Abstract
Genome-wide scans performed in affected sib pairs have revealed small and often inconsistent clues to the loci responsible for the inherited components of hypertension. Since blood pressure is a quantitative trait regulated by many loci, two siblings at opposite extremes of the blood pressure distribution are more likely to have inherited different alleles at any given locus. Hence, we investigated an extreme discordant sib pair strategy to analyse markers from two previous loci of interest: (1) the Gordons syndrome locus that includes the WNK4 gene and (2) the ROMK locus identified in our first genome-wide scan. For this study, 24 sib pairs with strong family histories of essential hypertension were selected from the top and bottom 10% of the blood pressure distribution and genotyped for highly polymorphic microsatellite markers on chromosomes 11 and 17. The mean age of the population was 39.8 ± 7.8 years. A significant inverse correlation was found between the squared difference in pulse pressure and the number of alleles shared by IBD between the siblings for the DS11925 marker (r = −0.44, p = 0.031), systolic pressure and chromosome 17 markers (D17S250: r = −0.42, p = 0.040; D17S799 (r = −0.51, p = 0.011), and this relationship persisted after correcting for age and gender. Markers on chromosome 17 (D17S250, D17S928 and D17S1301) and 11 (D11S1999) also correlated with diastolic pressure. These results illustrate the successful use of discordant sib pair analysis to detect linkage within relatively small numbers of pedigrees with hypertension. Further analysis of this cohort may be valuable in complementing findings from the large genome wide scans in affected sib pairs.
1. Introduction
High blood pressure is a common condition affecting one in four adults in the UK, and it is an important modifiable risk factor for cardiovascular, cerebrovascular and renal diseases globally [1]. Genetic and environmental factors impact variations in blood pressure amongst individuals; however, the mechanisms underlying this regulation are poorly understood. A number of studies using a genetic linkage approach identified several candidate genes influencing the blood pressure level, but dissecting these major genes is problematic due to blood pressure’s complex, polygenic nature. Since the advent of the genome era, a number of genome-wide scans (GWS) performed in hundreds of affected sib pairs (ASPs) [2,3] and in over hundred thousand individuals [4,5,6,7,8], including the UK Biobank sample cohort [9], have not revealed consistent clues to the loci responsible for hypertension. The reported genome-wide association studies (GWAS) identified over 1000 loci influencing blood pressure (BP), but the variants were generally limited to common and low frequencies that are mostly in the intergenic region of the genome, showing small associations with BP. On the other hand, several exome-sequencing and chip studies identified multiple rare variants in coding regions with large associations [10,11,12]. The most recent large-scale whole-genome-sequencing meta-analysis of blood pressure phenotypes (systolic, diastolic blood pressure and hypertension) in several multi-ancestry cohorts (51,456 participants) identified only two signals (rare and common variants) that achieved genome-wide significance [9]. This confirms the widely assumed genetically complex and polygenic nature of blood pressure.
In the mid-1990s, one approach received particular attention for dissecting polygenic traits including obesity, asthma, and age-related macular degeneration [13,14,15], and this is the extreme discordant sib pair (EDSP) strategy proposed by Risch and Zhang [16,17]. Here, individuals are sampled from both ends of the phenotype distribution, and because they share the same genetic background, EDSPs provide the most informative siblings for detecting genetic linkage. Using the extreme threshold strategy, Risch and Zhang showed that one can increase the power of a pair, and they estimated the number of required sib pairs to be as few as 10/25 at the two ends of the scale compared to the many hundreds required for the affected sib pairs needed for a linkage analysis [10,16,18] or the several hundreds of thousands required for GWAS [19,20]. We tested the hypothesis that if a genetic locus contributes to blood pressure regulation, then two siblings at opposite extremes of the blood pressure distribution will inherit different alleles at the locus.
