Identification of Hub Genes and Target miRNAs Crucial for Milk Production in Holstein Friesian Dairy Cattle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microarray Data Pre-Processing and Statistical Analysis
2.2. Network Module Creation Using the Integration of Protein–Protein Interactions (PPIs)
2.3. miRNAs, Biological Processes, and Pathway Analysis of DEGs
2.4. Relevance Network Design
3. Results
3.1. DEGs Identification and PPI Analysis
3.2. Network Analysis
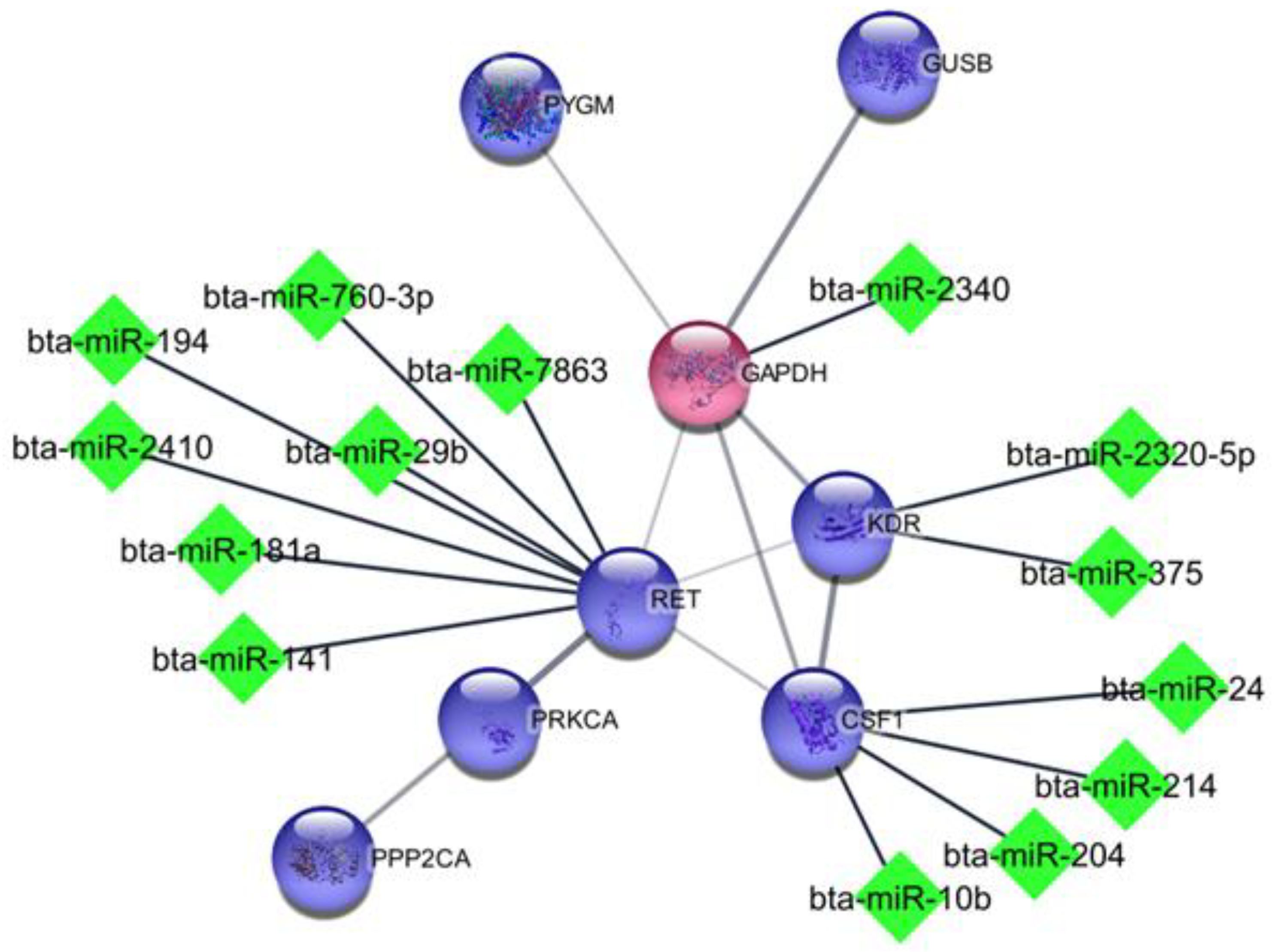
3.3. Biological Processes, Pathway Analysis, and miRNA Identification
4. Discussion
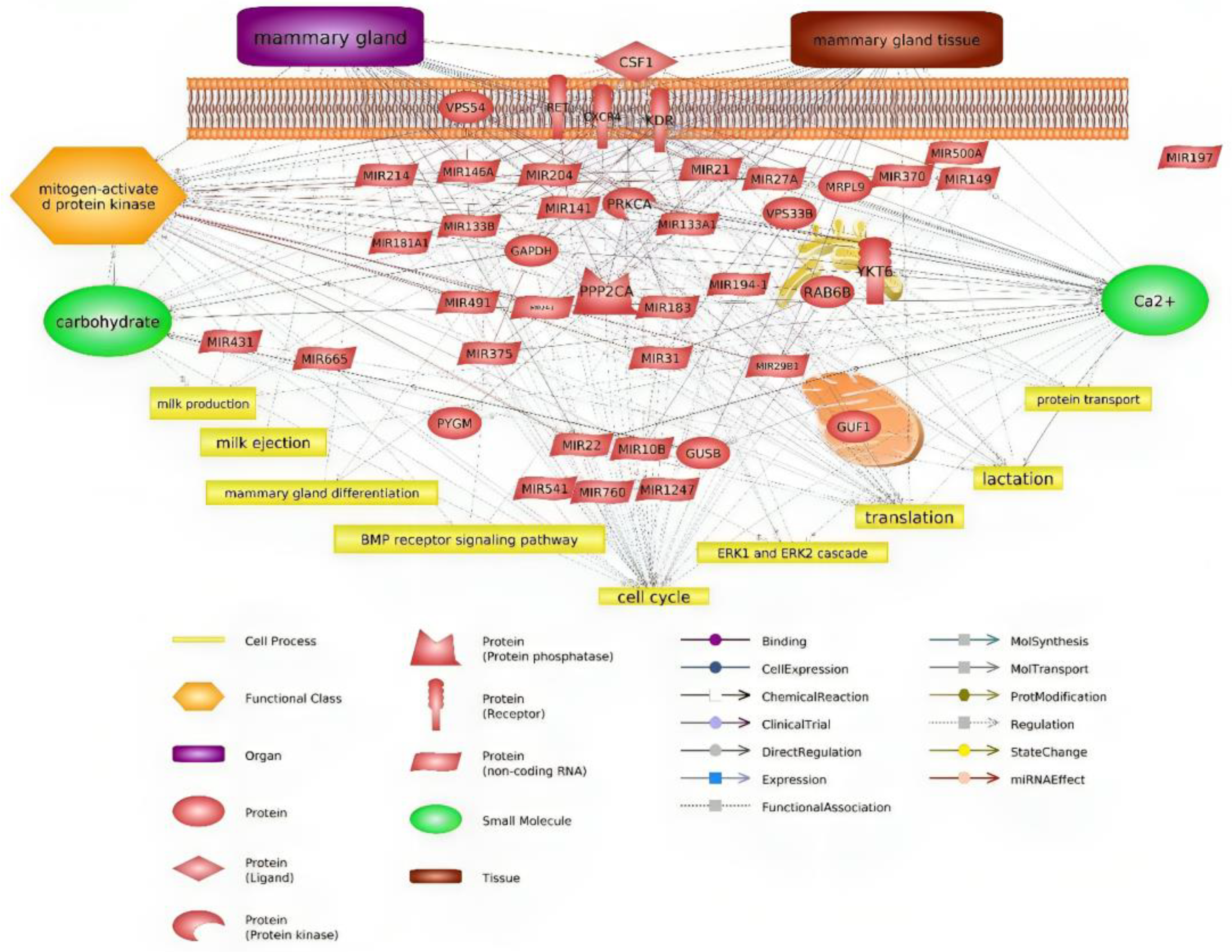
4.1. Hub Genes Related to Milk Production
4.2. miRNAs Related to Milk Production
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beuzen, N.D.; Stear, M.J.; Chang, K.C. Molecular markers and their use in animal breeding. Vet. J. 2000, 160, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Giosuè, A.; Calabrese, I.; Vitale, M.; Riccardi, G.; Vaccaro, O. Consumption of Dairy Foods and Cardiovascular Disease: A Systematic Review. Nutrients 2022, 14, 831. [Google Scholar] [CrossRef] [PubMed]
- Babio, N.; Becerra-Tomás, N.; Nishi, S.K.; López-González, L.; Paz-Graniel, I.; García-Gavilán, J.; Schröder, H.; Martín-Calvo, N.; Salas-Salvadó, J. Total dairy consumption in relation to overweight and obesity in children and adolescents: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2022, 23 (Suppl. S1), e13400. [Google Scholar] [CrossRef]
- Ma, Y.; Khan, M.Z.; Xiao, J.; Alugongo, G.M.; Chen, X.; Chen, T.; Liu, S.; He, Z.; Wang, J.; Shah, M.K. Genetic markers associated with milk production traits in dairy cattle. Agriculture 2021, 11, 1018. [Google Scholar] [CrossRef]
