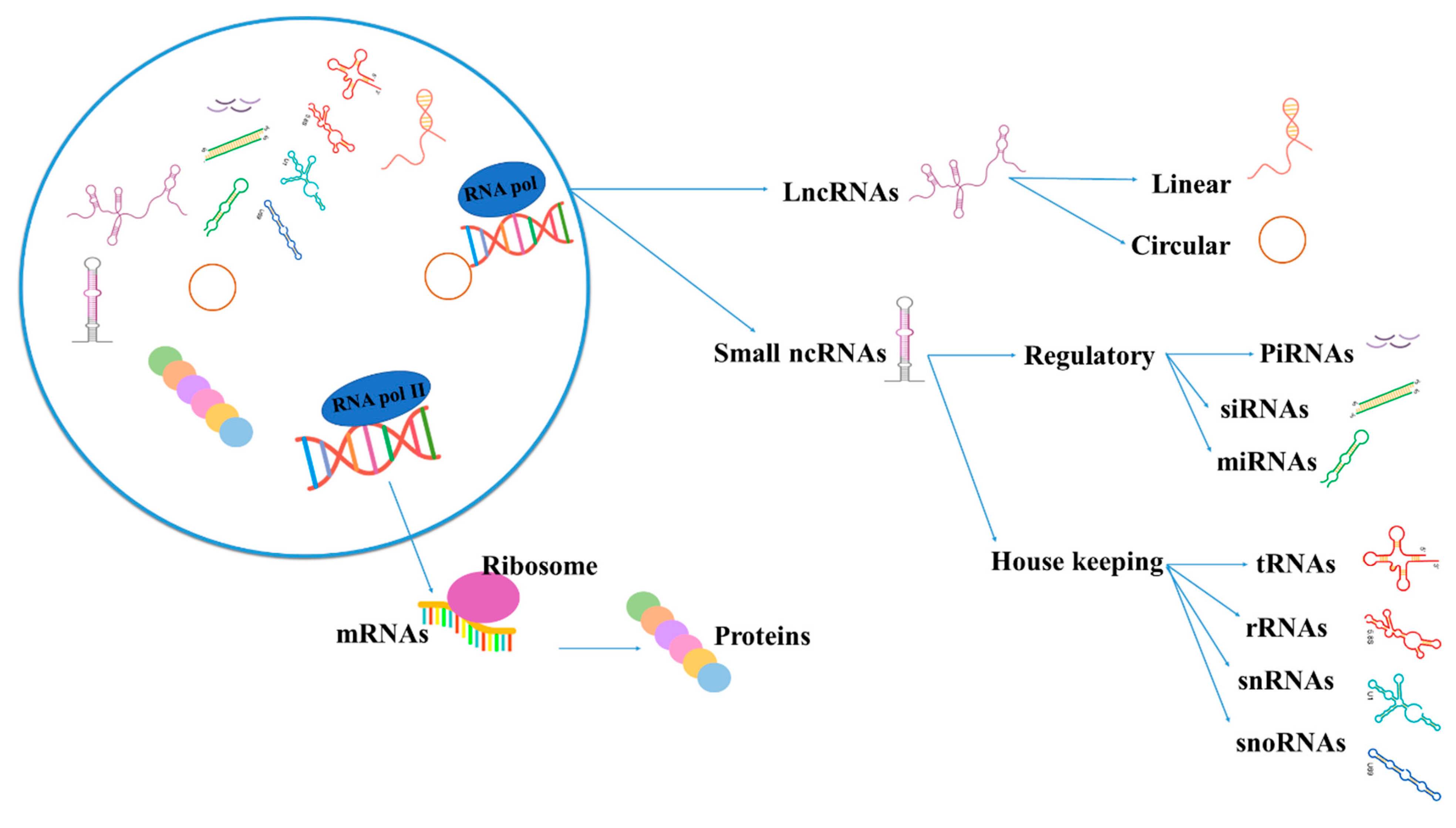
1.2. Noncoding RNAs (ncRNAs)
1.3. CircRNAs
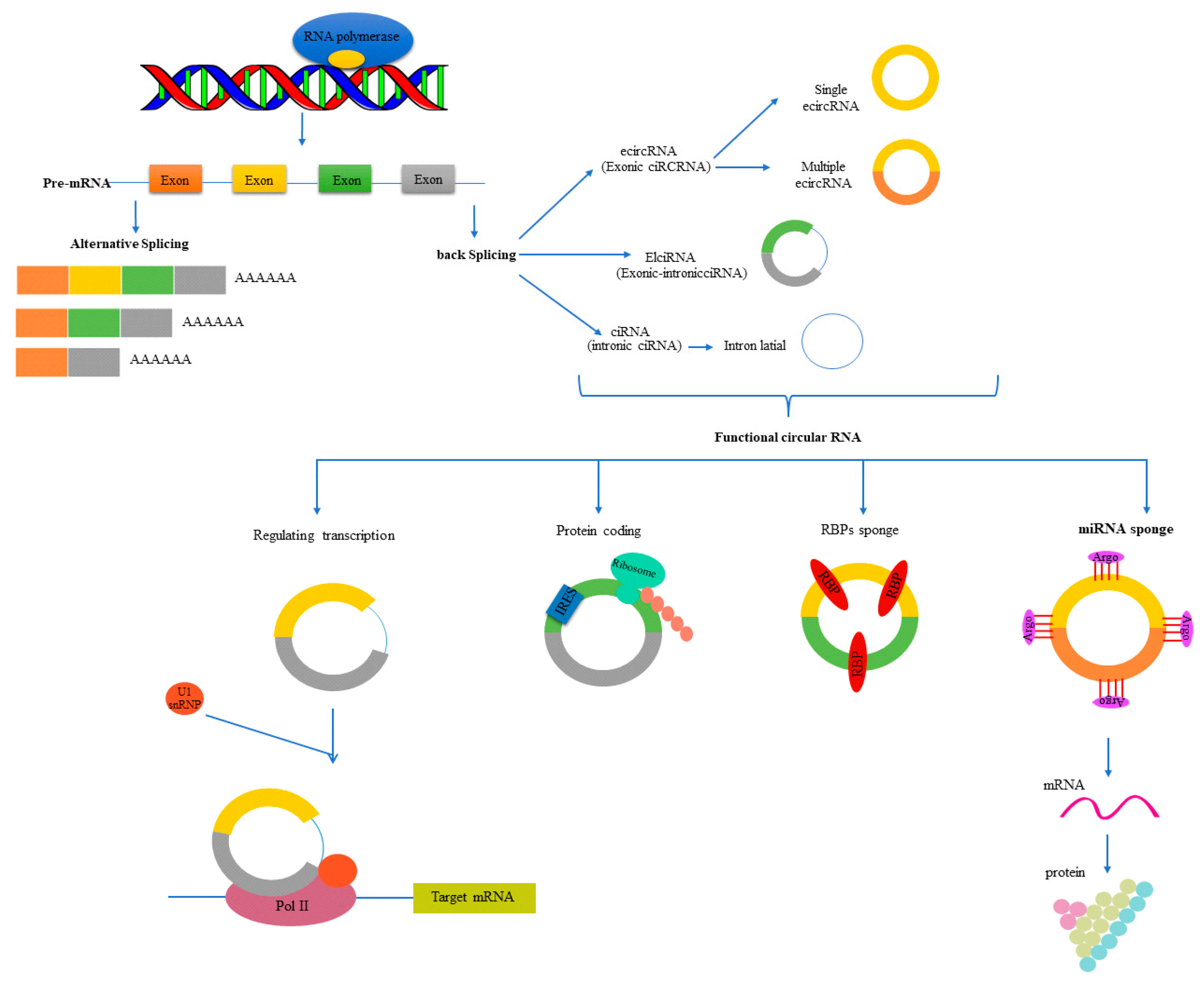
1.3.1. Formation (Biogenesis) and Classes of circRNAs
1.3.2. Nomenclature of circRNAs
1.3.3. Localization of circRNAs
1.3.4. Functions of circRNAs
2. CircRNAs in Cardiomyopathies
2.1. DCM
2.1.1. CircSLC8A1
2.1.2. Deregulated circRNAs in DCM
2.2. HCM
3. Diabetic Cardiomyopathy
3.1. Circ-HIPK3
3.2. Dysregulated circRNAs
4. Ischemic Cardiomyopathy (ICM)
Circ-Fndc3b
5. Doxorubicin-Induced Cardiomyopathy (DIC)
5.1. Circ-Amotl
5.2. Circ-FoxO3
5.3. Circ-ITCH
6. Cardiomyopathy Caused by Alcohol
7. CircRNAs in the Animal Model of Cardiomyopathy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| circRNAs | Circular RNAs |
| DCM | Dilated cardiomyopathy |
| HCM | Hypertrophic cardiomyopathy |
| RCM | Restrictive cardiomyopathy |
| ARVCM | Arrhythmogenic right ventricular cardiomyopathy |
| ncRNA | Noncoding RNA |
| piRNAs | Piwi-interacting RNAs |
| miRNAs | MicroRNAs |
| siRNAs | Small interfering RNAs |
| lncRNAs | Long noncoding RNAs |
| rRNAs | Ribosomal RNAs |
| tRNAs | Transfer RNAs |
| snRNAs | Small nuclear RNAs |
| snoRNAs | Small nucleolar RNAs |
| ecircRNA | Exonic circular RNAs |
| RCMs | Reverse complementary matches |
| ADAR | Adenosine deaminases acting on RNA |
| ciRNAs | Circular intronic RNAs |
| elciRNAs | Exon-intron circular RNAs |
| HUGO | Human genome organization |
| RBPs | RNA-binding proteins |
| Hectd1 | HECT domain E3 ubiquitin-protein ligase 1 |
| Ppp2r3 α | Protein phosphatase 2 regulatory subunit B’’ alpha |
| Slc8a1 | Solute carrier family 8 (SODIUM-CALCIUM EXCHANGER) member A1 |
| Dmd | Dystrophin |
| Ttn | Titin |
| CMs | Cardiomyocytes |
| ARID1A | AT-rich interaction domain 1A |
| FNDC3B | Fibronectin type III domain containing 3B |
| CACNA1D | Calcium voltage-gated channel subunit alpha1 D |
| SPHKAP | SPHK1 interactor, AKAP domain containing |
| ALPK2 | Alpha kinase 2 |
| AASS | Aminoadipate-semialdehyde synthase |
| FIRRE | Firre intergenic repeating RNA element |
| TMEFF1 | Transmembrane protein with EGF-like and two follistatin-like domains 1 |
| CHD7 | Chromodomain helicase DNA-binding protein 7 |
| ATXN10 | Ataxin 10 |
| DNAJ6C | DnaJ heat shock protein family (Hsp40) member B6 |
| ICA1 | Islet cell autoantigen 1 |
| BTBD7 | BTB domain containing 7 |
| FAT1 | FAT atypical cadherin 1 |
| LYPLAL1 | Lysophospholipase-like 1 |
| NHLRC2 | NHL repeat containing 2 |
| DHX40 | DEAH-box helicase 40 |
| PKN2 | Protein kinase N2 |
| MYH7 | Myosin heavy chain 7 |
| EBF1 | EBF transcription factor 1 |
| ZNF670 | Zinc finger protein 670 |
| SEC23A | SEC23 homolog A, COPII coat complex component |
| NBEA | Neurobeachin |
| PDE1C | Phosphodiesterase 1C |
| CTNND2 | Catenin delta 2 |
| ATRX | ATRX chromatin remodeler |
| OR2A1-AS1 | OR2A1 antisense RNA 1 |
| NPPA | Natriuretic peptide A |
| MYH6 | Myosin heavy chain 6 |
| RYR2 | Ryanodine receptor 2 |
| SCAF8 | SR-related CTD associated factor 8 |
| TIAM2 | TIAM Rac1 associated GEF 2 |
| RBM20 | RNA-binding motif protein 20 |
| MBOAT2 | Membrane bound O-acyltransferase domain containing 2 |
| TMEM56 | Transmembrane protein 56 (TLCD4 (TLC Domain Containing 4)) |
| HRCR | Heart-related circRNA |
| ARC | Activity-regulated cytoskeleton-associated protein |
| GO | Gene ontology |
| TRP | Transient receptor potential |
| HIPK3 | Homeodomain interacting protein kinase 3 |
| ceRNA | Competing endogenous RNA |
| COL1A1 | Collagen type I alpha 1 chain |
| COL3A1 | Collagen type III alpha 1 chain |
| CACR | Caspase-1-associated circRNA |
| Myo9a | Myosin IXA |
| COL1A2 | Collagen type I alpha 2 chain |
| α-SMA | α-smooth muscle actin |
| CTGF | Connective tissue growth factor |
| TGF-β1 | Transforming growth factor beta 1 |
| BCL2 | B-cell lymphoma 2 |
| ZNT7 | Zinc transporter 7 (SLC30A7) |
| Fndc3b | Fibronectin type III domain containing 3B |
| VEGF | Vascular endothelial growth factor |
| FUS | RNA-binding protein fused in the sarcoma |
| DIC | Doxorubicin-induced cardiomyopathy |
| DOX | Doxorubicin |
| ROS | Reactive oxygen species |
| TOP2β | Topoisomerase II-β |
| Amotl1 | Angiomotin-like 1 |
| Amotl2 | Angiomotin-like 2 |
| AKT | Protein kinase B (PKB) |
| pAKT | Phosphorylated AKT |
| PDK | Pyruvate dehydrogenase kinase |
| FOXO3 | Forkhead box O3 |
| RBPs | RNA-binding proteins |
| ITCH | Itchy E3 ubiquitin-protein ligase |
| hiPSC-CMs | Human-induced pluripotent stem-cell-derived cardiomyocytes |
| SIRT6 | Sirtuin 6 |
| SERCA2a | Sarco/endoplasmic reticulum calcium (Ca2+) ATPase |
