Hearing Loss in Stickler Syndrome: An Update
Abstract
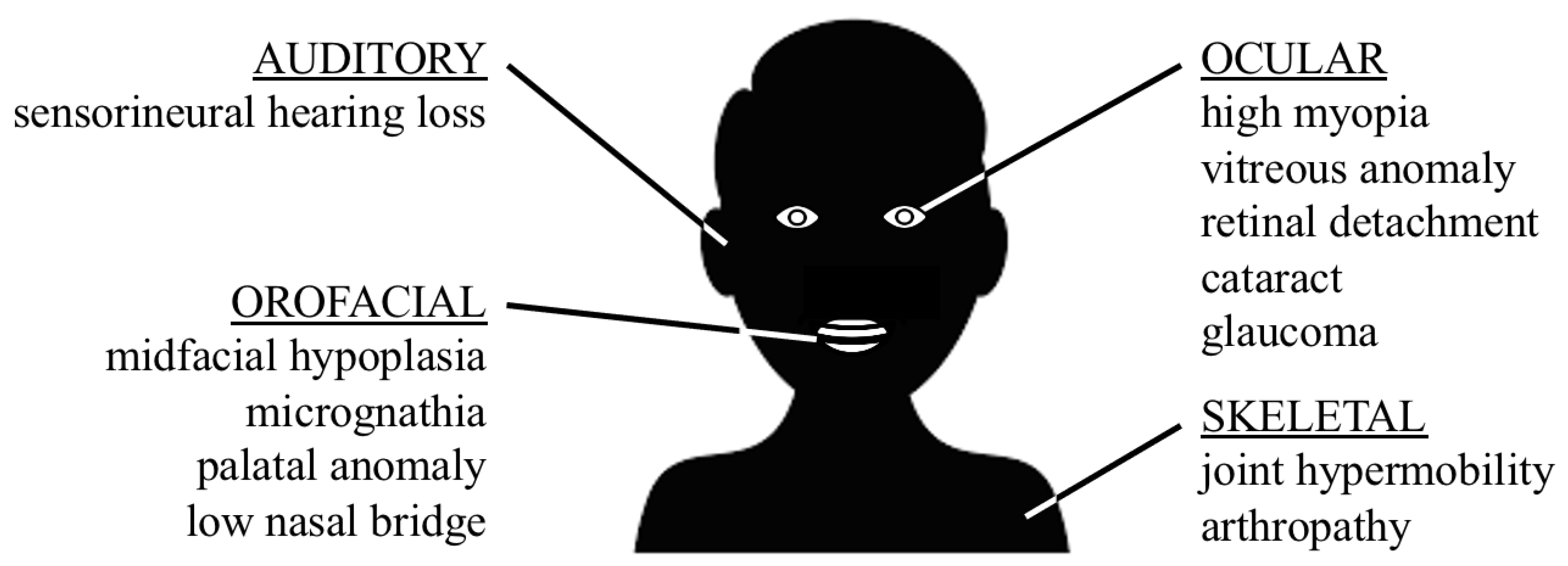
1. Introduction
2. Hearing Loss in Type 1 Stickler Syndrome (COL2A1)
3. Hearing Loss in Type 2–3 Stickler Syndrome (COL11A1 and COL11A2)
4. Hearing Loss in Type 4–6 Stickler Syndrome (COL9A1, COL9A2 and COL9A3)
5. Hearing Loss in Stickler Syndrome with Mutations in Non-Collagen Genes (BMP4, LOXL3 and LRP2)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stickler, G.B.; Belau, P.G.; Farrell, F.J.; Jones, J.D.; Pugh, D.G.; Steinberg, A.G.; Ward, L.E. Hereditary Progressive Arthro-Ophthalmopathy. Mayo Clin. Proc. 1965, 40, 433–455. [Google Scholar] [PubMed]
- Stickler, G.B.; Pugh, D.G. Hereditary Progressive Arthro-Ophthalmopathy. II. Additional Observations on Vertebral Abnormalities a Hearing Defect and a Report of a Similar Case. Mayo Clin. Proc. 1967, 42, 495–500. [Google Scholar]
- Acke, F.R.; Dhooge, I.J.; Malfait, F.; De Leenheer, E.M. Hearing impairment in Stickler syndrome: A systematic review. Orphanet J. Rare Dis. 2012, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Szymko-Bennett, Y.M.; Mastroianni, M.A.; Shotland, L.I.; Davis, J.; Ondrey, F.G.; Balog, J.Z.; Rudy, S.F.; McCullagh, L.; Levy, H.P.; Liberfarb, R.M.; et al. Auditory dysfunction in Stickler syndrome. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 1061–1068. [Google Scholar] [CrossRef][Green Version]
- Ahmad, N.N.; Ala-Kokko, L.; Knowlton, R.G.; Jimenez, S.A.; Weaver, E.J.; Maguire, J.I.; Tasman, W.; Prockop, D.J. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc. Natl. Acad. Sci. USA 1991, 88, 6624–6627. [Google Scholar] [CrossRef]
- Richards, A.J.; Yates, J.R.; Williams, R.; Payne, S.J.; Pope, F.M.; Scott, J.D.; Snead, M.P. A family with Stickler syndrome type 2 has a mutation in the COL11A1 gene resulting in the substitution of glycine 97 by valine in α 1 (XI) collagen. Hum. Mol. Genet. 1996, 5, 1339–1343. [Google Scholar] [CrossRef]
- Vikkula, M.; Mariman, E.C.; Lui, V.C.; Zhidkova, N.I.; Tiller, G.E.; Goldring, M.B.; van Beersum, S.E.; de Waal Malefijt, M.C.; van den Hoogen, F.H.; Ropers, H.H. Autosomal dominant and recessive osteochondrodysplasias associated with the COL11A2 locus. Cell 1995, 80, 431–437. [Google Scholar] [CrossRef]
- Richards, A.J.; McNinch, A.; Martin, H.; Oakhill, K.; Rai, H.; Waller, S.; Treacy, B.; Whitakker, J.; Meredith, S.; Poulson, A.; et al. Stickler syndrome and the vitreous phenotype: Mutations in COL2A1 and COL11A1. Hum. Mutat. 2010, 31, E1461–E1471. [Google Scholar] [CrossRef]
- Soh, Z.; Richard, A.J.; McNinch, A.; Alexander, P.; Martin, H.; Snead, M.P. Dominant Stickler Syndrome. Genes 2022, 13, 1089. [Google Scholar] [CrossRef]
- Nixon, T.R.W.; Richards, A.J.; Martin, H.; Alexander, P.; Snead, M. Autosomal Recessive Stickler Syndrome. Genes 2022, 13, 1135. [Google Scholar] [CrossRef]