Our aim was to recruit the untreated offspring of affected sibling pairs with essential hypertension from the East Anglia Region community [21] and determine what the detection rate would be for extreme discordant sib pairs in this cohort. We applied the EDSP strategy to the untreated offspring of hypertensive parents to validate two previous loci associated with hypertension: (i) the Gordon’s syndrome (psuedohypoaldosteronism type II, PHA type 2) locus on chromosome 17, including the WNK4 gene [22,23], and (ii) the ROMK locus on chromosome 11 which was identified in our first GWS for hypertension [21]. The two loci were selected from our published work in affected sib pairs [21] due to ongoing interest in the monogenic and polygenic nature of hypertension, in particular Gordon’s syndrome and WNK kinases [22,24,25].
2. Material and Methods
In total, 140 families with two or more untreated siblings with a family history of essential hypertension were recruited, of which 47 offspring pairs, referred to herein as sib pairs, had blood pressure (BP) differences of greater than 15/14 mmHg. Of the 47 sib pairs, 24 sib pairs (48 individuals) fitted the strictest definition of extreme discordance. Establishing the genetic architecture in individuals with extreme trait values is important because these individuals are at an increased risk of disease and are also the most likely to harbour rare variants of large effects due to natural selection. In these sib pairs, either one sib had high blood pressure (SBP >139 mmHg or DBP >85 mmHg) and the other sib had low blood pressure (SBP <112 mmHg or DBP <66 mmHg) or there was a difference of at least >25/20 mmHg in blood pressure between the sibs. Centiles of BP were defined from reference data previously gathered from 35,000 healthy subjects registered at their general practitioners and recruited to for cardiovascular risk screenings in the CLEAREST (Cholesterol Levels in the East Anglia Region Effective Screening and Treatment) study [26]. Ethical approval was obtained from the Local Research Ethics Committee (LREC 93/8), and written informed consent obtained from each participant.
At each participant’s clinic visit, basic demographic information (age, sex, self-reported birthweight in pounds, smoking status, alcohol consumption, personal illnesses and treatments) was acquired and a family history questionnaire was completed. Height and weight were recorded using standard methods in kilograms, and the body mass index was calculated in kg/m2. Peripheral blood pressure was recorded in the brachial artery of the non-dominant arm using a validated oscillometric technique (HEM-705CP; Omron Corporation, Kyoto, Japan). Blood pressure was measured twice unless there was discrepancy in the two measurements (>10 mmHg); in this case, a third reading was taken, and the average value was used in the subsequent analysis. All the sib pairs were healthy and were not taking any hypertensive or cardiovascular drugs. Peripheral venous blood samples were obtained for biochemical and genetic analysis.
Genomic DNA (gDNA) was isolated from whole blood in all 47 sib pairs recruited, but only individuals who fitted the strictest BP criteria i.e., 24 sib pairs, were genotyped for highly polymorphic microsatellite markers (all CA repeats) from the MRC panel and Gordon’s markers located on chromosomes 11 and 17, as listed in Table 1 [21,27]. Using previously investigated fluorescent-labelled primers and published methods, a polymerase chain reaction (PCR) carried out with minor modifications [21]. Specifically, microsatellite loci were amplified via the PCR in a 10μL reaction volume containing 10mM of Tris/HCl (at a pH of 8.3), 50 mM of KCl, and 0.2 mM of each dNTP, 2 pmol of each primer (forward and backward), an optimal concentration of MgCl2 (from 1.5 to 2.0mM; determined separately for each primer pair) and 50–100 ng of template gDNA. The polymerase chain reactions were carried out for 34 cycles at 94 °C for three minutes, 55 °C for 1 min and 72 °C for 45 s. The fluorescent-labelled PCR products were pooled and loaded onto an ABI 377 semi-automated sequencer (Perkin Elmer). The sizes of the markers, which reflected differences in the number of CA repeats, were assigned using version 2 of ABI Genotyper® software and corrected manually if necessary. To identify if any genetic polymorphisms in the WNK4 gene exons were involved in this cohort (n = 24 sib pairs), a mutation analysis of exons 7 and 17 was performed via a single-strand conformation polymorphism (SSCP) analysis and direct sequencing on an ABI 377 sequencer, and the data were analysed using Genescan and Genotyper software from ABI (Perkin Elmer).

