Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components
Abstract
1. Introduction
2. The Cytoskeleton
3. Microfilaments
4. Intermediate Filaments
5. Microtubules
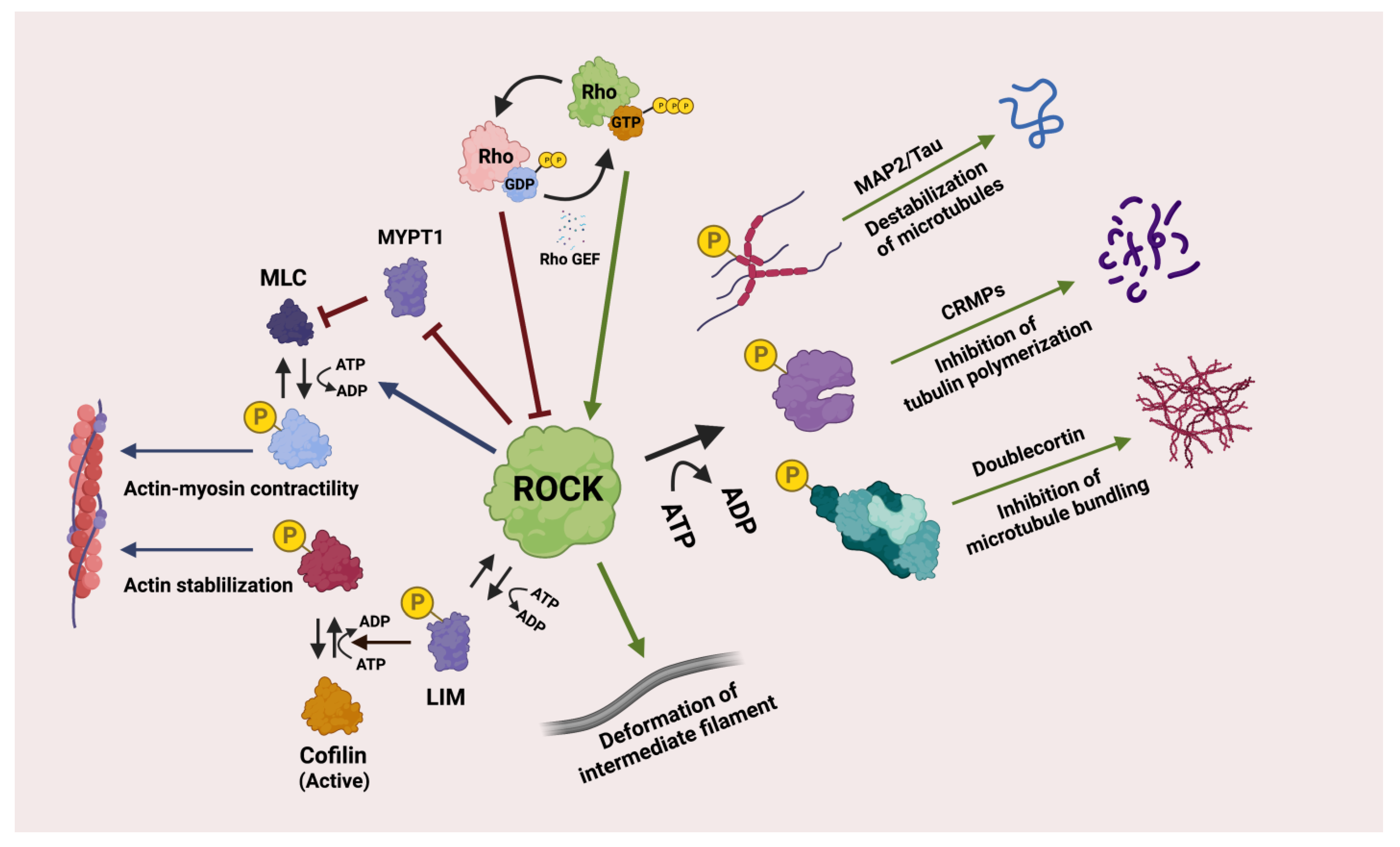
6. Rho-Associated Kinase/ROCK Pathway and Associated Genes
6.1. Rho GTPases
6.2. ROCK
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guan, G.; He, Y.; Mei, L. Atomic force microscopy: A nanobiotechnology for cellular research. Nano TransMed. 2022, 1, e9130004. [Google Scholar] [CrossRef]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bao, G.; Wang, N. Cell mechanics: Mechanical response, cell adhesion, and molecular deformation. Annu. Rev. Biomed. Eng. 2000, 2, 189–226. [Google Scholar] [CrossRef]
- Walker, M.; Rizzuto, P.; Godin, M.; Pelling, A.E. Structural and mechanical remodeling of the cytoskeleton maintains tensional homeostasis in 3D microtissues under acute dynamic stretch. Sci. Rep. 2020, 10, 7696. [Google Scholar] [CrossRef] [PubMed]
- Reichl, E.M.; Effler, J.C.; Robinson, D.N. The stress and strain of cytokinesis. Trends. Cell Biol. 2005, 15, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Dalous, J.; Burghardt, E.; Müller-Taubenberger, A.; Bruckert, F.; Gerisch, G.; Bretschneider, T. Reversal of cell polarity and actin-myosin cytoskeleton reorganization under mechanical and chemical stimulation. Biophys. J. 2008, 94, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, R.; Ehrlicher, A. The forces behind cell movement. Int. J. Biol. Sci. 2007, 3, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Pegoraro, A.F.; Janmey, P.; Weitz, D.A. Mechanical Properties of the Cytoskeleton and Cells. Cold Spring Harb. Perspect. Biol. 2017, 9, a022038. [Google Scholar] [CrossRef]
- Riento, K.; Ridley, A.J. ROCKs: Multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Maekawa, M.; Ishizaki, T.; Boku, S.; Watanabe, N.; Fujita, A.; Iwamatsu, A.; Obinata, T.; Ohashi, K.; Mizuno, K.; Narumiya, S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 1999, 285, 895–898. [Google Scholar] [CrossRef]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Schmandke, A.; Schmandke, A.; Strittmatter, S.M. ROCK and Rho: Biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist 2007, 13, 454–469. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.Y.; Li, C.Y.; Chen, J.; Pan, L.; Saito, S.; Terashita, T.; Saito, K.; Miyawaki, K.; Shigemoto, K.; Mominoki, K.; et al. Rho-ROCK signal pathway regulates microtubule-based process formation of cultured podocytes - Inhibition of ROCK promoted process elongation. Nephron Exp. Nephrol. 2004, 97, e49–e61. [Google Scholar] [CrossRef]
- Morgan-Fisher, M.; Wewer, U.M.; Yoneda, A. Regulation of ROCK activity in cancer. J. Histochem. Cytochem. 2013, 61, 185–198. [Google Scholar] [CrossRef]
- Lane, J.; Martin, T.A.; Watkins, G.; Mansel, R.E.; Jiang, W.G. The expression and prognostic value of ROCK I and ROCK II and their role in human breast cancer. Int. J. Oncol. 2008, 33, 585–593. [Google Scholar] [CrossRef]
