Archaeal Tubulin-like Proteins Modify Cell Shape in Haloferax volcanii during Early Biofilm Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Culture Methods
2.2. Strain Construction
2.3. Liquid Biofilm Formation, Fluorescent Microscopy Imaging, and Analysis
3. Results
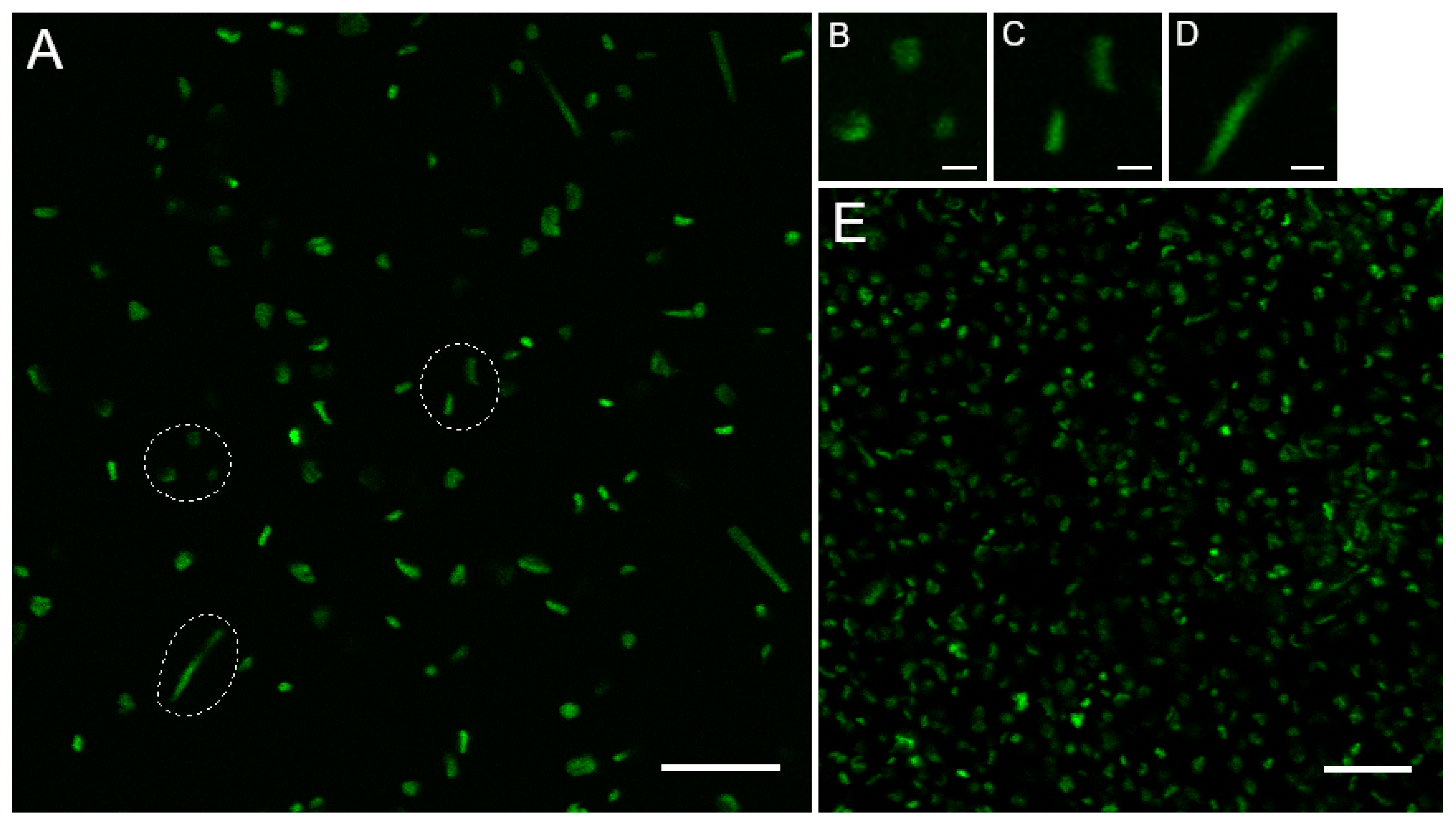
3.1. Haloferax volcanii Exhibits Three Main Morphologies during Early Liquid Biofilm Formation
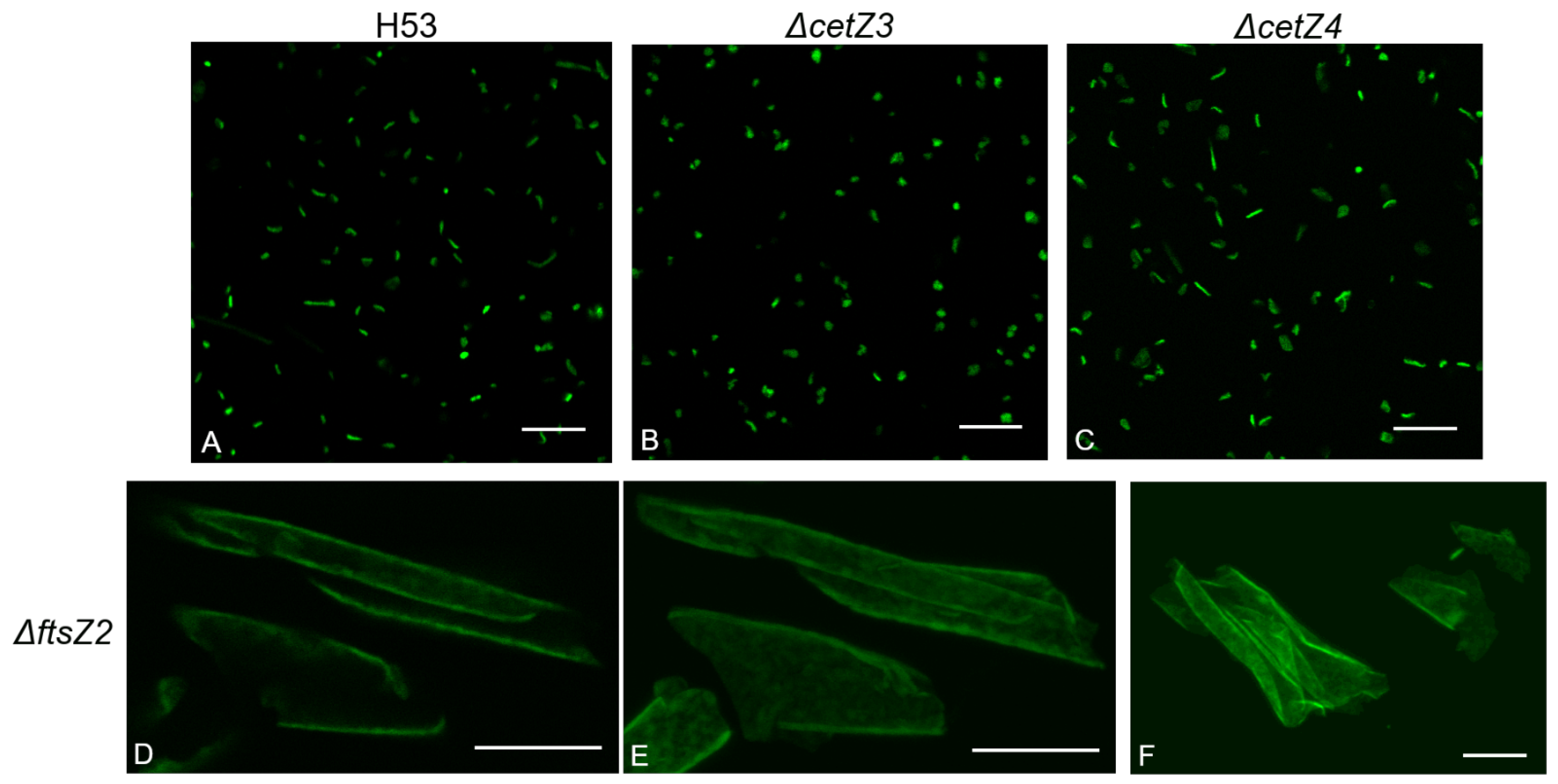
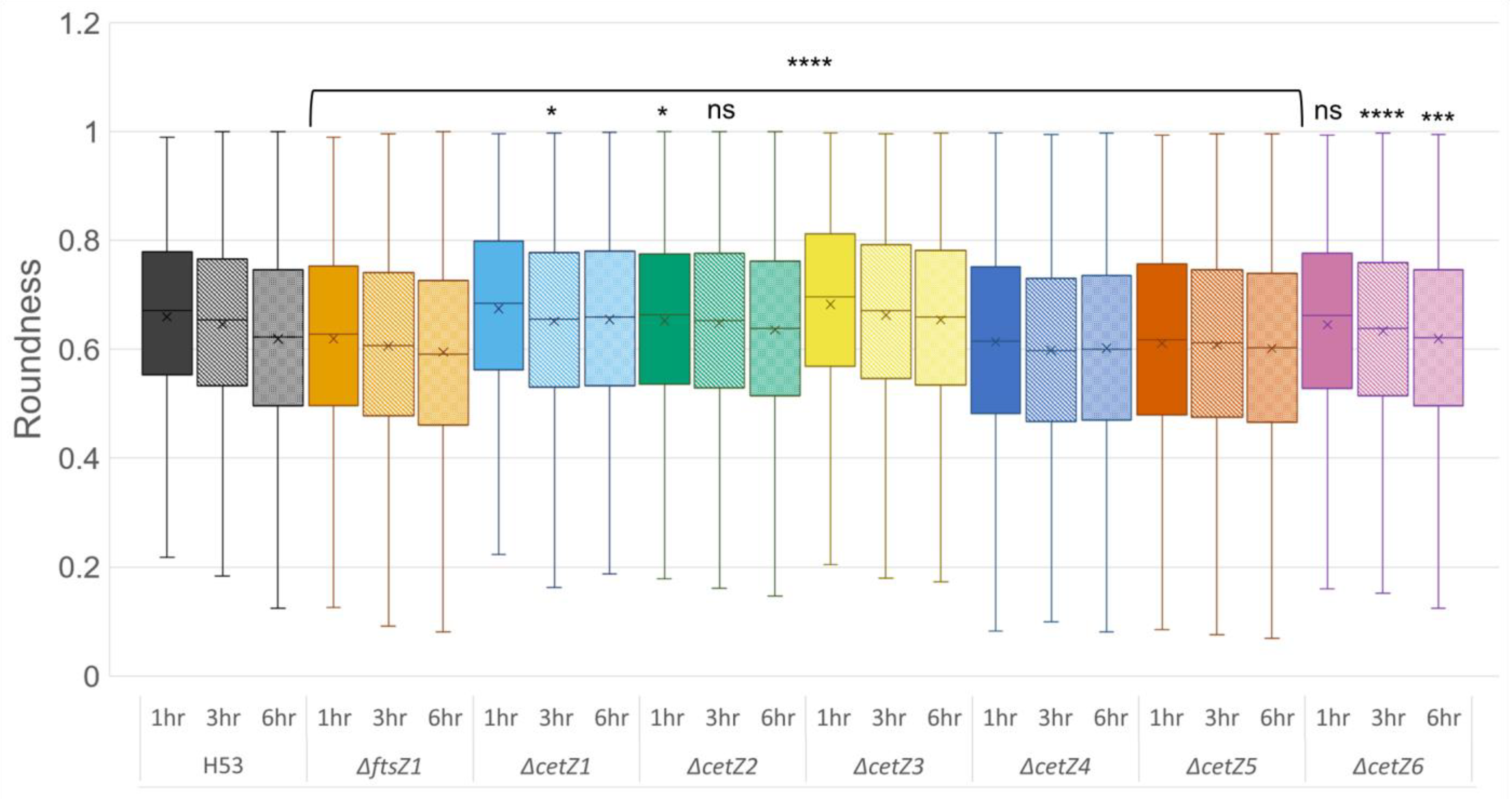
3.2. Deletion of ftsZ-cetZ Family Genes Causes Morphological Changes during Biofilm Development
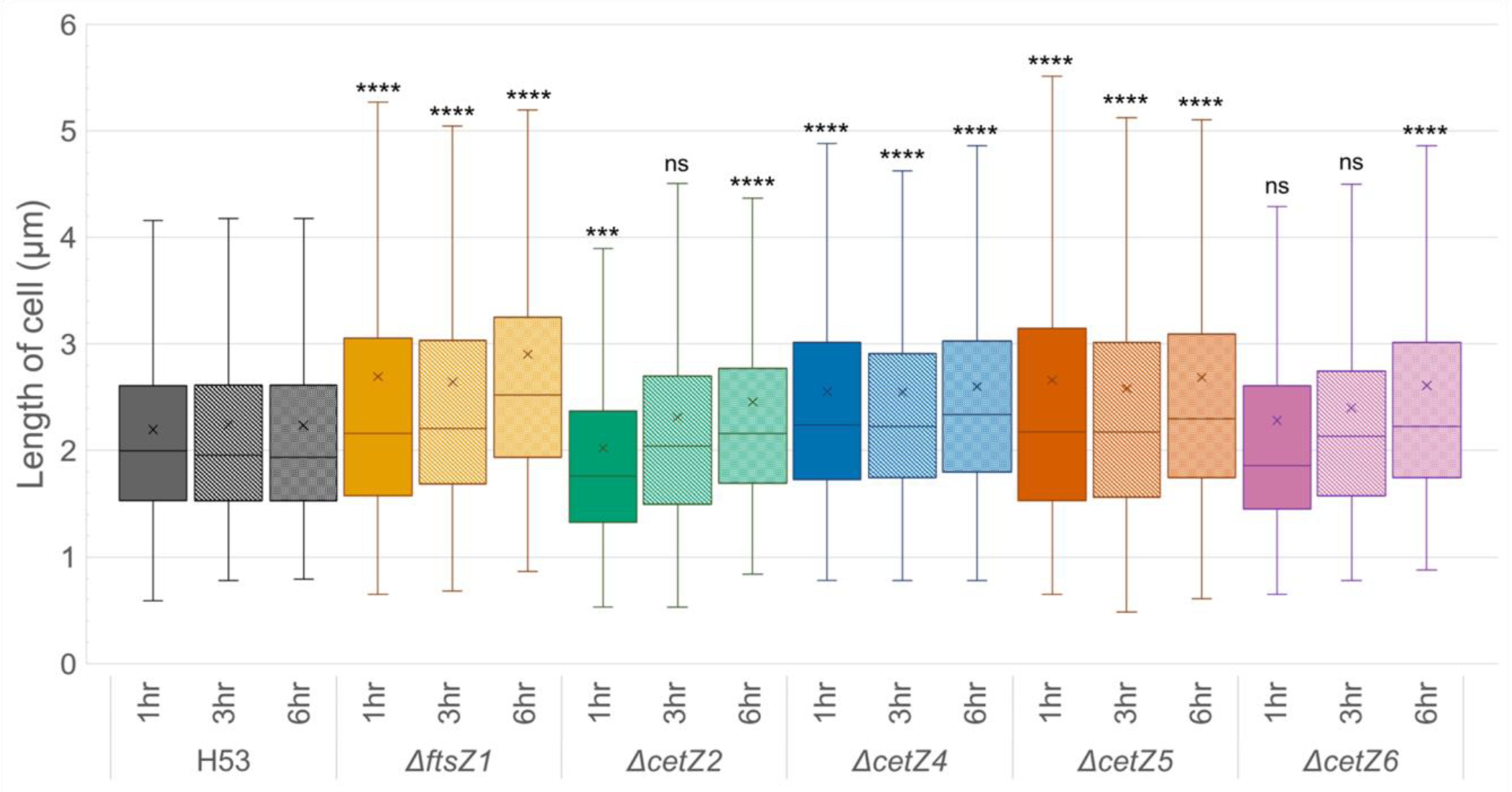
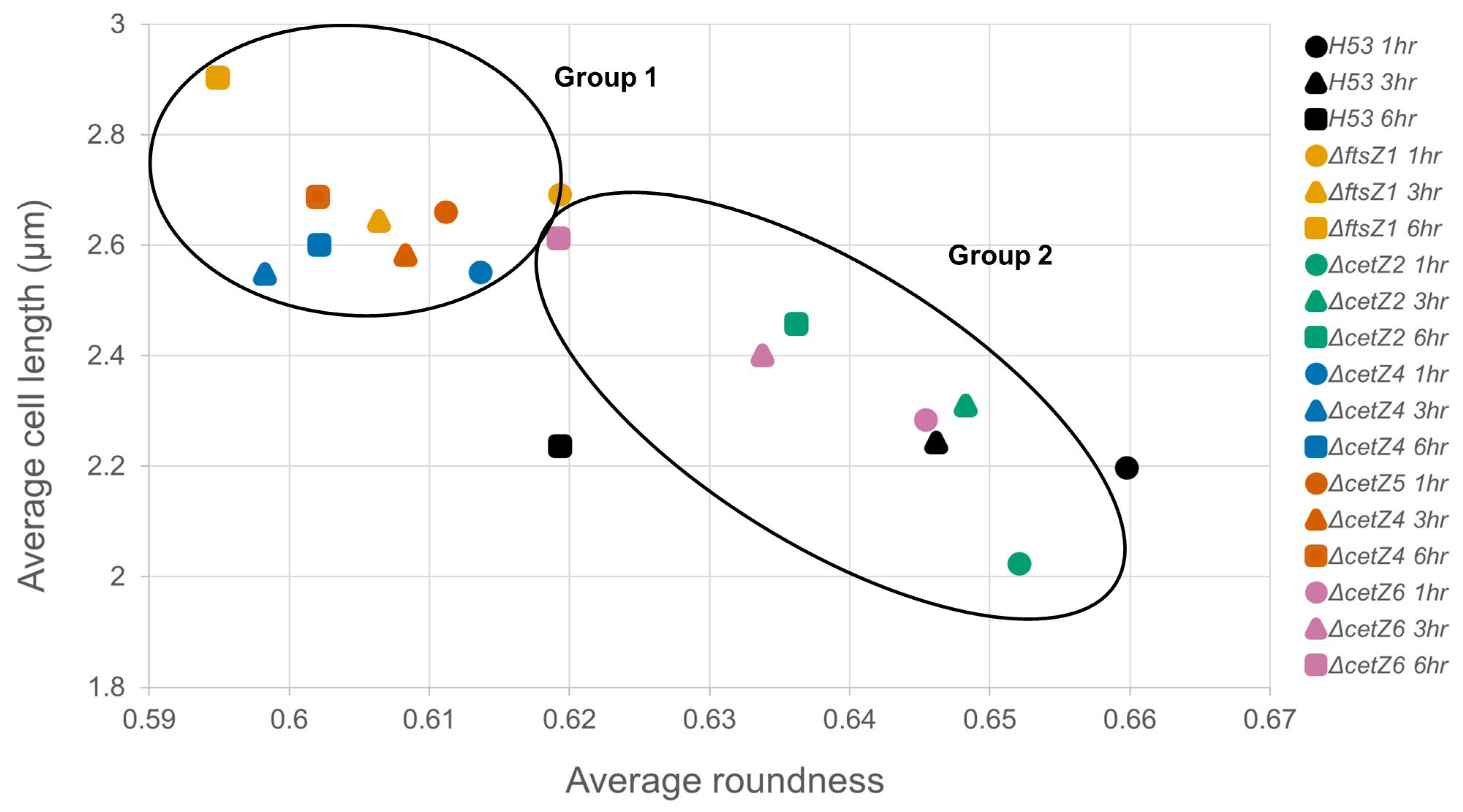
3.3. Mutant Cells Were Typically Longer Than Parental
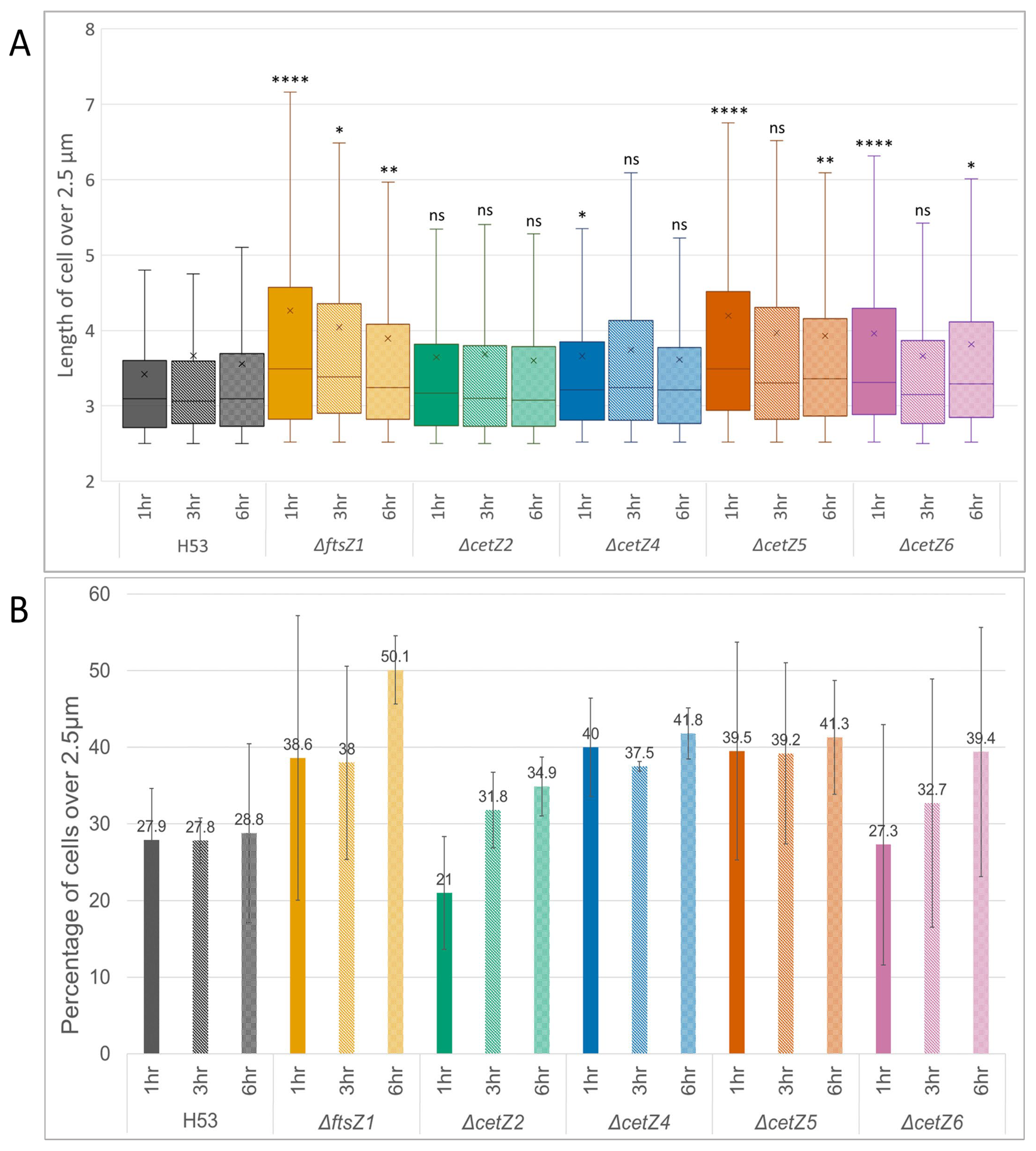
3.4. CetZ1 and CetZ3 Deletions Create More Coccoid or Discoid Morphologies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. 2020, 21, 307–326. [Google Scholar] [CrossRef]
- Vaughan, S.; Wickstead, B.; Gull, K.; Addinall, S.G. Molecular evolution of FtsZ protein sequences encoded within the genomes of Archaea, Bacteria, and Eukaryota. J. Mol. Evol. 2004, 58, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Bi, E.; Lutkenhaus, J. FtsZ ring structure associated with division in Escherichia coli. Nature 1991, 354, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Margolin, W. FtsZ and the division of prokaryotic cells and organelles. Nat. Rev. Mol. Cell Biol. 2005, 6, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Nagahisa, K.; Nakamura, T.; Fujiwara, S.; Imanaka, T.; Takagi, M. Characterization of FtsZ homolog from hyperthermophilic Archaeon Pyrococcus kodakaraensis KOD1. J. Biosci. Bioeng. 2000, 89, 181–187. [Google Scholar] [CrossRef]
- Aylett, C.H.S.; Duggin, I.G. The tubulin superfamily in Archaea. Subcell. Biochem. 2017, 84, 393–417. [Google Scholar] [CrossRef]
- Duggin, I.G.; Aylett, C.H.S.; Walsh, J.C.; Michie, K.A.; Wang, Q.; Turnbull, L.; Dawson, E.M.; Harry, E.J.; Whitchurch, C.B.; Amos, L.A.; et al. CetZ tubulin-like proteins control archaeal cell shape. Nature 2015, 519, 362–365. [Google Scholar] [CrossRef]
- Wang, X.; Lutkenhaus, J. FtsZ ring: The eubacterial division apparatus conserved in archaebacteria. Mol. Microbiol. 1996, 21, 313–319. [Google Scholar] [CrossRef]