Table 1.
DNA microsatellite markers investigated on chromosomes 11 and 17.
A statistical analysis was performed using SPSS software (version 28.0) to determine the relationship between blood pressure and the microsatellite markers (correlation and multiple regression analyses) and for graphical illustration. The data were also analysed using the Haseman–Elston quantitative trait loci regression method in which the squared difference in the phenotype (here, blood pressure) between sibs is regressed to the number of alleles shared by identity-by-descent (IBD) and by the use of the SPLINK package, a non-parametric likelihood ratio method. Since this was a small study of 24 sib pairs, correlation coefficients and unadjusted p-values are presented, and a p-value of <0.05 was considered statistically significant.
3. Results
The demographic details of this cohort are provided in Table 2. The mean age of the population was 39.8 ± 7.8 years (an age range 28–67 years), and the average blood pressure was 125 ± 18/77 ± 13 mmHg (Table 2). The two discordant groups of siblings were not significantly different in terms of their age, average height, birthweight or serum markers but differed in their body weight and body mass index. As expected, the affected siblings (high-blood-pressure tail) had significantly higher systolic, diastolic and pulse pressures compared to their unaffected siblings (low-blood-pressure tail). No significant differences were found between the two groups for any of the serum markers.

Table 2.
Demographic, haemodynamic and biochemical data in sib pairs.
Blood pressure levels correlated with chromosome 17 and 11 microsatellite markers, suggesting evidence of linkage. Significant inverse correlations were identified between the squared difference in the systolic blood pressure and the number of alleles shared between the siblings for the D17S799 (r = −0.51, p = 0.011) and D17S250 (r = −0.42, p = 0.04) markers, as illustrated in Figure 1. Diastolic blood pressure also correlated with markers on chromosome 17, D17S1301 (r = −0.42, p = 0.04; Figure 2), D17S250 (r = −0.39, p = 0.05; Figure 2) and D17S928 (r = 0.42, p = 0.04, Figure 3), and the D11S1999 marker on chromosome 11 (r = −0.46, p = 0.025; Figure 3). These relationships persisted even after correcting for confounding factors including age and sex for the markers associated with systolic and diastolic pressure (Table 3). A significant inverse correlation was also observed between the DS11925 marker and the squared difference in pulse pressure (Figure 4, r = −0.44, p = 0.031), a surrogate marker of arterial compliance and an independent predictor of cardiovascular complications.
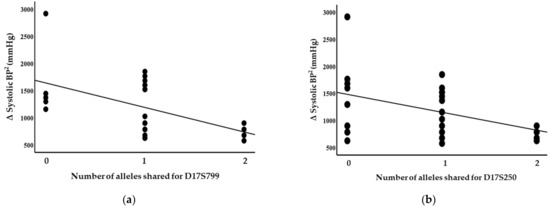
Figure 1.
Systolic blood pressure associated with chromosome 17 markers in siblings sharing alleles at IBD, suggesting evidence of linkages with (a) D17S799 (p = 0.011) and (b) D17S250 (p = 0.04).
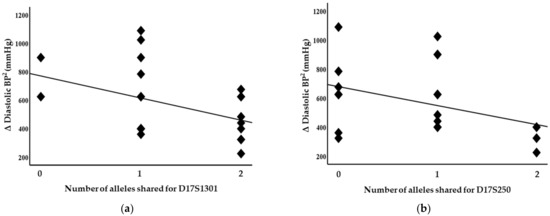
Figure 2.
Diastolic blood pressure associated with chromosome 17 markers in siblings sharing alleles at IBD, suggesting evidence of linkages with (a) D17S1301 (p = 0.04) and (b) D17S250 (p = 0.05).
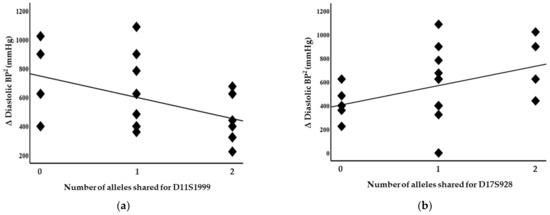
Figure 3.