- Liu, X.; Choy, E.; Hornicek, F.J.; Yang, S.; Yang, C.; Harmon, D.; Mankin, H.; Duan, Z. ROCK1 as a potential therapeutic target in osteosarcoma. J. Orthop. Res. 2011, 29, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Wong, C.M.; Tung, E.K.; Man, K.; Ng, I.O. Rho-kinase 2 is frequently overexpressed in hepatocellular carcinoma and involved in tumor invasion. Hepatology 2009, 49, 1583–1594. [Google Scholar] [CrossRef]
- Darling, E.M.; Di Carlo, D. High-Throughput Assessment of Cellular Mechanical Properties. Annu. Rev. Biomed. Eng. 2015, 17, 35–62. [Google Scholar] [CrossRef]
- Jiang, X.; Qin, Y.; Kun, L.; Zhou, Y. The Significant Role of the Microfilament System in Tumors. Front. Oncol. 2021, 11, 620390. [Google Scholar] [CrossRef]
- Falahzadeh, K.; Banaei-Esfahani, A.; Shahhoseini, M. The potential roles of actin in the nucleus. Cell Journal 2015, 17, 7–14. [Google Scholar] [PubMed]
- Gordón-Alonso, M.; Veiga, E.; Sánchez-Madrid, F. Actin dynamics at the immunological synapse. Cell Health Cytoskelet. 2010, 2, 33–47. [Google Scholar]
- Blessing, C.A.; Ugrinova, G.T.; Goodson, H.V. Actin and ARPs: Action in the nucleus. Trends Cell Biol. 2004, 14, 435–442. [Google Scholar] [CrossRef]
- Halpain, S. Actin in a supporting role. Nat. Neurosci. 2003, 6, 101–102. [Google Scholar] [CrossRef]
- Lanier, L.M.; Gertler, F.B. Actin cytoskeleton: Thinking globally, actin’ locally. Curr. Biol. 2000, 10, R655–R657. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Blanchoin, L.; Mullins, R.D. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 545–576. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Hall, M.N. Signaling to the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 1998, 14, 305–338. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, A. Microfilaments and membranes. Curr. Opin. Cell Biol. 1993, 5, 653–660. [Google Scholar] [CrossRef]
- Gül, D.; Habtemichael, N.; Dietrich, D.; Dietrich, J.; Gößwein, D.; Khamis, A.; Deuss, E.; Künzel, J.; Schneider, G.; Strieth, S.; et al. Identification of cytokeratin24 as a tumor suppressor for the management of head and neck cancer. Biol. Chem. 2022, 403, 869–890. [Google Scholar] [CrossRef]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types I and II Keratin Intermediate Filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, a018275. [Google Scholar] [CrossRef]
- Saitoh, T.; Sato, K.; Tonogi, M.; Tanaka, Y.; Yamane, G.Y. Expression of Cytokeratin 13, 14, 17, and 19 in 4-nitroquinoline-1-oxide-induced Oral Carcinogenesis in Rat. Bull. Tokyo Dent. Coll. 2016, 57, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, J.; Bowden, P.E.; Coulombe, P.A.; Langbein, L.; Lane, E.B.; Magin, T.M.; Maltais, L.; Omary, M.B.; Parry, D.A.; Rogers, M.A.; et al. New consensus nomenclature for mammalian keratins. J. Cell Biol. 2006, 174, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Koike, K.; Kubo, Y.; Masuko, S.; Arakawa, Y.; Ando, S. In vitro assembly properties of human type I and II hair keratins. Cell Struct. Funct. 2014, 39, 31–43. [Google Scholar] [CrossRef]