- Buitenhuis, A.J.; Sundekilde, U.K.; Poulsen, N.A.; Bertram, H.C.; Larsen, L.B.; Sørensen, P. Estimation of genetic parameters and detection of quantitative trait loci for metabolites in Danish Holstein milk. J. Dairy Sci. 2013, 96, 3285–3295. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.M.; Nie, Q.H.; Peng, X.; Zhang, D.X.; Zhang, X.Q. Single nucleotide polymorphisms of the chicken insulin-like factor binding protein 2 gene associated with chicken growth and carcass traits. Poult. Sci. 2005, 84, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Bar-Joseph, Z. Analyzing time series gene expression data. Bioinformatics 2004, 20, 2493–2503. [Google Scholar] [CrossRef]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef]
- Dysin, A.P.; Barkova, O.Y.; Pozovnikova, M.V. The Role of microRNAs in the Mammary Gland Development, Health, and Function of Cattle, Goats, and Sheep. Non-Coding RNA 2021, 7, 78. [Google Scholar] [CrossRef]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, M.; Wang, J.; Pan, Y.; Wu, F.-X. CytoNCA: A cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Biosystems 2015, 127, 67–72. [Google Scholar] [CrossRef]
- Nepusz, T.; Yu, H.; Paccanaro, A. Detecting overlapping protein complexes in protein-protein interaction networks. Nat. Methods 2012, 9, 471–472. [Google Scholar] [CrossRef] [PubMed]
- Bader, G.D.; Hogue, C.W.V. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef]
- Lemay, D.G.; Lynn, D.J.; Martin, W.F.; Neville, M.C.; Casey, T.M.; Rincon, G.; Kriventseva, E.V.; Barris, W.C.; Hinrichs, A.S.; Molenaar, A.J.; et al. The bovine lactation genome: Insights into the evolution of mammalian milk. Genome Biol. 2009, 10, R43. [Google Scholar] [CrossRef]
- Lim, J.; Hao, T.; Shaw, C.; Patel, A.J.; Szabó, G.; Rual, J.-F.; Fisk, C.J.; Li, N.; Smolyar, A.; Hill, D.E.; et al. A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration. Cell 2006, 125, 801–814. [Google Scholar] [CrossRef]