Diastolic blood pressure associated with chromosome 11 and 17 markers in siblings sharing alleles at IBD, suggesting evidence of linkages with (a) D11S1999 (p = 0.025) and (b) D17S928 (p = 0.04).

Table 3.
Genetic markers independently associated with blood pressure phenotypes.
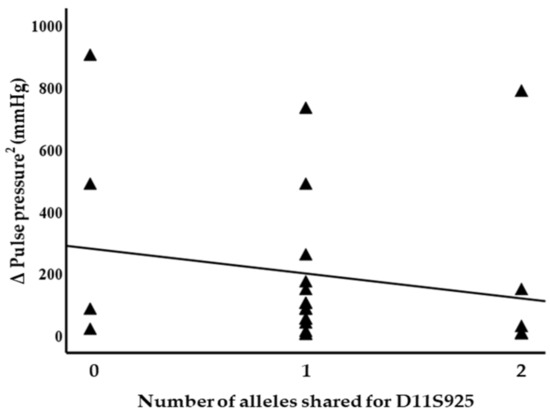
Figure 4.
Pulse pressure associated with chromosome 11 marker in siblings sharing alleles at IBD, suggesting evidence of linkage with D11S925 (p = 0.03).
However, we did not find any polymorphisms in exons 7 and 17 of the WNK4 gene in this cohort, suggesting that within the population from which sib pairs were drawn (48 individuals genotyped), there were no common polymorphisms in the coding region of the WNK4 gene that influenced the blood pressure level.
4. Discussion
High blood pressure affects over 25% of adults and is the major cause of cardiovascular morbidity and mortality. Despite over half a century of multiple lines of investigations, the genetic basis remains poorly understood, with each genetic loci contributing to a minor proportion of blood pressure inheritance. We investigated the feasibility of exploiting the continuous nature of the blood pressure distribution, using extreme discordant sib pairs rather than regarding hypertension as a categorical abnormality. This strategy is widely used in experimental hypertension genetics in which two rodent strains of opposite phenotypes are crossed. However, this approach is most useful when there are large differences in the continuous variable between sib pairs. The extreme discordant strategy, in which a lack of alleles at contributing loci is sought, is predicted to reduce the number of sib ships required by several orders of magnitude (from 10- to 40-fold) [16,17] but depends on identifying families in which the candidate gene is sufficiently important for extreme discordance in blood pressure to exist between sibs with different alleles. The main impetus for this approach is a practical one: it reduces the effort, time and genotyping costs versus a whole-genome scan.
For the first time, we were able to identify that extreme discordant sib pairs do indeed exist in the general population, although their identification is even more labour intensive clinically because of the low return for each proband when compared to that of the concordant sibs required for affected sib pair studies. In theory, our present cohort approached the number required in humans, when the number of loci responsible for hypertension is itself unknown. We were able to recruit double the minimum target proposed by Risch. However, the identification and phenotyping process carried out to identify 24 sib pairs with extreme blood pressure differences took 2 years. Our East Anglian population also has a low prevalence of cardiovascular risk compared to London and other parts of the UK. All the sib pairs studied, including the original probands (parents), were local residents from a region of low CV risk with little or no influence of environmental factors. Nevertheless, this resource will be useful to replicate loci of modest linkage on genome-wide scans and will help to exclude false positives.
In our previously published genome-wide search for the susceptibility loci of hypertension in affected sib pairs (ASPs) [21], we identified microsatellite markers on a chromosome 11q locus that associated with hypertension. The D11S934 marker showed significant two-point linkage (p = 0.004) in the affected sib-pairs [21]. This region contains the ROMK gene that encodes the KCNJ1 potassium channel and is mutated in Bartter’s syndrome (type 2), a monogenic hypertension disorder [28]. In our study here, the D11S934 marker did not associate with any blood pressure phenotype (systolic, diastolic or pulse pressure). It is possible ROMK does not influence blood pressure in our East Anglian population, although other genes in the region around D11S934 may be operating. In fact, we found several other highly polymorphic genomic markers in the chromosome 11 region associated with diastolic pressure (D11S1999, D11S1301 and D11S928) and pulse pressure (D11S925) phenotypes.