| ACM | Alcoholic cardiomyopathy |
| circ-ITCH | CircRNA-itchy E3 ubiquitin-protein ligase |
| qRT-PCR | Quantitative reverse transcription-polymerase chain reaction |
| rt-circRNAs | Read-through circRNAs |
References
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [PubMed]
- Beqqali, A. Alternative splicing in cardiomyopathy. Biophys. Rev. 2018, 10, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [PubMed]
- Braunwald, E. Cardiomyopathies: An overview. Circ. Res. 2017, 121, 711–721. [Google Scholar] [CrossRef]
- Salemi, V.M.C.; Mohty, D.; Altavila, S.L.; Melo, M.D.; Kalil Filho, R.; Bocchi, E.A. Insights into the Classification of Cardiomyopathies: Past, Present, and Future Directions. SciELO Bras. 2021, 76, e2808. [Google Scholar] [CrossRef]
- Malakootian, M.; Bagheri Moghaddam, M.; Kalayinia, S.; Farrashi, M.; Maleki, M.; Sadeghipour, P.; Amin, A. Dilated cardiomyopathy caused by a pathogenic nucleotide variant in RBM20 in an Iranian family. BMC Med. Genom. 2022, 15, 106. [Google Scholar] [CrossRef]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef]
- Arbustini, E.; Narula, N.; Dec, G.W.; Reddy, K.S.; Greenberg, B.; Kushwaha, S.; Marwick, T.; Pinney, S.; Bellazzi, R.; Favalli, V.; et al. The MOGE (S) classification for a phenotype–genotype nomenclature of cardiomyopathy: Endorsed by the World Heart Federation. J. Am. Coll. Cardiol. 2013, 62, 2046–2072. [Google Scholar] [CrossRef]
- Amaral, P.P.; Mattick, J.S. Noncoding RNA in development. Mamm. Genome 2008, 19, 454–492. [Google Scholar] [CrossRef]
- Lander, E.S.; Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860. [Google Scholar]
- Consortium, E.P. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Azad, F.M.; Naeli, P.; Pakzad, M.; Fouani, Y.; Bajgan, E.T.; Baharvand, H.; Mowla, S.J. Novel spliced variants of OCT4, OCT4C and OCT4C1, with distinct expression patterns and functions in pluripotent and tumor cell lines. Eur. J. Cell Biol. 2017, 96, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Khraiwesh, B.; Arif, M.A.; Seumel, G.I.; Ossowski, S.; Weigel, D.; Reski, R.; Frank, W. Transcriptional control of gene expression by microRNAs. Cell 2010, 140, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Bonasio, R.; Shiekhattar, R. Regulation of transcription by long noncoding RNAs. Annu. Rev. Genet. 2014, 48, 433–455. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Hombach, S.; Kretz, M. Non-coding RNAs: Classification, biology and functioning. Non-Coding RNAs Colorectal Cancer 2016, 937, 3–17. [Google Scholar]
- La Ferlita, A.; Battaglia, R.; Andronico, F.; Caruso, S.; Cianci, A.; Purrello, M.; Di Pietro, C. Non-coding RNAs in endometrial physiopathology. Int. J. Mol. Sci. 2018, 19, 2120. [Google Scholar] [CrossRef]
- Amin, N.; McGrath, A.; Chen, Y.-P.P. Evaluation of deep learning in non-coding RNA classification. Nat. Mach. Intell. 2019, 1, 246–256. [Google Scholar] [CrossRef]
- Malakootian, M.; Naeli, P.; Mowla, S.J.; Seidah, N.G. Post-Transcriptional Effects of miRNAs on PCSK7 Expression and Function: miR-125a-5p, miR-143-3p and miR-409-3p as Negative Regulators. Metabolites 2022, 12, 588. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Chen, L.-L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-L.; Yang, L. Regulation of circRNA biogenesis. RNA Biol. 2015, 12, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Vicens, Q.; Westhof, E. Biogenesis of circular RNAs. Cell 2014, 159, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Zaphiropoulos, P.G. Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: Correlation with exon skipping. Proc. Natl. Acad. Sci. USA 1996, 93, 6536–6541. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef]
- Li, X.; Liu, S.; Zhang, L.; Issaian, A.; Hill, R.C.; Espinosa, S.; Shi, S.; Cui, Y.; Kappel, K.; Das, R.; et al. A unified mechanism for intron and exon definition and back-splicing. Nature 2019, 573, 375–380. [Google Scholar] [CrossRef]
- Yang, L.; Fu, J.; Zhou, Y. Circular RNAs and their emerging roles in immune regulation. Front. Immunol. 2018, 9, 2977. [Google Scholar] [CrossRef]
- Lim, T.B.; Aliwarga, E.; Luu, T.D.; Li, Y.P.; Ng, S.L.; Annadoray, L.; Sian, S.; Ackers-Johnson, M.A.; Foo, R.S. Targeting the highly abundant circular RNA circSlc8a1 in cardiomyocytes attenuates pressure overload induced hypertrophy. Cardiovasc. Res. 2019, 115, 1998–2007. [Google Scholar] [CrossRef]
- Altesha, M.A.; Ni, T.; Khan, A.; Liu, K.; Zheng, X. Circular RNA in cardiovascular disease. J. Cell. Physiol. 2019, 234, 5588–5600. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.O.; Chen, T.; Xiang, J.F.; Yin, Q.F.; Xing, Y.H.; Zhu, S.; Yang, L.; Chen, L.L. Circular intronic long noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef]