- Infante, C.; Ponce, M.; Asensio, E.; Zerolo, R.; Manchado, M. Molecular characterization of a novel type II keratin gene (sseKer3) in the Senegalese sole (Solea senegalensis): Differential expression of keratin genes by salinity. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 160, 15–23. [Google Scholar] [CrossRef]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Bowden, P.E.; Quinlan, R.A.; Breitkreutz, D.; Fusenig, N.E. Proteolytic modification of acidic and basic keratins during terminal differentiation of mouse and human epidermis. Eur. J. Biochem. 1984, 142, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ridge, K.M.; Eriksson, J.E.; Pekny, M.; Goldman, R.D. Roles of vimentin in health and disease. Genes Dev. 2022, 36, 391–407. [Google Scholar] [CrossRef]
- Yue, Q.; Feng, L.; Cao, B.; Liu, M.; Zhang, D.; Wu, W.; Jiang, B.; Yang, M.; Liu, X.; Guo, D. Proteomic analysis revealed the important role of vimentin in human cervical carcinoma HeLa cells treated with gambogic acid. Mol. Cell. Proteom. 2016, 15, 26–44. [Google Scholar] [CrossRef]
- Kidd, M.E.; Shumaker, D.K.; Ridge, K.M. The role of Vimentin intermediate filaments in the progression of lung cancer. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1–6. [Google Scholar] [CrossRef]
- Menko, A.S.; Bleaken, B.M.; Libowitz, A.A.; Zhang, L.; Stepp, M.A.; Walker, J.L. A central role for vimentin in regulating repair function during healing of the lens epithelium. Mol. Biol. Cell 2014, 25, 776–790. [Google Scholar] [CrossRef]
- Herrmann, H.; Bär, H.; Kreplak, L.; Strelkov, S.V.; Aebi, U. Intermediate filaments: From cell architecture to nanomechanics. Nat. Rev. Mol. Cell Biol. 2007, 8, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, Q.; Tang, D.D. Role of vimentin in smooth muscle force development. Am. J. Physiol. Cell Physiol. 2006, 291, C483–C489. [Google Scholar] [CrossRef] [PubMed]
- Didonna, A.; Opal, P. The role of neurofilament aggregation in neurodegeneration: Lessons from rare inherited neurological disorders. Mol. Neurodegener. 2019, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Nixon, R.A. Specialized roles of neurofilament proteins in synapses: Relevance to neuropsychiatric disorders. Brain Res. Bull. 2016, 126, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. A. Neurofilaments at a glance. J. Cell Sci. 2012, 125, 3257–3263. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Schlaepfer, W.W. Role of neurofilament aggregation in motor neuron disease. Ann. Neurol. 2006, 60, 399–406. [Google Scholar] [CrossRef]
- Hoffman, P.N. Distinct roles of neurofilament and tubulin gene expression in axonal growth. Ciba Found. Symp. 1988, 138, 192–204. [Google Scholar]
- Khadija, S.G.; Chen, F.; Hadden, T.; Commissaris, R.L.; Kowluru, A. Biology and regulatory roles of nuclear lamins in cellular function and dysfunction. Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 111–120. [Google Scholar] [CrossRef]