- Liao, Y.; Ithurbide, S.; Evenhuis, C.; Löwe, J.; Duggin, I.G. Cell division in the archaeon Haloferax volcanii relies on two FtsZ proteins with distinct functions in division ring assembly and constriction. Nat. Microbiol. 2021, 6, 594–605. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- van Wolferen, M.; Orell, A.; Albers, S.-V. Archaeal biofilm formation. Nat. Rev. Microbiol. 2018, 16, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Chimileski, S.; Franklin, M.J.; Papke, R.T. Biofilms formed by the archaeon Haloferax volcanii exhibit cellular differentiation and social motility, and facilitate horizontal gene transfer. BMC Biol. 2014, 12, 65. [Google Scholar] [CrossRef]
- Mevarech, M.; Werczberger, R. Genetic transfer in Halobacterium volcanii. J. Bacteriol. 1985, 162, 461–462. [Google Scholar] [CrossRef]
- Naor, A.; Gophna, U. Cell fusion and hybrids in Archaea. Bioengineered 2013, 4, 126–129. [Google Scholar] [CrossRef]
- Makkay, A.M.; Louyakis, A.S.; Ram-Mohan, N.; Gophna, U.; Gogarten, J.P.; Papke, R.T. Insights into gene expression changes under conditions that facilitate horizontal gene transfer (mating) of a model archaeon. Sci. Rep. 2020, 10, 22297. [Google Scholar] [CrossRef]