- Ashwal-Fluss, R.; Meyer, M.; Pamudurti, N.R.; Ivanov, A.; Bartok, O.; Hanan, M.; Evantal, N.; Memczak, S.; Rajewsky, N.; Kadener, S. circRNA biogenesis competes with pre-mRNA splicing. Mol. Cell 2014, 56, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Conn, S.J.; Pillman, K.A.; Toubia, J.; Conn, V.M.; Salmanidis, M.; Phillips, C.A.; Roslan, S.; Schreiber, A.W.; Gregory, P.A.; Goodall, G.J. The RNA binding protein quaking regulates formation of circRNAs. Cell 2015, 160, 1125–1134. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Zhong, Y.; Shang, R.; Zhang, X.; Song, W.; Kjems, J.; Li, H. The emerging landscape of circular RNA in life processes. RNA Biol. 2017, 14, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Q.; Shen, J.; Yang, B.B.; Ding, X. Circbank: A comprehensive database for circRNA with standard nomenclature. RNA Biol. 2019, 16, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Salzman, J.; Gawad, C.; Wang, P.L.; Lacayo, N.; Brown, P.O. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS ONE 2012, 7, e30733. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef]
- Prestes, P.R.; Maier, M.C.; Woods, B.A.; Charchar, F.J. A guide to the short, long and circular RNAs in hypertension and cardiovascular disease. Int. J. Mol. Sci. 2020, 21, 3666. [Google Scholar] [CrossRef]
- Yang, Q.; Du, W.W.; Wu, N.; Yang, W.; Awan, F.M.; Fang, L.; Ma, J.; Li, X.; Zeng, Y.; Yang, Z.; et al. A circular RNA promotes tumorigenesis by inducing c-myc nuclear translocation. Cell Death Differ. 2017, 24, 1609–1620. [Google Scholar] [CrossRef]
- Wang, L.; Long, H.; Zheng, Q.; Bo, X.; Xiao, X.; Li, B. Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression. Mol. Cancer 2019, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, J.; Deng, H.; Ma, R.; Liao, J.Y.; Liang, H.; Hu, J.; Li, J.; Guo, Z.; Cai, J.; et al. Targeting mitochondria-located circRNA SCAR alleviates NASH via reducing mROS output. Cell 2020, 183, 76–93.e22. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, H.; Wang, C.; Liu, W.; Liu, M.; Zhu, Y.; Xu, W.; Jin, H.; Li, J. Mitochondrial genome-derived circRNA mc-COX2 functions as an oncogene in chronic lymphocytic leukemia. Mol. Ther. Nucleic Acids 2020, 20, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, K.; Panda, A.C.; Munk, R.; Grammatikakis, I.; Dudekula, D.B.; De, S.; Kim, J.; Noh, J.H.; Kim, K.M.; Martindale, J.L.; et al. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 2017, 14, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.-S.; Ai, Y.; Wilusz, J.E. Biogenesis and functions of circular RNAs come into focus. Trends Cell Biol. 2020, 30, 226–240. [Google Scholar] [CrossRef]
- Li, M.-L.; Wang, W.; Jin, Z.-B. Circular RNAs in the Central Nervous System. Front. Mol. Biosci. 2021, 8, 169. [Google Scholar] [CrossRef]
- Malakootian, M.; Gholipour, A.; Bagheri Moghaddam, M.; Arabian, M.; Oveisee, M. Potential roles of circular rnas and environmental and clinical factors in intervertebral disc degeneration. Environ. Health Eng. Manag. J. 2022, 9, 189–200. [Google Scholar] [CrossRef]
- Zhang, N.; Nan, A.; Chen, L.; Li, X.; Jia, Y.; Qiu, M.; Dai, X.; Zhou, H.; Zhu, J.; Zhang, H.; et al. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol. Cancer 2020, 19, 101. [Google Scholar] [CrossRef]
- Yang, B.; Li, L.; Tong, G.; Zeng, Z.; Tan, J.; Su, Z.; Liu, Z.; Lin, J.; Gao, W.; Chen, J.; et al. Circular RNA circ_001422 promotes the progression and metastasis of osteosarcoma via the miR-195-5p/FGF2/PI3K/Akt axis. J. Exp. Clin. Cancer Res. 2021, 40, 235. [Google Scholar] [CrossRef]
- Cen, J.; Liang, Y.; Huang, Y.; Pan, Y.; Shu, G.; Zheng, Z.; Liao, X.; Zhou, M.; Chen, D.; Fang, Y.; et al. Circular RNA circSDHC serves as a sponge for miR-127-3p to promote the proliferation and metastasis of renal cell carcinoma via the CDKN3/E2F1 axis. Mol. Cancer 2021, 20, 19. [Google Scholar] [CrossRef]
- Wang, S.; Tong, H.; Shi, W.; Ma, F.; Quan, Z. CircPVT1 promotes gallbladder cancer growth by sponging miR-339-3p and regulates MCL-1 expression. Cell Death Discov. 2021, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Y.; Liang, G.; Ling, Y.; Tan, W.; Tan, L.; Andrews, R.; Zhong, W.; Zhang, X.; Song, E.; et al. Circular RNA hsa_circ_001783 regulates breast cancer progression via sponging miR-200c-3p. Cell Death Dis. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.; Machill, K.; Schulzki, I.; Schumacher, D.; Werner, D. Acute reversible cardiomyopathy with cardiogenic shock in a patient with Addisonian crisis: A case report. Int. J. Cardiol. 2007, 116, e71–e73. [Google Scholar] [CrossRef] [PubMed]