- Roudbari, Z.; Coort, S.L.; Kutmon, M.; Eijssen, L.; Melius, J.; Sadkowski, T.; Evelo, C.T. Identification of Biological Pathways Contributing to Marbling in Skeletal Muscle to Improve Beef Cattle Breeding. Front. Genet. 2019, 10, 1370. [Google Scholar] [CrossRef]
- Varshney, N.; Mohanty, A.K.; Kumar, S.; Kaushik, J.K.; Dang, A.K.; Mukesh, M.; Mishra, B.P.; Kataria, R.; Kimothi, S.P.; Mukhopadhyay, T.K.; et al. Selection of suitable reference genes for quantitative gene expression studies in milk somatic cells of lactating cows (Bos indicus). J. Dairy Sci. 2012, 95, 2935–2945. [Google Scholar] [CrossRef]
- Corbin, I.R.; Gong, Y.; Zhang, M.; Minuk, G.Y. Proliferative and nutritional dependent regulation of glyceraldehyde-3-phosphate dehydrogenase expression in the rat liver. Cell Prolif. 2002, 35, 173–182. [Google Scholar] [CrossRef]
- Mattmiller, S.A.; Corl, C.M.; Gandy, J.C.; Loor, J.J.; Sordillo, L.M. Glucose transporter and hypoxia-associated gene expression in the mammary gland of transition dairy cattle. J. Dairy Sci. 2011, 94, 2912–2922. [Google Scholar] [CrossRef]
- Sapi, E. The role of CSF-1 in normal physiology of mammary gland and breast cancer: An update. Exp. Biol. Med. Maywood NJ 2004, 229, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Morandi, A.; Barbetti, V.; Riverso, M.; Dello Sbarba, P.; Rovida, E. The colony-stimulating factor-1 (CSF-1) receptor sustains ERK1/2 activation and proliferation in breast cancer cell lines. PLoS ONE 2011, 6, e27450. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.M.; Mulligan, L.M. The RET receptor is linked to stress response pathways. Cancer Res. 2004, 64, 4453–4463. [Google Scholar] [CrossRef]
- Gao, Y.-E.; Wang, Y.; Chen, F.-Q.; Feng, J.-Y.; Yang, G.; Feng, G.-X.; Yang, Z.; Ye, L.-H.; Zhang, X.-D. Post-transcriptional modulation of protein phosphatase PPP2CA and tumor suppressor PTEN by endogenous siRNA cleaved from hairpin within PTEN mRNA 3′UTR in human liver cells. Acta Pharmacol. Sin. 2016, 37, 898–907. [Google Scholar] [CrossRef]