- Ferrari, M.C.; Cerletti, M.; Paggi, R.A.; Troetschel, C.; Poetsch, A.; De Castro, R.E. The LonB protease modulates the degradation of CetZ1 involved in rod-shape determination in Haloferax volcanii. J. Proteomics. 2020, 211, 103546. [Google Scholar] [CrossRef]
- Allers, T.; Ngo, H.-P.; Mevarech, M.; Lloyd, R.G. Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based on the leuB and trpA genes. Appl. Environ. Microbiol. 2004, 70, 943–953. [Google Scholar] [CrossRef]
- Blaby, I.K.; Phillips, G.; Blaby-Haas, C.E.; Gulig, K.S.; El Yacoubi, B.; de Crécy-Lagard, V. Towards a systems approach in the genetic analysis of Archaea: Accelerating mutant construction and phenotypic analysis in Haloferax volcanii. Archaea 2010, 2010, 426239. [Google Scholar] [CrossRef] [PubMed]
- Dyall-Smith, M. The Halohandbook: Protocols for Haloarchaeal Genetics. Available online: https://haloarchaea.com/halohandbook/ (accessed on 31 August 2020).
- Reuter, C.J.; Maupin-Furlow, J.A. Analysis of proteasome-dependent proteolysis in Haloferax volcanii cells, using short-lived green fluorescent proteins. Appl. Environ. Microbiol. 2004, 70, 7530–7538. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Reudan, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Walsby, A.E. A square bacterium. Nature 1980, 283, 69–71. [Google Scholar] [CrossRef]