- Devaux, Y.; Creemers, E.E.; Boon, R.A.; Werfel, S.; Thum, T.; Engelhardt, S.; Dimmeler, S.; Squire, I.; Cardiolinc Network. Circular RNAs in heart failure. Eur. J. Heart Fail. 2017, 19, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Weng, X.; Zhao, Y.; Chen, W.; Gan, T.; Xu, D. Circular RNAs in cardiovascular disease: An overview. BioMed Res. Int. 2017, 2017, 5135781. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Gu, L.; Guo, Y.; Zhou, Y.; Hua, L.; Chen, J.; He, S.; Zhang, S.; Jia, Q.; Zhao, C.; et al. Association between circular RNA expression content and severity of coronary atherosclerosis in human coronary artery. J. Clin. Lab. Anal. 2020, 34, e23552. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.-Y.; Zhao, C.H.; Yuan, J.X.; Zhang, Y.J.; Jin, J.L.; Gu, M.F.; Mao, Z.Y.; Sun, H.J.; Jia, Q.W.; Ji, M.Y.; et al. Circular RNA profile in coronary artery disease. Am. J. Transl. Res. 2019, 11, 7115. [Google Scholar]
- Vilades, D.; Martínez-Camblor, P.; Ferrero-Gregori, A.; Bär, C.; Lu, D.; Xiao, K.; Vea, À.; Nasarre, L.; Sanchez Vega, J.; Leta, R.; et al. Plasma circular RNA hsa_circ_0001445 and coronary artery disease: Performance as a biomarker. FASEB J. 2020, 34, 4403–4414. [Google Scholar] [CrossRef]
- Chen, C.; Shen, H.; Huang, Q.; Li, Q. The circular RNA CDR1as regulates the proliferation and apoptosis of human cardiomyocytes through the miR-135a/HMOX1 and miR-135b/HMOX1 axes. Genet. Test. Mol. Biomark. 2020, 24, 537–548. [Google Scholar] [CrossRef]
- .Jakobi, T.; Czaja-Hasse, L.F.; Reinhardt, R.; Dieterich, C. Profiling and validation of the circular RNA repertoire in adult murine hearts. Genom. Proteom. Bioinform. 2016, 14, 216–223. [Google Scholar] [CrossRef]
- Kokot, K.E.; Kneuer, J.M.; John, D.; Rebs, S.; Möbius-Winkler, M.N.; Erbe, S.; Müller, M.; Andritschke, M.; Gaul, S.; Sheikh, B.N.; et al. Reduction of A-to-I RNA editing in the failing human heart regulates formation of circular RNAs. Basic Res. Cardiol. 2022, 117, 32. [Google Scholar] [CrossRef]
- Fischer, J.W.; Leung, A.K. CircRNAs: A regulator of cellular stress. Crit. Rev. Biochem. Mol. Biol. 2017, 52, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.U.; Agarwal, V.; Guo, H.; Bartel, D.P. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014, 15, 409. [Google Scholar] [CrossRef] [PubMed]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of circRNAs. Mol. Cell 2017, 66, 9–21.e7. [Google Scholar] [CrossRef] [PubMed]
- Vo, J.N.; Cieslik, M.; Zhang, Y.; Shukla, S.; Xiao, L.; Zhang, Y.; Wu, Y.M.; Dhanasekaran, S.M.; Engelke, C.G.; Cao, X.; et al. The landscape of circular RNA in cancer. Cell 2019, 176, 869–881.e13. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.Q.; Zhao, Q.; Wang, F.; Lan, P.; Wang, Z.; Zuo, Z.X.; Wu, Q.N.; Fan, X.J.; Mo, H.Y.; Chen, L.; et al. A circRNA signature predicts postoperative recurrence in stage II/III colon cancer. EMBO Mol. Med. 2019, 11, e10168. [Google Scholar] [CrossRef]
- McNally, E.M.; Mestroni, L. Dilated cardiomyopathy: Genetic determinants and mechanisms. Circ. Res. 2017, 121, 731–748. [Google Scholar] [CrossRef]
- Hänselmann, A.; Veltmann, C.; Bauersachs, J.; Berliner, D. Dilated cardiomyopathies and non-compaction cardiomyopathy. Herz 2020, 45, 212–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Xu, W.; Chen, J.; Zhou, X. The long non-coding RNA H19 promotes cardiomyocyte apoptosis in dilated cardiomyopathy. Oncotarget 2017, 8, 28588. [Google Scholar] [CrossRef]
- Zhang, X.; Nie, X.; Yuan, S.; Li, H.; Fan, J.; Li, C.; Sun, Y.; Zhao, Y.; Hou, H.; Wang, D.W.; et al. Circulating long non-coding RNA ENST00000507296 is a prognostic indicator in patients with dilated cardiomyopathy. Mol. Ther. Nucleic Acids 2019, 16, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, F.; Tong, J.; Li, Y.; Cai, J.; Wang, Y.; Li, P.; Hao, Y.; Tian, W.; Lv, Y.; et al. Circulating microRNAs as novel biomarkers for dilated cardiomyopathy. Cardiol. J. 2017, 24, 65–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mirzadeh Azad, F.; Arabian, M.; Maleki, M.; Malakootian, M. Small molecules with big impacts on cardiovascular diseases. Biochem. Genet. 2020, 58, 359–383. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-F.; Lytton, J. A circularized sodium-calcium exchanger exon 2 transcript. J. Biol. Chem. 1999, 274, 8153–8160. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.L.; Lim, B.T.; Anene-Nzelu, C.G.; Ackers-Johnson, M.; Dashi, A.; See, K.; Tiang, Z.; Lee, D.P.; Chua, W.W.; Luu, T.D.; et al. A landscape of circular RNA expression in the human heart. Cardiovasc. Res. 2017, 113, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Jahn, C.; Bär, C.; Thum, T. CircSlc8a1, breaking a vicious circle in cardiac hypertrophy. Cardiovasc. Res. 2019, 115, 1946–1947. [Google Scholar] [CrossRef]