- Van Camp, G.; Snoeckx, R.L.; Hilgert, N.; van den Ende, J.; Fukoka, H.; Wagatsuma, M.; Suzuki, H.; Erica Smets, R.M.; Vanhoenacker, F.; Declau, F.; et al. A new autosomal recessive form of Stickler syndrome is caused by a mutation in the COL9A1 gene. Am. J. Hum. Genet. 2006, 79, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Booth, C.; Fillman, C.; Shapiro, M.; Blair, M.P.; Hyland, J.C.; Ala-Kooko, L. A loss of function mutation in the COL9A2 gene causes autosomal recessive Stickler syndrome. Am. J. Med. Genet. A 2011, 155, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Faletra, F.; D’Adamo, A.P.; Bruno, I.; Athanasakis, E.; Biskup, S.; Esposito, L.; Gasparini, P. Autosomal recessive Stickler syndrome due to a loss of function mutation in the COL9A3 gene. Am. J. Med. Genet. A 2014, 164, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, I.; Sommen, M.; Claes, C.; Pinner, J.; Flaherty, M.; Collins, F.; Van Camp, G. Broadening the phenotype of LRP2 mutations: A new mutation in LRP2 causes a predominantly ocular phenotype suggestive of Stickler syndrome. Clin. Genet. 2014, 86, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, F.; Al Hazzaa, S.A.; Tayeb, H.; Alkuraya, F.S. LOXL3, encoding lysyl oxidase-like 3, is mutated in a family with autosomal recessive Stickler syndrome. Hum. Genet. 2015, 134, 451–453. [Google Scholar] [CrossRef]
- Nixon, T.R.W.; Richards, A.; Towns, L.K.; Fuller, G.; Abbs, S.; Alexander, P.; McNinch, A.; Sanford, R.N.; Snead, M.P. Bone morphogenetic protein 4 (BMP4) loss-of-function variant associated with autosomal dominant Stickler syndrome and renal dysplasia. Eur. J. Hum. Genet. 2019, 27, 369–377. [Google Scholar] [CrossRef]
- Acke, F.R.; Swinnen, F.K.; Malfait, F.; Dhooge, I.J.; De Leenheer, E.M.R. Auditory phenotype in Stickler syndrome: Results of audiometric analysis in 20 patients. Eur. Arch. Otorhinolaryngol. 2016, 273, 3025–3034. [Google Scholar] [CrossRef]
- Bath, F.; Swanson, D.; Zavala, H.; Chinnadurai, S.; Roby, B.R. Hearing Outcomes in Stickler Syndrome: Variation Due to COL2A1 and COL11A1. Cleft Palate Craniofac. J. 2021, 59, 970–975. [Google Scholar] [CrossRef]
- Baijens, L.W.; De Leenheer, E.M.R.; Weekamp, H.H.; Cruysberg, J.R.M.; Mortier, G.R.; Cremers, C.W.R.J. Stickler syndrome type I and Stapes ankylosis. Int. J. Pediatr. Otorhinolaryngol. 2004, 68, 1573–1580. [Google Scholar] [CrossRef]
- Swinnen, F.K.; De Leenheer, E.M.R.; Coucke, P.J.; Cremers, C.W.R.J.; Dhooge, I.J.M. Stapes surgery in osteogenesis imperfecta: Retrospective analysis of 34 operated ears. Audiol. Neurootol. 2012, 17, 198–206. [Google Scholar] [CrossRef]
- Alexander, P.; Gomersall, P.; Stancel-Lewis, J.; Fincham, G.S.; Poulson, A.; Richards, A.; McNinch, A.; Baguley, D.M.; Snead, M. Auditory dysfunction in type 2 Stickler Syndrome. Eur. Arch. Otorhinolaryngol. 2021, 278, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Kannu, P.; Bateman, J.; Savarirayan, R. Clinical phenotypes associated with type II collagen mutations. J. Paediatr. Child. Health 2012, 48, E38–E43. [Google Scholar] [CrossRef] [PubMed]