- Carmosino, M.; Torretta, S.; Procino, G.; Gerbino, A.; Forleo, C.; Favale, S.; Svelto, M. Role of nuclear Lamin A/C in cardiomyocyte functions. Biol. Cell 2014, 106, 346–358. [Google Scholar] [CrossRef]
- Burke, B.; Stewart, C.L. The nuclear lamins: Flexibility in function. Nat. Rev. Mol. Cell Biol. 2013, 14, 13–24. [Google Scholar] [CrossRef]
- Lopez-Soler, R.I.; Moir, R.D.; Spann, T.P.; Stick, R.; Goldman, R.D. A role for nuclear lamins in nuclear envelope assembly. J. Cell Biol. 2001, 154, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Parnaik, V.K. Role of Nuclear Lamins in Nuclear Organization, Cellular Signaling, and Inherited Diseases. Int. Rev. Cell Mol. Biol. 2008, 266, 157–206. [Google Scholar] [CrossRef]
- Oka, M.; Kudo, H.; Sugama, N.; Asami, Y.; Takehana, M. The function of filensin and phakinin in lens transparency. Mol. Vis. 2008, 14, 815–822. [Google Scholar] [PubMed]
- Pittenger, J.T.; Hess, J.F.; FitzGerald, P.G. Identifying the role of specific motifs in the lens fiber cell-specific intermediate filament phakosin. Invest. Ophthalmol. Vis. Sci. 2007, 48, 5132–5141. [Google Scholar] [CrossRef] [PubMed]
- Blankenship, T.N.; Hess, J.F.; FitzGerald, P.G. Development- and differentiation-dependent reorganization of intermediate filaments in fiber cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 735–742. [Google Scholar] [PubMed]
- Goulielmos, G.; Remington, S.; Schwesinger, F.; Georgatos, S.D.; Gounari, F. Contributions of the structural domains of filensin in polymer formation and filament distribution. J. Cell Sci. 1996, 109, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Georgatos, S.D.; Gounari, F.; Remington, S. The beaded intermediate filaments and their potential functions in eye lens. Bioessays 1994, 16, 413–418. [Google Scholar] [CrossRef]
- Hlavaty, D.; Lechler, T. Roles for microtubules in the proliferative and differentiated cells of stratified epithelia. Curr. Opin. Cell Biol. 2021, 68, 98–104. [Google Scholar] [CrossRef]
- Chang, W.; Gu, J.G. Role of microtubules in Piezo2 mechanotransduction of mouse Merkel cells. J. Neurophysiol. 2020, 124, 1824–1831. [Google Scholar] [CrossRef]
- Matis, M. The Mechanical Role of Microtubules in Tissue Remodeling. Bioessays 2020, 42, 1900244. [Google Scholar] [CrossRef]
- Logan, C.M.; Menko, A.S. Microtubules: Evolving roles and critical cellular interactions. Exp. Biol. Med. 2019, 244, 1240–1254. [Google Scholar] [CrossRef]
- Cirillo, L.; Gotta, M.; Meraldi, P. The elephant in the room: The role of microtubules in cancer. Adv. Exp. Med. Biol. 2017, 1002, 93–124. [Google Scholar]
- Ganguly, A.; Yang, H.; Sharma, R.; Patel, K.D.; Cabral, F. The role of microtubules and their dynamics in cell migration. J. Biol. Chem. 2012, 287, 43359–43369. [Google Scholar] [CrossRef] [PubMed]