- Werfel, S.; Nothjunge, S.; Schwarzmayr, T.; Strom, T.M.; Meitinger, T.; Engelhardt, S. Characterization of circular RNAs in human, mouse and rat hearts. J. Mol. Cell. Cardiol. 2016, 98, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Morey, R.; Palpant, N.J.; Wang, P.L.; Afari, N.; Jiang, C.; Parast, M.M.; Murry, C.E.; Laurent, L.C.; Salzman, J. Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development. Genome Biol. 2015, 16, 126. [Google Scholar] [CrossRef]
- Lei, W.; Feng, T.; Fang, X.; Yu, Y.; Yang, J.; Zhao, Z.A.; Liu, J.; Shen, Z.; Deng, W.; Hu, S. Signature of circular RNAs in human induced pluripotent stem cells and derived cardiomyocytes. Stem Cell Res. Ther. 2018, 9, 56. [Google Scholar] [CrossRef]
- Siede, D.; Rapti, K.; Gorska, A.A.; Katus, H.A.; Altmüller, J.; Boeckel, J.N.; Meder, B.; Maack, C.; Völkers, M.; Müller, O.J.; et al. Identification of circular RNAs with host gene-independent expression in human model systems for cardiac differentiation and disease. J. Mol. Cell. Cardiol. 2017, 109, 48–56. [Google Scholar] [CrossRef]
- Dong, K.; He, X.; Su, H.; Fulton, D.J.; Zhou, J. Genomic analysis of circular RNAs in heart. BMC Med. Genom. 2020, 13, 167. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhao, Y.; Dai, F.; Su, E.; Li, F.; Yan, Y. Analysis of changes in circular RNA expression and construction of ceRNA networks in human dilated cardiomyopathy. J. Cell. Mol. Med. 2021, 25, 2572–2583. [Google Scholar] [CrossRef] [PubMed]
- Brauch, K.M.; Karst, M.L.; Herron, K.J.; de Andrade, M.; Pellikka, P.A.; Rodeheffer, R.J.; Michels, V.V.; Olson, T.M. Mutations in ribonucleic acid binding protein gene cause familial dilated cardiomyopathy. J. Am. Coll. Cardiol. 2009, 54, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Reckman, Y.J.; Aufiero, S.; van den Hoogenhof, M.M.; van der Made, I.; Beqqali, A.; Koolbergen, D.R.; Rasmussen, T.B.; van der Velden, J. RBM20 regulates circular RNA production from the titin gene. Circ. Res. 2016, 119, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Maatz, H.; Jens, M.; Liss, M.; Schafer, S.; Heinig, M.; Kirchner, M.; Adami, E.; Rintisch, C.; Dauksaite, V.; Radke, M.H.; et al. RNA-binding protein RBM20 represses splicing to orchestrate cardiac pre-mRNA processing. J. Clin. Investig. 2014, 124, 3419–3430. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Schafer, S.; Greaser, M.L.; Radke, M.H.; Liss, M.; Govindarajan, T.; Maatz, H.; Schulz, H.; Li, S.; Parrish, A.M.; et al. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat. Med. 2012, 18, 766–773. [Google Scholar] [CrossRef]
- Tuohy, C.V.; Kaul, S.; Song, H.K.; Nazer, B.; Heitner, S.B. Hypertrophic cardiomyopathy: The future of treatment. Eur. J. Heart Fail. 2020, 22, 228–240. [Google Scholar] [CrossRef]
- Morrow, A.G.; Braunwald, E. Functional aortic stenosis: A malformation characterized by resistance to left ventricular outflow without anatomic obstruction. Circulation 1959, 20, 181–189. [Google Scholar] [CrossRef]
- Teare, D. Asymmetrical hypertrophy of the heart in young adults. Br. Heart J. 1958, 20, 1–8. [Google Scholar] [CrossRef]
- Geske, J.B.; Ommen, S.R.; Gersh, B.J. Hypertrophic cardiomyopathy: Clinical update. JACC Heart Fail. 2018, 6, 364–375. [Google Scholar] [CrossRef]
- Maron, B.J.; Gardin, J.M.; Flack, J.M.; Gidding, S.S.; Kurosaki, T.T.; Bild, D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: Echocardiographic analysis of 4111 subjects in the CARDIA study. Circulation 1995, 92, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Sonnenschein, K.; Wilczek, A.L.; de Gonzalo-Calvo, D.; Pfanne, A.; Derda, A.A.; Zwadlo, C.; Bavendiek, U.; Bauersachs, J.; Fiedler, J.; Thum, T. Serum circular RNAs act as blood-based biomarkers for hypertrophic obstructive cardiomyopathy. Sci. Rep. 2019, 9, 20350. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, J.; Sun, R.; He, Z.; Chen, Q.; Liu, W.; Wu, M.; Bao, J.; Liu, Z.; Wang, J.; et al. Comprehensive construction of a circular RNA-associated competing endogenous RNA network identified novel circular RNAs in hypertrophic cardiomyopathy by integrated analysis. Front. Genet. 2020, 11, 764. [Google Scholar] [CrossRef]