- Pawłowski, K.; Pires, J.A.A.; Faulconnier, Y.; Chambon, C.; Germon, P.; Boby, C.; Leroux, C. Mammary Gland Transcriptome and Proteome Modifications by Nutrient Restriction in Early Lactation Holstein Cows Challenged with Intra-Mammary Lipopolysaccharide. Int. J. Mol. Sci. 2019, 20, 1156. [Google Scholar] [CrossRef]
- Oshima, A.; Kyle, J.W.; Miller, R.D.; Hoffmann, J.W.; Powell, P.P.; Grubb, J.H.; Sly, W.S.; Tropak, M.; Guise, K.S.; Gravel, R.A. Cloning, sequencing, and expression of cDNA for human β-glucuronidase. Proc. Natl. Acad. Sci. USA 1987, 84, 685–689. [Google Scholar] [CrossRef]
- Paraboschi, E.M.; Rimoldi, V.; Soldà, G.; Tabaglio, T.; Dall’Osso, C.; Saba, E.; Vigliano, M.; Salviati, A.; Leone, M.; Benedetti, M.D.; et al. Functional variations modulating PRKCA expression and alternative splicing predispose to multiple sclerosis. Hum. Mol. Genet. 2014, 23, 6746–6761. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, Y.; Wang, C.; Li, Q. Let-7g-5p regulates mouse mammary cells differentiation and function by targeting PRKCA. J. Cell. Physiol. 2019, 234, 10101–10110. [Google Scholar] [CrossRef]
- Migocka-Patrzałek, M.; Lewicka, A.; Elias, M.; Daczewska, M. The effect of muscle glycogen phosphorylase (Pygm) knockdown on zebrafish morphology. Int. J. Biochem. Cell Biol. 2020, 118, 105658. [Google Scholar] [CrossRef]
- Oliver, C.H.; Watson, C.J. Making milk: A new link between STAT5 and Akt1. JAK-STAT 2013, 2, e23228. [Google Scholar] [CrossRef] [PubMed]