- van der Vaart, B.; Akhmanova, A.; Straube, A. Regulation of microtubule dynamic instability. Biochem. Soc. Trans. 2009, 37, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Kulić, I.M.; Brown, A.E.X.; Kim, H.; Kural, C.; Blehm, B.; Selvin, P.R.; Nelson, P.C.; Gelfand, V.I. The role of microtubule movement in bidirectional organelle transport. Proc. Natl. Acad. Sci. USA 2008, 105, 10011–10016. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.C. Viscoelasticity of Concentrated Isotropic Solutions of Semiflexible Polymers. 1. Model and Stress Tensor. Macromolecules 1998, 31, 7030–7043. [Google Scholar] [CrossRef]
- Wen, Q.; Janmey, P.A. Polymer physics of the cytoskeleton. Curr. Opin. Solid. State Mater. Sci. 2011, 15, 177–182. [Google Scholar] [CrossRef]
- Wen, Q.; Janmey, P.A. Effects of non-linearity on cell-ECM interactions. Exp. Cell Res. 2013, 319, 2481–2489. [Google Scholar] [CrossRef]
- Gittes, F.; Mickey, B.; Nettleton, J.; Howard, J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell. Biol. 1993, 120, 923–934. [Google Scholar] [CrossRef]
- De La Cruz, E.M.; Gardel, M.L. Actin Mechanics and Fragmentation. J. Biol. Chem. 2015, 290, 17137–17144. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2013. [Google Scholar]
- De La Cruz, E.M.; Roland, J.; McCullough, B.R.; Blanchoin, L.; Martiel, J.L. Origin of twist-bend coupling in actin filaments. Biophys. J. 2010, 99, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.J.; Hosford, M.A.; Molitoris, B.A. Mechanism of Actin Polymerization in Cellular ATP Depletion*. J. Biol. Chem. 2004, 279, 5194–5199. [Google Scholar] [CrossRef] [PubMed]
- Kudryashov, D.S.; Reisler, E. ATP and ADP actin states. Biopolymers 2013, 99, 245–256. [Google Scholar] [CrossRef]
- Janmey, P.A.; Hvidt, S.; Oster, G.F.; Lamb, J.; Stossel, T.P.; Hartwig, J.H. Effect of ATP on actin filament stiffness. Nature 1990, 347, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Brieher, W. Mechanisms of actin disassembly. Mol. Biol. Cell 2013, 24, 2299–2302. [Google Scholar] [CrossRef] [PubMed]
- Wegner, A. Treadmilling of actin at physiological salt concentrations: An analysis of the critical concentrations of actin filaments. J. Mol. Biol. 1982, 161, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Miller, R.T. Mechanisms of mechanical signaling in development and disease. J. Cell Sci. 2011, 124, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Schwarz, W.H.; Käs, J.A.; Stossel, T.P.; Janmey, P.A.; Pollard, T.D. Mechanical Properties of Actin Filament Networks Depend on Preparation, Polymerization Conditions, and Storage of Actin Monomers. Biophys. J. 1998, 74, 2731–2740. [Google Scholar] [CrossRef]
- Haase, K.; Pelling, A.E. The role of the actin cortex in maintaining cell shape. Commun. Integr. Biol. 2013, 6, e26714. [Google Scholar] [CrossRef]
- Qin, Z.; Kreplak, L.; Buehler, M.J. Hierarchical structure controls nanomechanical properties of vimentin intermediate filaments. PLoS ONE 2009, 4, e7294. [Google Scholar] [CrossRef]
- Qin, Z.; Buehler, M.J. Flaw tolerance of nuclear intermediate filament lamina under extreme mechanical deformation. ACS Nano 2011, 5, 3034–3042. [Google Scholar] [CrossRef]