- Wang, K.; Long, B.O.; Liu, F.; Wang, J.X.; Liu, C.Y.; Zhao, B.; Zhou, L.Y.; Sun, T.; Wang, M.; Yu, T.; et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur. Heart J. 2016, 37, 2602–2611. [Google Scholar] [CrossRef]
- Honke, N. Die Bedeutung einer Forcierenden Virusreplikation auf die Aktivierung des Adaptiven Immunsystems und Zerstörung der Selbsttoleranz; Universitäts-und Landesbibliothek der Heinrich-Heine-Universität Düsseldorf: Düsseldorf, Germany, 2014. [Google Scholar]
- Parim, B.; Uddandrao, V.S.; Saravanan, G. Diabetic cardiomyopathy: Molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail. Rev. 2019, 24, 279–299. [Google Scholar] [CrossRef]
- Rubler, S.; Dlugash, J.; Yuceoglu, Y.Z.; Kumral, T.; Branwood, A.W.; Grishman, A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972, 30, 595–602. [Google Scholar] [CrossRef]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016, 12, 144–153. [Google Scholar] [CrossRef]
- Hu, X.; Bai, T.; Xu, Z.; Liu, Q.; Zheng, Y.; Cai, L. Pathophysiological fundamentals of diabetic cardiomyopathy. Compr. Physiol. 2011, 7, 693–711. [Google Scholar]
- Li, Y.; Zheng, F.; Xiao, X.; Xie, F.; Tao, D.; Huang, C.; Liu, D.; Wang, M.; Wang, L.; Zeng, F.; et al. Circ HIPK 3 sponges miR-558 to suppress heparanase expression in bladder cancer cells. EMBO Rep. 2017, 18, 1646–1659. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Liao, Q. CircHIPK3: A promising cancer-related circular RNA. Am. J. Transl. Res. 2020, 12, 6694. [Google Scholar] [PubMed]
- Wang, W.; Zhang, S.; Xu, L.; Feng, Y.; Wu, X.; Zhang, M.; Yu, Z.; Zhou, X. Involvement of circHIPK3 in the pathogenesis of diabetic cardiomyopathy in mice. Diabetologia 2021, 64, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, A.; Qin, Y.; Che, H.; Wang, Y.; Lv, J.; Li, Y.; Li, H.; Yue, E.; Ding, X.; et al. A novel circular RNA mediates pyroptosis of diabetic cardiomyopathy by functioning as a competing endogenous RNA. Mol. Ther. Nucleic Acids 2019, 17, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-M.; Zhang, M.; Huang, L.; Hu, Z.Q.; Zhu, J.N.; Xiao, Z.; Zhang, Z.; Lin, Q.X.; Zheng, X.L.; Yang, M.; et al. CircRNA_000203 enhances the expression of fibrosis-associated genes by derepressing targets of miR-26b-5p, Col1a2 and CTGF, in cardiac fibroblasts. Sci. Rep. 2017, 7, 40342. [Google Scholar] [CrossRef]
- Zhou, B.; Yu, J.-W. A novel identified circular RNA, circRNA_010567, promotes myocardial fibrosis via suppressing miR-141 by targeting TGF-β1. Biochem. Biophys. Res. Commun. 2017, 487, 769–775. [Google Scholar] [CrossRef]
- Dong, S.; Tu, C.; Ye, X.; Li, L.; Zhang, M.; Xue, A.; Chen, S.; Zhao, Z.; Cong, B.; Lin, J.; et al. Expression profiling of circular RNAs and their potential role in early-stage diabetic cardiomyopathy. Mol. Med. Rep. 2020, 22, 1958–1968. [Google Scholar] [CrossRef]
- Schuster, A.; Morton, G.; Chiribiri, A.; Perera, D.; Vanoverschelde, J.L.; Nagel, E. Imaging in the management of ischemic cardiomyopathy: Special focus on magnetic resonance. J. Am. Coll. Cardiol. 2012, 59, 359–370. [Google Scholar] [CrossRef]
- Hasenfuss, G.; Pieske, B. Calcium cycling in congestive heart failure. J. Mol. Cell. Cardiol. 2002, 34, 951–969. [Google Scholar] [CrossRef]
- Cicconi, S.; Ventura, N.; Pastore, D.; Bonini, P.; Nardo, P.D.; Lauro, R.; Marlier, L.N. Characterization of apoptosis signal transduction pathways in HL-5 cardiomyocytes exposed to ischemia/reperfusion oxidative stress model. J. Cell. Physiol. 2003, 195, 27–37. [Google Scholar] [CrossRef]
- Rajabi, M.; Kassiotis, C.; Razeghi, P.; Taegtmeyer, H. Return to the fetal gene program protects the stressed heart: A strong hypothesis. Heart Fail. Rev. 2007, 12, 331–343. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Xie, F.; Tao, D.; Xiao, X.; Huang, C.; Wang, M.; Gu, C.; Zhang, X.; Jiang, G. Hsa_circ_0001361 promotes bladder cancer invasion and metastasis through miR-491-5p/MMP9 axis. Oncogene 2020, 39, 1696–1709. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.N.S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat. Commun. 2019, 10, 4317. [Google Scholar] [CrossRef]
- Suter, T.M.; Ewer, M.S. Cancer drugs and the heart: Importance and management. Eur. Heart J. 2013, 34, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S. Genetics of Anthracycline Cardiomyopathy in Cancer Survivors: JACC: CardioOncology State-of-the-Art Review. Cardio Oncol. 2020, 2, 539–552. [Google Scholar]