Similarly, two of the markers investigated for chromosome 17 correlated with systolic blood pressure (D17S799 and D17S250) suggesting genetic linkage. We previously demonstrated evidence of linkage for the D17S250 marker (with a maximum LOD score of around 2.3) with Gordon syndrome in a large, well-characterised Brisbane family with seven offspring [22]. This suggests that the same genetic locus for hypertension is involved in both “Gordon syndrome” and “essential hypertension”. So far, there is no evidence to corroborate our findings with this EDSP strategy in other populations or other complex phenotypes using microsatellite markers. Numerous studies sought to identify genes for hypertension and blood pressure using genome-wide scans via discordant sib pairs and general populations. But using the genome scan, the only study that reported regions containing five genomic markers on various chromosomes (D3S2387, D11S2019, D15S657, D16S3396 and D17S1303) for systolic and diastolic blood pressure in their primary analysis and eight regions in a secondary analysis (D4S3248, D72195, D101423, D20S470, D20S482, D21S2052, PAH and AGT) highlighted chromosomes 11 and 17 as putative regions for harbouring hypertension genes [29]. This study used extremely discordant, highly concordant and low concordant sib pairs in China [29], unlike the American, Quebec and San Antonio studies [30,31,32].
5. Conclusions
Significant inverse correlations between blood pressure (systolic, diastolic and pulse pressures) and several microsatellite markers suggests evidence of linkage on chromosomes 11 and 17, as noted previously in our studies on affected sib pairs with essential hypertension and the Brisbane family with Gordon’s syndrome. The results also illustrate the use of a discordant sib pair analysis to detect linkage within small numbers of pedigrees with essential hypertension. Further analysis of this cohort may be valuable in complementing findings from large genome-wide scans in affected sib pairs.
Author Contributions
Conceptualization, Y. and K.M.O.; Methodology, Y. and K.M.O.; Formal analysis, Y.; Investigation, Y. and K.M.O.; Resources, Y.; Data curation, Y.; Writing—original draft, Y.; Writing—review & editing, K.M.O.; Visualization, Y.; Supervision, K.M.O.; Project administration, Y. and K.M.O.; Funding acquisition, Y. and K.M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a project grant from the British Heart Foundation (PG95169).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Local Ethics Committee, Cambridge.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We thank all study participants for their time with this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kulkarni, S.; Glover, M.; Kapil, V.; Abrams, S.M.L.; Partridge, S.; McCormack, T.; Sever, P.; Delles, C.; Wilkinson, I.B. Management of hypertensive crisis: British and Irish Hypertension Society. J. Hum. Hypertens. 2022, 14, 1–17. [Google Scholar]
- Province, M.A.; Kardia, S.L.; Ranade, K.; Rao, D.C.; Thiel, B.A.; Cooper, R.S.; Risch, N.; Turner, S.T.; Cox, D.R.; Hunt, S.C.; et al. A meta-analysis of genome-wide linkage scans for hypertension: The national heart, lung and blood institute family blood pressure program. Am. J. Hypertens. 2003, 16, 144–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caulfield, M.; Munroe, P.; Pembroke, J.; Samani, N.; Dominiczak, A.; Brown, M.; Webster, J.; Ratcliffe, P.; O’Shea, S.; Papp, J.; et al. Genome-wide mapping of human loci for essential hypertension. Lancet 2003, 261, 118–2123. [Google Scholar]