- Hesse, M.; Magin, T.M.; Weber, K. Genes for intermediate filament proteins and the draft sequence of the human genome: Novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J. Cell Sci. 2001, 114, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Strnad, P.; Stumptner, C.; Zatloukal, K.; Denk, H. Intermediate filament cytoskeleton of the liver in health and disease. Histochem. Cell Biol. 2008, 129, 735–749. [Google Scholar] [CrossRef]
- Sanghvi-Shah, R.; Weber, G.F. Intermediate Filaments at the Junction of Mechanotransduction, Migration, and Development. Front. Cell Dev. Biol. 2017, 5, 81. [Google Scholar] [CrossRef] [PubMed]
- Oshima, R.G. Intermediate filaments: A historical perspective. Exp. Cell Res. 2007, 313, 1981–1994. [Google Scholar] [CrossRef]
- Karantza, V. Keratins in health and cancer: More than mere epithelial cell markers. Oncogene 2011, 30, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.L.; Chou, C.C. Effect of mutations on the hydrophobic interactions of the hierarchical molecular structure and mechanical properties of epithelial keratin 1/10. Int. J. Biol. Macromol. 2022, 212, 442–450. [Google Scholar] [CrossRef]
- Lowery, J.; Kuczmarski, E.R.; Herrmann, H.; Goldman, R.D. Intermediate Filaments Play a Pivotal Role in Regulating Cell Architecture and Function. J. Biol. Chem. 2015, 290, 17145–17153. [Google Scholar] [CrossRef]
- FitzGerald, P.; Sun, N.; Shibata, B.; Hess, J.F. Expression of the type VI intermediate filament proteins CP49 and filensin in the mouse lens epithelium. Mol. Vis. 2016, 22, 970–989. [Google Scholar]
- Fudge, D.; Russell, D.; Beriault, D.; Moore, W.; Lane, E.B.; Vogl, A.W. The intermediate filament network in cultured human keratinocytes is remarkably extensible and resilient. PLoS ONE 2008, 3, e2327. [Google Scholar] [CrossRef]
- Nolting, J.-F.; Möbius, W.; Köster, S. Mechanics of individual keratin bundles in living cells. Biophys. J. 2014, 107, 2693–2699. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xu, J.; Coulombe, P.A.; Wirtz, D. Keratin Filament Suspensions Show Unique Micromechanical Properties *. J. Biol. Chem. 1999, 274, 19145–19151. [Google Scholar] [CrossRef] [PubMed]
- Beil, M.; Micoulet, A.; von Wichert, G.; Paschke, S.; Walther, P.; Omary, M.B.; Van Veldhoven, P.P.; Gern, U.; Wolff-Hieber, E.; Eggermann, J.; et al. Sphingosylphosphorylcholine regulates keratin network architecture and visco-elastic properties of human cancer cells. Nat. Cell Biol. 2003, 5, 803–811. [Google Scholar] [CrossRef]
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef]
- Roll-Mecak, A. The Tubulin Code in Microtubule Dynamics and Information Encoding. Dev. Cell 2020, 54, 7–20. [Google Scholar] [CrossRef]
- Tubulins. HGNC Database, HUGO Gene Nomenclature Committee (HGNC), European Molecular Biology Laboratory, European Bi-oinformatics Institute (EMBL-EBI), Wellcome Genome Campus, Hinxton, Cambridge CB10 1SD, United Kingdom. 2022. Available online: https://www.genenames.org/data/genegroup/#!/group/778. (accessed on 21 December 2022).