- Singal, P.K.; Iliskovic, N. Doxorubicin-induced cardiomyopathy. N. Engl. J. Med. 1998, 339, 900–905. [Google Scholar] [CrossRef]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Ferrans, V.; Clark, J.R.; Zhang, J.; Yu, Z.X.; Herman, E.H. Pathogenesis and prevention of doxorubicin cardiomyopathy. Tsitologiia 1997, 39, 928–937. [Google Scholar] [PubMed]
- Jones, R.L.; Swanton, C.; Ewer, M.S. Anthracycline cardiotoxicity. Expert Opin. Drug Saf. 2006, 5, 791–809. [Google Scholar] [CrossRef]
- Šimůnek, T.; Štěrba, M.; Popelová, O.; Adamcová, M.; Hrdina, R.; Geršl, V. Anthracycline-induced cardiotoxicity: Overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol. Rep. 2009, 61, 154–171. [Google Scholar] [CrossRef]
- Nishi, M.; Wang, P.-Y.; Hwang, P.M. Protective role of p53 in doxorubicin-induced cardiomyopathy as a mitochondrial disease. Mol. Cell. Oncol. 2020, 7, 1724598. [Google Scholar] [CrossRef]
- Ferreira, A.L.d.A.; Matsubara, L.S.; Matsubara, B.B. Anthracycline-induced cardiotoxicity. Cardiovasc. Hematol. Agents Med. Chem. 2008, 6, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Angsutararux, P.; Luanpitpong, S.; Issaragrisil, S. Chemotherapy-induced cardiotoxicity: Overview of the roles of oxidative stress. Oxidative Med. Cell. Longev. 2015, 2015, 795602. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-W.; Shi, J.; Li, Y.J.; Wei, L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch. Immunol. Ther. Exp. 2009, 57, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, D.B. Anthracyclines and heart failure. N. Engl. J. Med. 2013, 368, 1154–1156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Olson, R.D.; Gambliel, H.A.; Vestal, R.E.; Shadle, S.E.; Charlier, H.A.; Cusack, B.J. Doxorubicin cardiac dysfunction. Cardiovasc. Toxicol. 2005, 5, 269–283. [Google Scholar] [CrossRef]
- Zheng, Y.; Vertuani, S.; Nyström, S.; Audebert, S.; Meijer, I.; Tegnebratt, T.; Borg, J.P.; Uhlén, P.; Majumdar, A.; Holmgren, L. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circ. Res. 2009, 105, 260–270. [Google Scholar] [CrossRef]
- Moleirinho, S.; Guerrant, W.; Kissil, J.L. The Angiomotins–from discovery to function. FEBS Lett. 2014, 588, 2693–2703. [Google Scholar] [CrossRef]
- Bratt, A.; Birot, O.; Sinha, I.; Veitonmäki, N.; Aase, K.; Ernkvist, M.; Holmgren, L. Angiomotin regulates endothelial cell-cell junctions and cell motility. J. Biol. Chem. 2005, 280, 34859–34869. [Google Scholar] [CrossRef]
- Zeng, Y.; Du, W.W.; Wu, Y.; Yang, Z.; Awan, F.M.; Li, X.; Yang, W.; Zhang, C.; Yang, Q.; Yee, A.; et al. A circular RNA binds to and activates AKT phosphorylation and nuclear localization reducing apoptosis and enhancing cardiac repair. Theranostics 2017, 7, 3842. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-K.; Wang, G.; Chen, Z.; Teruya-Feldstein, J.; Liu, Y.; Chan, C.H.; Yang, W.L.; Erdjument-Bromage, H.; Nakayama, K.I.; Nimer, S.; et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat. Cell Biol. 2009, 11, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Viars, C.S.; Czekay, S.; Cavenee, W.K.; Arden, K.C. Cloning and characterization of three human forkhead genes that comprise an FKHR-like gene subfamily. Genomics 1998, 47, 187–199. [Google Scholar] [CrossRef]
- Fibbe, W.E.; Shi, Y. FOXO3, a molecular search for the fountain of youth. Cell Stem Cell 2019, 24, 351–352. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, L.Y.; Tang, X.; Zhang, J.; Zhai, L.L.; Yi, Y.Y.; Yi, J.; Lin, J.; Qian, J.; Deng, Z.Q. Circ-Foxo3 is positively associated with the Foxo3 gene and leads to better prognosis of acute myeloid leukemia patients. BMC Cancer 2019, 19, 930. [Google Scholar] [CrossRef]
- Du, W.W.; Yang, W.; Liu, E.; Yang, Z.; Dhaliwal, P.; Yang, B.B. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016, 44, 2846–2858. [Google Scholar] [CrossRef]
- Segura, M.F.; Hanniford, D.; Menendez, S.; Reavie, L.; Zou, X.; Alvarez-Diaz, S.; Zakrzewski, J.; Blochin, E.; Rose, A.; Bogunovic, D.; et al. Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proc. Natl. Acad. Sci. USA 2009, 106, 1814–1819. [Google Scholar] [CrossRef]
- Du, W.W.; Yang, W.; Chen, Y.; Wu, Z.K.; Foster, F.S.; Yang, Z.; Li, X.; Yang, B.B. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur. Heart J. 2017, 38, 1402–1412. [Google Scholar] [CrossRef]
- Han, D.; Wang, Y.; Wang, Y.; Dai, X.; Zhou, T.; Chen, J.; Tao, B.; Zhang, J.; Cao, F. The tumor-suppressive human circular RNA CircITCH sponges miR-330-5p to ameliorate doxorubicin-induced cardiotoxicity through upregulating SIRT6, survivin, and SERCA2a. Circ. Res. 2020, 127, e108–e125. [Google Scholar] [CrossRef]