- Khetarpal, U.; Robertson, N.G.; Yoo, T.J.; Morton, C.C. Expression and localization of COL2A1 mRNA and type II collagen in human fetal cochlea. Hear. Res. 1994, 79, 59–73. [Google Scholar] [CrossRef]
- Hoornaert, K.P.; Vereecke, I.; Dewinter, C.; Rosenberg, T.; Beemer, F.A.; Leroy, J.G.; Bendix, L.; Björk, E.; Bonduelle, M.; Boute, O.; et al. Stickler syndrome caused by COL2A1 mutations: Genotype-phenotype correlation in a series of 100 patients. Eur. J. Hum. Genet. 2010, 18, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Admiraal, R.J.; Brunner, H.G.; Dijkstra, T.L.; Huygen, P.L.; Cremers, C.W. Hearing loss in the nonocular Stickler syndrome caused by a COL11A2 mutation. Laryngoscope 2000, 110, 457–461. [Google Scholar] [CrossRef]
- Iwasa, Y.; Moteki, H.; Hattori, M.; Sato, R.; Nishio, S.-Y.; Takumi, Y.; Usami, S.-I. Non-ocular Stickler syndrome with a novel mutation in COL11A2 diagnosed by massively parallel sequencing in Japanese hearing loss patients. Ann. Otol. Rhinol. Laryngol. 2015, 124, 111S–117S. [Google Scholar] [CrossRef]
- Majava, M.; Hoornaert, K.P.; Bartholdi, D.; Bouma, M.C.; Bouman, K.; Carrera, M.; Devriendt, K.; Hurts, J.; Kitsos, G.; Niedrist, D.; et al. A report on 10 new patients with heterozygous mutations in the COL11A1 gene and a review of genotype-phenotype correlations in type XI collagenopathies. Am. J. Med. Genet. A 2007, 143, 258–264. [Google Scholar] [CrossRef]
- Van Beelen, E.; Leijendeckers, J.M.; Huygen, P.L.M.; Admiraal, R.J.C.; Hoefsloot, L.H.; Lichtenbelt, K.D.; Stöbe, L.; Pennings, R.J.E.; Leuwer, R.; Snik, A.F.M.; et al. Audiometric characteristics of two Dutch families with non-ocular Stickler syndrome (COL11A2). Hear. Res. 2012, 291, 15–23. [Google Scholar] [CrossRef]
- Griffith, A.J.; Gebarski, S.S.; Shepard, N.T.; Kileny, P.R. Audiovestibular phenotype associated with a COL11A1 mutation in Marshall syndrome. Arch. Otolaryngol. Head Neck Surg. 2000, 126, 891–894. [Google Scholar] [CrossRef]
- Acke, F.R.; Malfait, F.; Vanakker, O.M.; Steyaert, W.; De Leeneer, K.; Mortier, G.; Dhooge, I.; De Paepe, A.; De Leenheer, E.M.R.; Coucke, P.J. Novel pathogenic COL11A1/COL11A2 variants in Stickler syndrome detected by targeted NGS and exome sequencing. Mol. Genet. Metab. 2014, 113, 230–235. [Google Scholar] [CrossRef]
- Richards, A.J.; Fincham, G.S.; McNinch, A.; Hill, D.; Poulson, A.V.; Castle, B.; Lees, M.M.; Moore, A.T.; Scott, J.D.; Snead, M.P. Alternative splicing modifies the effect of mutations in COL11A1 and results in recessive type 2 Stickler syndrome with profound hearing loss. J. Med. Genet. 2013, 50, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kahrizi, K.; Meyer, N.C.; Riazalhosseini, Y.; Van Camp, G.; Najmabadi, H.; Smith, R.H.J. Mutation of COL11A2 causes autosomal recessive non-syndromic hearing loss at the DFNB53 locus. J. Med. Genet. 2005, 42, e61. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Iyama, K.; Inoguchi, K.; Khaleduzzaman, M.; Ninomyia, Y.; Ramirez, F. Developmental pattern of expression of the mouse α 1 (XI) collagen gene (Col11a1). Dev. Dyn. 1995, 204, 41–47. [Google Scholar] [CrossRef]