- Warren, H.R.; Evangelou, E.; Cabrera, C.P.; Gao, H.; Ren, M.; Mifsud, B.; Ntalla, I.; Surendran, P.; Liu, C.; Cook, J.P.; et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat. Genet. 2017, 49, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Ehret, G.B.; Ferreira, T.; Chasman, D.I.; Jackson, A.U.; Schmidt, E.M.; Johnson, T.; Thorleifsson, G.; Luan, J.A.; Donnelly, L.A.; Kanoni, S.; et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat. Genet. 2016, 48, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Hellwege, J.N.; Keaton, J.; Park, J.; Qiu, C.; Warren, H.R.; Torstenson, E.S.; Kovesdy, C.P.; Sun, Y.V.; Wilson, O.D.; et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet. 2019, 51, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Jia, P.; Zhan, N.; Bat, B.K.K.; Feng, Q.; Tsoi, K.K.F. The genetic architecture of blood pressure variability: A genome-wide association study of 9370 participants from UK Biobank. J. Clin. Hyperens. 2022, 24, 1370–1380. [Google Scholar] [CrossRef]
- Kelly, T.N.; Sun, X.; He, K.Y.; Brown, M.R.; Taliun, S.A.G.; Hellwege, J.N.; Irvin, M.R.; Mi, X.; Brody, J.A.; Franceschini, N.; et al. Insights from a large-scale whole-genome sequencing study of systolic blood pressure, diastolic blood pressure and hypertension. Hypertension 2022, 79, 1656–1667. [Google Scholar] [CrossRef]
- Surendran, P.; Drenos, F.; Young, R.; Warren, H.; Cook, J.P.; Manning, A.K.; Grarup, N.; Sim, X.; Barnes, D.R.; Witkowska, K.; et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat. Genet. 2016, 48, 1151–1161. [Google Scholar] [CrossRef]
- Yu, B.; Pulit, S.L.; Hwang, S.J.; Brody, J.A.; Amin, N.; Auer, P.L.; Bis, J.C.; Boerwinkle, E.; Burke, G.L.; Chakravarti, A.; et al. Rare exome sequence variants in CLCN6 reduce blood pressure levels and hypertension risk. Circ. Cardiovasc. Genet. 2016, 9, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kraja, A.T.; Smith, J.A.; Brody, J.A.; Franceschini, N.; Bis, J.C.; Rice, K.; Morrison, A.C.; Lu, Y.; Weiss, S.; et al. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat. Genet. 2016, 48, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Price, R.A.; Li, W.-D.; Zhao, H. FTO gene SNPs associated with extreme obesity in cases, controls and extremely discordant sister pairs. BMC Med. Genet. 2008, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Dalman, C.; Karlsson, H.; Lewis, G.; Osborn, D.P.L.; Gardner, R.; Hayes, J.F. Childhood and parental asthma, future risk of bipolar disorder and schizophrenia spectrum disorder: A population-based cohort study. Schizophr. Bull. 2019, 45, 360–368. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, M.M.; Lane, A.M.; Shah, C.P.; Ott, J.; Dryja, T.P.; Miller, J.W. Extremely discordant sib-pair study design to determine risk factors for neovascular age-related macular degeneration. Arch. Ophthalmol. 2004, 122, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Risch, N.; Zhang, H. Extreme discordant sib pairs for mapping quantitative trait loci in humans. Science 1995, 268, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Risch, N.; Zhang, H. Mapping quantitative trait loci with extreme discordant sib pairs: Sampling considerations. Am. J. Hum. Genet. 1996, 58, 836–843. [Google Scholar]
- Rao, D.C.; Province, M.A.; Leppert, M.F.; Oberman, A.; Heiss, G.; Ellison, C.; Arnett, D.K.; Eckfeldt, J.H.; Schwander, K.; Mockrin, S.; et al. A genome-wide affected sibpair linkage analysis of hypertension: The HyperGEN network. Am. J. Hypertens. 2003, 16, 148–150. [Google Scholar] [CrossRef]