- Santana-Molina, C.; Del Saz-Navarro, D.; Devos, D.P. Early origin and evolution of the FtsZ/tubulin protein family. Front. Microbiol. 2023, 13, 1100249. [Google Scholar] [CrossRef] [PubMed]
- Yutin, N.; Koonin, E.V. Archaeal origin of tubulin. Biol. Direct 2012, 7, 10. [Google Scholar] [CrossRef]
- Vlamakis, H.; Chai, Y.; Beauregard, P.; Losick, R.; Kolter, R. Sticking together: Building a biofilm the Bacillus subtilis way. Nat. Rev. Microbiol. 2013, 11, 157–168. [Google Scholar] [CrossRef]
- van Gestel, J.; Vlamakis, H.; Kolter, R. From cell differentiation to cell collectives: Bacillus subtilis uses division of labor to migrate. PLoS Biol. 2015, 13, e1002141. [Google Scholar] [CrossRef]
- Meeks, J.C.; Elhai, J. Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiol. Mol. Biol. Rev. 2002, 66, 94–121. [Google Scholar] [CrossRef]
- Bolhuis, H.; Palm, P.; Wende, A.; Falb, M.; Rampp, M.; Rodriguez-Valera, F.; Pfeiffer, F.; Oesterhelt, D. The genome of the square archaeon Haloquadratum walsbyi: Life at the limits of water activity. BMC Genom. 2006, 7, 169. [Google Scholar] [CrossRef]