- Fang, M.; Adams, J.S.; McMahan, B.L.; Brown, R.J.; Oxford, J.T. The expression patterns of minor fibrillar collagens during development in zebrafish. Gene Expr. Patterns 2010, 10, 315–322. [Google Scholar] [CrossRef]
- Shpargel, K.B.; Makishima, T.; Griffith, A.J. Col11a1 and Col11a2 mRNA expression in the developing mouse cochlea: Implications for the correlation of hearing loss phenotype with mutant type XI collagen genotype. Acta Otolaryngol. 2004, 124, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Takanosu, M.; Arita, M.; Bao, Y.; Ren, Z.X.; Maier, A.; Prockop, D.J.; Mayne, R. Targeted disruption of Col11a2 produces a mild cartilage phenotype in transgenic mice: Comparison with the human disorder otospondylomegaepiphyseal dysplasia (OSMED). Dev. Dyn. 2001, 222, 141–152. [Google Scholar] [CrossRef]
- McGuirt, W.T.; Prasad, S.D.; Griffith, A.J.; Kunst, H.P.; Green, G.E.; Shpargel, K.B.; Runge, C.; Huybrechts, C.; Mueller, R.F.; Lynch, E.; et al. Mutations in COL11A2 cause non-syndromic hearing loss (DFNA13). Nat. Genet. 1999, 23, 413–419. [Google Scholar] [CrossRef]
- Szymko-Bennett, Y.M.; Kurima, K.; Olsen, B.; Seegmiller, R.; Griffith, A.J. Auditory function associated with Col11a1 haploinsufficiency in chondrodysplasia (cho) mice. Hear. Res. 2003, 175, 178–182. [Google Scholar] [CrossRef]
- Hanson-Kahn, A.; Li, B.; Cohn, D.H.; Nickerson, D.A.; Bamshad, M.J.; Hudgins, L. Autosomal recessive Stickler syndrome resulting from a COL9A3 mutation. Am. J. Med. Genet. A 2018, 176, 2887–2891. [Google Scholar] [CrossRef]
- Nikopoulos, K.; Schrauwen, I.; Simon, M.; Collin, R.W.J.; Veckeneer, M.; Keymolen, K.; Van Camp, G.; Cremers, F.P.M.; Born, L.I.V.D. Autosomal recessive Stickler syndrome in two families is caused by mutations in the COL9A1 gene. Invest. Ophthalmol. Vis. Sci. 2011, 52, 4774–4779. [Google Scholar] [CrossRef]
- Nixon, T.R.W.; Alexander, P.; Richards, A.; McNinch, A.; Bearcroft, P.W.P.; Cobben, J.; Snead, M.P. Homozygous Type IX collagen variants (COL9A1, COL9A2, and COL9A3) causing recessive Stickler syndrome-Expanding the phenotype. Am. J. Med. Genet. A 2019, 179, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Rad, A.; Najafi, M.; Suri, F.; Abedini, S.; Loum, S.; Karimiani, E.G.; Daftarian, N.; Murphy, D.; Doosti, M.; Moghaddasi, A.; et al. Identification of three novel homozygous variants in COL9A3 causing autosomal recessive Stickler syndrome. Orphanet J. Rare Dis. 2022, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Asamura, K.; Abe, S.; Imamura, Y.; Aszodi, A.; Suzuki, N.; Hashimoto, S.; Takumi, Y.; Hayashi, T.; Fässler, R.; Nakamura, Y.; et al. Type IX collagen is crucial for normal hearing. Neuroscience 2005, 132, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Asamura, K.; Kikuchi, Y.; Takumi, Y.; Abe, S.; Imamura, Y.; Hayashi, T.; Aszodi, A.; Fässler, R.; Usami, S.