- Van Herwijnen, M.J.C.; Driedonks, T.A.P.; Snoek, B.L.; Kroon, A.M.T.; Kleinjan, M.; Jorritsma, R.; Pieterse, C.M.J.; Hoen, E.N.M.N.; Wauben, M.H.M. Abundantly Present miRNAs in Milk-Derived Extracellular Vesicles Are Conserved Between Mammals. Front. Nutr. 2018, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Luoreng, Z.-M.; Wang, X.-P.; Mei, C.-G.; Zan, L.-S. Comparison of microRNA Profiles between Bovine Mammary Glands Infected with Staphylococcus aureus and Escherichia coli. Int. J. Biol. Sci. 2018, 14, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Modepalli, V.; Kumar, A.; Hinds, L.A.; Sharp, J.A.; Nicholas, K.R.; Lefevre, C. Differential temporal expression of milk miRNA during the lactation cycle of the marsupial tammar wallaby (Macropus eugenii). BMC Genom. 2014, 15, 1012. [Google Scholar] [CrossRef]
- Schanzenbach, C.I.; Kirchner, B.; Ulbrich, S.E.; Pfaffl, M.W. Can milk cell or skim milk miRNAs be used as biomarkers for early pregnancy detection in cattle? PLoS ONE 2017, 12, e0172220. [Google Scholar] [CrossRef]
- Li, Q.; Yang, C.; Du, J.; Zhang, B.; He, Y.; Hu, Q.; Li, M.; Zhang, Y.; Wang, C.; Zhong, J. Characterization of miRNA profiles in the mammary tissue of dairy cattle in response to heat stress. BMC Genom. 2018, 19, 975. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Patel, A.B.; Joshi, C. Comparative analysis of SNP candidates in disparate milk yielding river buffaloes using targeted sequencing. PeerJ 2016, 4, e2147. [Google Scholar] [CrossRef] [PubMed]
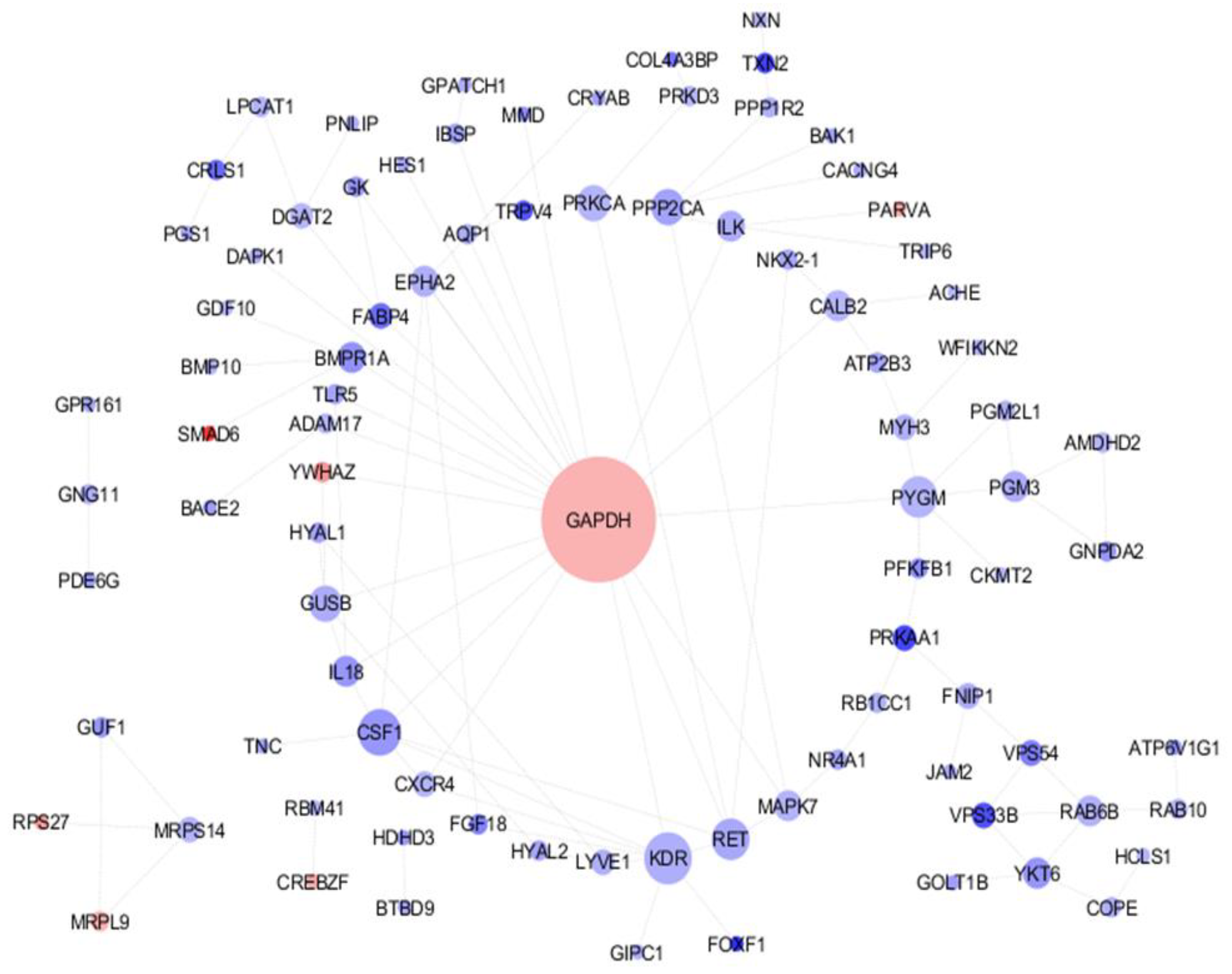

| No. | Genes | p-Value |
|---|---|---|
| Cluster 1 | GUF1, MRPS14, MRPL9, RPS27, YKT6, RAB6B, VPS54, VPS33B | 5.8 × 10−9 |
| Cluster 2 | GAPDH, KDR, CSF1, PYGM, GUSB, CXCR4, FGF18, FABP4, GDF10, BMP10, SMAD6 | 3.4 × 10−10 |
| Cluster 3 | PPP2CA, PRKCA, CACNG4, MAPK7, CCNI, BAK1, ANKLE2, PRKCA | 3.26 × 10−7 |
| Cluster 4 | PRKAA1, AMDHD2, GNPDA2, PGM3, PGM2L1, PFKFB1, PDE6G | 0.000151 |
| Gene | Gene Name | Expression Log FC | Degree | Betweenness | Closeness |
|---|---|---|---|---|---|
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase | −1.07139 | 22 | 4384.83 | 0.074 |