- Mullakhanbhai, M.F.; Larsen, H. Halobacterium volcanii spec. nov., a Dead Sea halobacterium with a moderate salt requirement. Arch. Microbiol. 1975, 104, 207–214. [Google Scholar] [CrossRef]
- Megaw, J.; Gilmore, B.F. Archaeal persisters: Persister cell formation as a stress response in Haloferax volcanii. Front. Microbiol. 2017, 8, 1589. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, J.; Kimyon, O.; Manefield, M.; Beloe, C.J.; Burns, B.P. Archaea join the conversation: Detection of AHL-like activity across a range of archaeal isolates. FEMS Microbiol. Lett. 2020, 367, fnaa123. [Google Scholar] [CrossRef] [PubMed]
- Quax, T.E.F.; Altegoer, F.; Rossi, F.; Li, Z.; Rodriguez-Franco, M.; Kraus, F.; Bange, G.; Albers, S.-V. Structure and function of the archaeal response regulator CheY. Proc. Natl. Acad. Sci. USA 2017, 115, E1259–E1268. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Ithurbide, S.; de Silva, R.T.; Erdmann, S.; Duggin, I.G. Archaeal cell biology: Diverse functions of tubulin-like cytoskeletal proteins at the cell envelope. Emerg. Top. Life Sci. 2018, 2, 547–559. [Google Scholar] [CrossRef]