- Andersson, C.; Schou, M.; Gustafsson, F.; Torp-Pedersen, C. Alcohol Intake in Patients With Cardiomyopathy and Heart Failure: Consensus and Controversy. Circ. Heart Fail. 2022, 15, e009459. [Google Scholar] [CrossRef] [PubMed]
- Piano, M.R.; Phillips, S.A. Alcoholic cardiomyopathy: Pathophysiologic insights. Cardiovasc. Toxicol. 2014, 14, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, H.; Ding, N.; Wang, S.; Duan, Z.; Birnbaum, Y.; Ye, Y.; Qian, J. Expression profiling of circular RNAs and micrornas in heart tissue of mice with alcoholic cardiomyopathy. Cell. Physiol. Biochem. 2018, 46, 2284–2296. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gan, T.Y.; Li, N.; Liu, C.Y.; Zhou, L.Y.; Gao, J.N.; Chen, C.; Yan, K.W.; Ponnusamy, M.; Zhang, Y.H.; et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 2017, 24, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
| Circular RNAs | Related Disease | Expression | Methods | Samples | Ref |
|---|---|---|---|---|---|
| Circ-SLC8A1 | DCM | Up | RNA high-throughput sequencing and qRT-PCR | Heart samples | [78] |
| circ-SLC8A1 circ-CHD7 circ-ATXN10 | DCM | Up | RNA sequencing and qRT-PCR | Heart samples | [79] |
| Circ-DNA6JC | DCM | Down | RNA sequencing and qRT-PCR | Heart samples | [79] |
| circTTN_70 circTTN_132 circTTN_34 circTTN_52 circRYR2_71 circRYR2_95 | DCM | Down | Read-through circRNA | Heart samples | [80] |
| circSLC8A1_11 circ-SLC8A1_12 | DCM | Up | Read-through circRNA | Heart samples | [80] |
| circ- EBF1 circ- ZNF670 circ- NBEA | DCM | Down | RNA high-throughput sequencing and qRT-PCR | Heart samples | [81] |
| circ- FAT1 circ- ICA1 circ- LYPLAL1 | DCM | UP | RNA high-throughput sequencing and qRT-PCR | Heart samples | [81] |
| circ- MYH7 circ- SEC23A circ- TTN circ- PDE1C circ- CTNND2 circ- ATRX and OR2A1-AS1 | DCM | Down | RNA high-throughput sequencing | Heart samples | [81] |
| circ- TTN circ- BTBD7 circ- NHLRC2 circ-DHX40 circ- G083903 circ- PKN2 | DCM | UP | RNA high-throughput sequencing | Heart samples | [81] |
| Circular RNAs | Related Disease | Expression | Methods | Samples | Ref |
|---|---|---|---|---|---|
| circDNAJC6 circMBOAT2 circTMEM56 | HCM | Down | qRT-PCR | Serum samples | [92] |
| hsa_circ_0043762 hsa_circ_0036248 hsa_circ_0071269 | HCM | - | Microarray | Plasma samples | [93] |
| HRCR | Cardiac hypertrophy and heart failure | Down | Microarray and qRT-PCR | Animals model | [94] |
| Circular RNAs | Related Disease | Expression | Methods | Samples | Ref |
|---|---|---|---|---|---|
| circHIPK3 | Diabetic cardiomyopathy | Up | qRT-PCR | Animals model | [102] |
| CACR | Diabetic cardiomyopathy | Up | qRT-PCR | Serum samples and cell culture | [103] |
| circRNA_000203 | Diabetic cardiomyopathy | Up | CircRNA microarray and qRT-PCR | Animals model | [104] |
| circRNA_010567 | Diabetic cardiomyopathy | Up | CircRNA microarray and qRT-PCR | Animals model | [105] |
| mmu_circ_0001697 mmu_circ_0001160 novel_circ_0008273 novel_circ_0009344 mmu_circ_0001625 mmu_circ_0000431 | Diabetic cardiomyopathy | Up | RNA sequencing and qRT-PCR | Animals model | [106] |
| mmu_circ_0000652 mmu_circ_0000058 mmu_circ_0001058 mmu_circ_0000680 novel_circ_0000824 mmu_circ_0000547 novel_circ_0004285 | Diabetic cardiomyopathy | Down | RNA sequencing and qRT-PCR | Animals model | [106] |
| Circular RNAs | Related Disease | Expression | Methods | Samples | Ref |
|---|---|---|---|---|---|
| circ-Amotl1 | DIC | Up | Microarray and qRT-PCR | Human cardiac tissues and Animals model | [130] |
| circ-Foxo3 | DIC | Up | Circular RNA sequencing and qRT-PCR | Animals model | [138] |
| CircITCH | DIC | Down | qRT-PCR | hiPSC-CMs and heart samples and animals model | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagheri Moghaddam, M.; Maleki, M.; Oveisee, M.; Bagheri Moghaddam, M.; Arabian, M.; Malakootian, M. Circular RNAs: New Players in Cardiomyopathy. Genes 2022, 13, 1537. https://doi.org/10.3390/genes13091537
Bagheri Moghaddam M, Maleki M, Oveisee M, Bagheri Moghaddam M, Arabian M, Malakootian M. Circular RNAs: New Players in Cardiomyopathy. Genes. 2022; 13(9):1537. https://doi.org/10.3390/genes13091537
Chicago/Turabian StyleBagheri Moghaddam, Maedeh, Majid Maleki, Maziar Oveisee, Mahrokh Bagheri Moghaddam, Maedeh Arabian, and Mahshid Malakootian. 2022. "Circular RNAs: New Players in Cardiomyopathy" Genes 13, no. 9: 1537. https://doi.org/10.3390/genes13091537
APA StyleBagheri Moghaddam, M., Maleki, M., Oveisee, M., Bagheri Moghaddam, M., Arabian, M., & Malakootian, M. (2022). Circular RNAs: New Players in Cardiomyopathy. Genes, 13(9), 1537. https://doi.org/10.3390/genes13091537