| KDR | Kinase insert domain receptor | −0.99096 | 8 | 539.66 | 0.070 |
| CSF1 | Colony-stimulating factor1 | −0.49033 | 8 | 180.5 | 0.070 |
| PYGM | phosphorylase, glycogen, muscle associated | −0.4587 | 6 | 2199.0 | 0.072 |
| RET | Ret proto-oncogene | −0.39795 | 6 | 397.33 | 0.071 |
| PPP2CA | Protein phosphatase 2 catalytic subunit alpha | 0.66955 | 5 | 763.0 | 0.068 |
| GUSB | Glucuronidase beta | 0.371806 | 5 | 272.33 | 0.070 |
| PRKCA | Protein kinase C alpha | −0.33072 | 5 | 418.66 | 0.068 |
| Biological Activity Analysis of Clustered Genes | |
|---|---|
| Cluster 1 | Translation, mitochondrial translational elongation, mitochondrial translational initiation, protein transport. |
| Cluster 2 | BMP signalling pathway, regulation of MAPK cascade, fat cell differentiation, carbohydrate metabolic process, cell development, SMAD protein signal transduction, positive regulation of ERK1 and ERK2 cascade, and positive regulation of gene expression. |
| Cluster 3 | Regulation of cell cycle, cell proliferation, calcium ion transmembrane transport, peptidyl-serine phosphorylation, and positive regulation of calcium ion transport into cytosol. |
| Cluster 4 | Carbohydrate metabolic process, N-acetylglucosamine metabolic process, N-acetylglucosamine catabolic process, N-acetylneuraminate catabolic process, glycogen catabolic process, and lipid biosynthetic process. |
| Pathway Analysis of Clustered Gene | |
|---|---|
| Cluster 1 | Ribosome, SNARE interactions in vesicular transport. |
| Cluster 2 | Rap1 signalling pathway, Ras signalling pathway, Chemokine signalling pathway, PI3K-Akt signalling pathway. |
| Cluster 3 | Protein processing in endoplasmic reticulum, MAPK signalling pathway, ErbB signalling pathway, Ras signalling pathway, Rap1 signalling pathway, Calcium signalling pathway, HIF-1 signalling pathway. |