- Straube, A.; Merdes, A. EB3 regulates microtubule dynamics at the cell cortex and is required for myoblast elongation and fusion. Curr. Biol. 2007, 17, 1318–1325. [Google Scholar] [CrossRef]
- Zhang, T.; Zaal, K.J.M.; Sheridan, J.; Mehta, A.; Gundersen, G.G.; Ralston, E. Microtubule plus-end binding protein EB1 is necessary for muscle cell differentiation, elongation and fusion. J. Cell Sci. 2009, 122, 1401–1409. [Google Scholar] [CrossRef]
- Abdul-Halim, M.F.; Schulze, S.; DiLucido, A.; Pfeiffer, F.; Filho, A.W.B.; Pohlschroder, M. Lipid anchoring of archaeosortase substrates and midcell growth in Haloarchaea. MBio 2020, 11, e00349-20. [Google Scholar] [CrossRef]
- Nußbaum, P.; Gerstner, M.; Dingethal, M.; Erb, C.; Albers, S.-V. The archaeal protein SepF is essential for cell division in Haloferax volcanii. Nat. Commun. 2021, 12, 3469. [Google Scholar] [CrossRef]
- Liao, Y.; Vogel, V.; Hauber, S.; Bartel, J.; Alkhnbashi, O.S.; Maaß, S.; Schwarz, T.S.; Backofen, R.; Becher, D.; Duggin, I.G.; et al. CdrS is a global transcriptional regulator influencing cell division in Haloferax volcanii. MBio 2021, 12, e01416-21. [Google Scholar] [CrossRef]