- Newton-Cheh, C.; Johnson, T.; Gateva, V.; Tobin, M.D.; Bochud, M.; Coin, L.; Najjar, S.S.; Zhao, J.H.; Heath, S.C.; Eyheramendy, S.; et al. Genome wide association study identifies eight loci associated with blood pressure. Nat. Genet. 2009, 41, 666–676. [Google Scholar] [CrossRef]
- Levy, D.; Ehret, G.B.; Rice, K.; Verwoert, G.C.; Launer, L.J.; Dehghan, A.; Glazer, N.L.; Morrison, A.C.; Johnson, A.D.; Aspelund, T.; et al. Genome wide association study of blood pressure and hypertension. Nat. Genet. 2009, 41, 677–687. [Google Scholar] [CrossRef]
- Sharma, P.; Fatibene, J.; Ferraro, F.; Jia, H.; Monteith, S.; Brown, C.; Clayton, D.; O’Shaughnessy, K.M.; Brown, M.J. A genome-wide search for susceptible loci to human essential hypertension. Hypertension 2000, 35, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, K.M.; Fu, B.; Johnson, A.; Gordon, R.D. Linkage of Gordon’s syndrome to the long arm of chromosome 17 in a region recently linked to familial essential hypertension. J. Hum. Hypertens. 1998, 12, 675–678. [Google Scholar] [CrossRef][Green Version]
- Cope, G.; Golbang, A.; O’Shaughnessy, K.M. WNK kinases and the control of blood pressure. Pharmacol. Ther. 2005, 106, 221–231. [Google Scholar] [CrossRef]
- Murthy, M.; Cope, G.; O’Shaughnessy, K.M. The acidic motif of WNK4 is crucial for its interaction with the K channel ROMK. Biochem. Biophys. Res. Commun. 2008, 375, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Glover, M.; O’Shaughnessy, K.M. Molecular insights from dysregulation of the thiazide-sensitive WNK/SPAK/NCC pathway in the kidney: Gordon syndrome and thiazide-induced hyponatraemia. Clin. Exp. Pharmacol. Physiol. 2013, 40, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Yasmin; Mascie-Taylor, C.G.N.; Brown, M.; Hughes, N. The effect of dietary intervention on changes in total cholesterol, blood pressure and weight in a Cambridge study. Int. J. Clin. Pract. 1998, 52, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Reed, P.W.; Davies, J.L.; Copeman, J.B.; Bennett, S.T.; Palmer, S.M.; Pritchard, L.E.; Gough, S.C.L.; Kawaguchi, Y.; Cordell, H.J.; Balfour, K.M.; et al. Chromosome specific microsatellite sets for flourescent-based, semi-automated genome mapping. Nat. Genet. 1994, 7, 390–395. [Google Scholar] [CrossRef]
- Simon, D.B.; Karet, F.E.; Rodriguez-Soriano, J.; Hamdan, J.H.; DiPietro, A.; Trachtman, H.; Sanjad, S.A.; Lifton, R.P. Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel. ROMK Nat. Genet. 1996, 14, 152–156. [Google Scholar]
- Xu, X.; Rogus, J.J.; Terwedow, H.A.; Yang, J.; Wang, Z.; Chen, C.; Niu, T.; Wang, B.; Xu, H.; Weiss, S.; et al. An extreme-sib-par genome scan for genes regulating blood pressure. Am. J. Hum. Genet. 1999, 64, 1694–1701. [Google Scholar] [CrossRef]
- Krushkal, J.; Ferrell, R.; Mockrin, S.C.; Turner, S.T.; Sing, C.F.; Boerwinkle, E. Genome-wide linkage analyses of systolic blood pressure using highly discordant siblings. Circulation 1999, 99, 1407–1410. [Google Scholar] [CrossRef]
- Rice, T.; Rankinen, T.; Province, M.A.; Chagnon, Y.C.; Perusse, L.; Boreck, I.B.; Bouchard, C.; Rao, D.C. Genome wide linkage analysis of systolic and diastolic blood pressure. The Quebec family study. Circulation 2000, 102, 1956–1963. [Google Scholar] [CrossRef]
- Atwood, L.D.; Samollow, P.B.; Hixson, J.E.; Stern, M.P.; MacCluer, J.W. Genome-wide linkage analysis of blood pressure in Mexican Americans. Genet. Epidemiol. 2001, 20, 373–382. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).