| Cluster 4 | Amino sugar and nucleotide sugar metabolism, Glucagon signalling pathway, AMPK signalling pathway. |
| Target Genes | miRNAs |
|---|---|
| RET | bta-miR-141, bta-miR-181a, bta-miR-194, bta-miR-2410, bta-miR-29b, bta-miR-760-3p, bta-miR-7863 |
| CXCR4 | bta-miR-2344 |
| GAPDH | bta-miR-2340 |
| CSF1 | bta-miR-10b, bta-miR-204, bta-miR-214, bta-miR-24, bta-miR-375, bta-miR-665, bta-miR-760-3p, bta-miR-7863 |
| KDR | bta-miR-375, bta-miR-2320-5p |
| VPS54 | bta-miR-146a, bta-miR-22-3p, bta-miR-2413, bta-miR-31 |
| RAB6B | bta-miR-149-5p, bta-miR-21-3p, bta-miR-2357, bta-miR-29b, bta-miR-27a-5p, bta-miR-431, bta-miR-491, bta-miR-541, bta-miR-760-3p |
| VPS33B | bta-miR-183, bta-miR-1247-3p, bta-miR-7863 |
| YKT6 | bta-miR-197, bta-miR-500, bta-miR-133a, bta-miR-133b |
| MRPL9 | bta-miR-370, bta-miR-665 |
| GUF1 | bta-miR-10b, bta-miR-204, bta-miR-214, bta-miR-24, bta-miR-375, bta-miR-665, bta-miR-760-3p, bta-miR-7863 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roudbari, Z.; Mokhtari, M.; Ebrahimpour Gorji, A.; Sadkowski, T.; Sadr, A.S.; Shirali, M. Identification of Hub Genes and Target miRNAs Crucial for Milk Production in Holstein Friesian Dairy Cattle. Genes 2023, 14, 2105. https://doi.org/10.3390/genes14112105
Roudbari Z, Mokhtari M, Ebrahimpour Gorji A, Sadkowski T, Sadr AS, Shirali M. Identification of Hub Genes and Target miRNAs Crucial for Milk Production in Holstein Friesian Dairy Cattle. Genes. 2023; 14(11):2105. https://doi.org/10.3390/genes14112105
Chicago/Turabian StyleRoudbari, Zahra, Morteza Mokhtari, Abdolvahab Ebrahimpour Gorji, Tomasz Sadkowski, Ayeh Sadat Sadr, and Masoud Shirali. 2023. "Identification of Hub Genes and Target miRNAs Crucial for Milk Production in Holstein Friesian Dairy Cattle" Genes 14, no. 11: 2105. https://doi.org/10.3390/genes14112105