- Rosenshine, I.; Tchelet, R.; Mevarech, M. The mechanism of DNA transfer in the mating system of an archaebacterium. Science 1989, 245, 1387–1389. Available online: https://www.jstor.org/stable/1704277 (accessed on 28 September 2019). [CrossRef]
- Naor, A.; Lapierre, P.; Mevarech, M.; Papke, R.T.; Gophna, U. Low species barriers in halophilic archaea and the formation of recombinant hybrids. Curr. Biol. 2012, 22, 1444–1448. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooper, A.; Makkay, A.M.; Papke, R.T. Archaeal Tubulin-like Proteins Modify Cell Shape in Haloferax volcanii during Early Biofilm Development. Genes 2023, 14, 1861. https://doi.org/10.3390/genes14101861
Cooper A, Makkay AM, Papke RT. Archaeal Tubulin-like Proteins Modify Cell Shape in Haloferax volcanii during Early Biofilm Development. Genes. 2023; 14(10):1861. https://doi.org/10.3390/genes14101861
Chicago/Turabian StyleCooper, Alexei, Andrea M. Makkay, and R. Thane Papke. 2023. "Archaeal Tubulin-like Proteins Modify Cell Shape in Haloferax volcanii during Early Biofilm Development" Genes 14, no. 10: 1861. https://doi.org/10.3390/genes14101861