-I. Type IX collagen knock-out mouse shows progressive hearing loss. Neurosci. Res. 2005, 51, 293–298. [Google Scholar] [CrossRef]
- König, O.; Rüttiger, L.; Müller, M.; Zimmermann, U.; Erdmann, B.; Kalbacher, H.; Gross, M.; Knipper, M. Estrogen and the inner ear: Megalin knockout mice suffer progressive hearing loss. FASEB J. 2008, 22, 410–417. [Google Scholar] [CrossRef]
- Chan, T.K.; Alkaabi, M.K.; ElBarky, A.M.; El-Hattab, A.W. LOXL3 novel mutation causing a rare form of autosomal recessive Stickler syndrome. Clin. Genet. 2019, 95, 325–328. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, X.; Wan, P.; Mo, F.; Chen, G.; Zhang, J.; Gao, J. Targeted Deletion of Loxl3 by Col2a1-Cre Leads to Progressive Hearing Loss. Front. Cell Dev. Biol. 2021, 9, 683495. [Google Scholar] [CrossRef]
- Blauwkamp, M.N.; Beyer, L.A.; Kabara, L.; Takemura, K.; Buck, T.; King, W.; Dolan, D.F.; Barald, K.F.; Raphael, Y.; Koenig, R.J. The role of bone morphogenetic protein 4 in inner ear development and function. Hear. Res. 2007, 225, 71–79. [Google Scholar] [CrossRef][Green Version]


| Type of Stickler Syndrome | Gene | Inheritance | Main Distinguishing Features | First Description |
|---|---|---|---|---|
| Type 1 | COL2A1 | AD | Membranous vitreous, Mild hearing loss | [5] |
| Type 2 | COL11A1 | AD/AR | Beaded vitreous, Moderate (AD) to severe (AR) hearing loss | [6] |
| Type 3 | COL11A2 | AD | No ocular symptoms Moderate hearing loss | [7] |
| Type 4 | COL9A1 | AR | Hypoplastic vitreous Moderate to severe hearing loss | [11] |
| Type 5 | COL9A2 | AR | Hypoplastic vitreous Moderate to severe hearing loss | [12] |
| Type 6 | COL9A3 | AR | Hypoplastic vitreous Moderate to severe hearing loss | [13] |
| - | LRP2 | AR | Microglobulinuria Normal hearing | [14] |
| - | LOXL3 | AR | Orofacial defects Normal hearing | [15] |
| - | BMP4 | AD | Hypoplastic vitreous Renal dysplasia | [16] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acke, F.R.E.; De Leenheer, E.M.R. Hearing Loss in Stickler Syndrome: An Update. Genes 2022, 13, 1571. https://doi.org/10.3390/genes13091571
Acke FRE, De Leenheer EMR. Hearing Loss in Stickler Syndrome: An Update. Genes. 2022; 13(9):1571. https://doi.org/10.3390/genes13091571
Chicago/Turabian StyleAcke, Frederic R. E., and Els M. R. De Leenheer. 2022. "Hearing Loss in Stickler Syndrome: An Update" Genes 13, no. 9: 1571. https://doi.org/10.3390/genes13091571
APA StyleAcke, F. R. E., & De Leenheer, E. M. R. (2022). Hearing Loss in Stickler Syndrome: An Update. Genes, 13(9), 1571. https://doi.org/10.3390/genes13091571