- Sept, D.; MacKintosh, F.C. Microtubule elasticity: Connecting all-atom simulations with continuum mechanics. Phys. Rev. Lett. 2010, 104, 018101. [Google Scholar] [CrossRef]
- Schaap, I.A.T.; Carrasco, C.; de Pablo, P.J.; MacKintosh, F.C.; Schmidt, C.F. Elastic Response, Buckling, and Instability of Microtubules under Radial Indentation. Biophys. J. 2006, 91, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Avila, J. Microtubule functions. Life Sci. 1992, 50, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Kent, I.A.; Lele, T.P. Microtubule-based force generation. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1428. [Google Scholar] [CrossRef]
- Akhmanova, A.; Steinmetz, M.O. Control of microtubule organization and dynamics: Two ends in the limelight. Nat. Rev. Mol. Cell Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef]
- van Haren, J.; Wittmann, T. Microtubule Plus End Dynamics—Do We Know How Microtubules Grow?: Cells boost microtubule growth by promoting distinct structural transitions at growing microtubule ends. Bioessays 2019, 41, e1800194. [Google Scholar] [CrossRef]
- MacRae, T.H. Towards an understanding of microtubule function and cell organization: An overview. Biochem. Cell Biol. 1992, 70, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Zwetsloot, A.J.; Tut, G.; Straube, A. Measuring microtubule dynamics. Essays Biochem. 2018, 62, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Sept, D.; Baker, N.A.; McCammon, J.A. The physical basis of microtubule structure and stability. Protein Sci. 2003, 12, 2257–2261. [Google Scholar] [CrossRef]
- McIntosh, J.R.; Grishchuk, E.L.; West, R.R. Chromosome-microtubule interactions during mitosis. Annu. Rev. Cell Dev. Biol. 2002, 18, 193–219. [Google Scholar] [CrossRef]
- Xiao, H.; Verdier-Pinard, P.; Fernandez-Fuentes, N.; Burd, B.; Angeletti, R.; Fiser, A.; Horwitz, S.B.; Orr, G.A. Insights into the mechanism of microtubule stabilization by Taxol. Proc. Natl. Acad. Sci. USA 2006, 103, 10166–10173. [Google Scholar] [CrossRef] [PubMed]
- Müsch, A. Microtubule Organization and Function in Epithelial Cells. Traffic 2004, 5, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-L.; Chien, A.J.; Moon, R.T. Wnt/Fz signaling and the cytoskeleton: Potential roles in tumorigenesis. Cell Res. 2009, 19, 532–545. [Google Scholar] [CrossRef]
- Hoffman, L.; Jensen, C.C.; Yoshigi, M.; Beckerle, M. Mechanical signals activate p38 MAPK pathway-dependent reinforcement of actin via mechanosensitive HspB1. Mol. Biol. Cell 2017, 28, 2661–2675. [Google Scholar] [CrossRef]
- Sauzeau, V.; Le Jeune, H.; Cario-Toumaniantz, C.; Smolenski, A.; Lohmann, S.M.; Bertoglio, J.; Chardin, P.; Pacaud, P.; Loirand, G. Cyclic GMP-dependent Protein Kinase Signaling Pathway Inhibits RhoA-induced Ca2+ Sensitization of Contraction in Vascular Smooth Muscle*. J. Biol. Chem. 2000, 275, 21722–21729. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, J.; Han, J.; Li, W.; Su, F.; Xu, X.; Zhai, Z.; Xiao, F. cGMP interacts with tropomyosin and downregulates actin-tropomyosin-myosin complex interaction. Respir. Res. 2018, 19, 201. [Google Scholar] [CrossRef]
- James, R.G.; Conrad, W.H.; Moon, R.T. Beta-catenin-independent Wnt pathways: Signals, core proteins, and effectors. Methods Mol. Biol. 2008, 468, 131–144. [Google Scholar] [CrossRef]
- Roarty, K.; Pfefferle, A.D.; Creighton, C.J.; Perou, C.M.; Rosen, J.M. Ror2-mediated alternative Wnt signaling regulates cell fate and adhesion during mammary tumor progression. Oncogene 2017, 36, 5958–5968. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Leong, H.C.; Datta, A.; Gopal, V.; Kumar, A.P.; Yap, C.T. PI3K/AKT Signaling Tips the Balance of Cytoskeletal Forces for Cancer Progression. Cancers 2022, 14, 1652. [Google Scholar] [CrossRef]
- May-Simera, H.L.; Kelley, M.W. Cilia, Wnt signaling, and the cytoskeleton. Cilia 2012, 1, 7. [Google Scholar] [CrossRef]
- Shapiro, L. The multi-talented beta-catenin makes its first appearance. Structure 1997, 5, 1265–1268. [Google Scholar] [CrossRef]
- Shi, J.; Wei, L. Rho Kinases in Embryonic Development and Stem Cell Research. Arch. Immunol. Ther. Exp. 2022, 70, 4. [Google Scholar] [CrossRef]
- Tang, L.; Dai, F.; Liu, Y.; Yu, X.; Huang, C.; Wang, Y.; Yao, W. RhoA/ROCK signaling regulates smooth muscle phenotypic modulation and vascular remodeling via the JNK pathway and vimentin cytoskeleton. Pharmacol. Res. 2018, 133, 201–212. [Google Scholar] [CrossRef]
- Schofield, A.V.; Bernard, O. Rho-associated coiled-coil kinase (ROCK) signaling and disease. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 301–316. [Google Scholar] [CrossRef]
- Sit, S.-T.; Manser, E. Rho GTPases and their role in organizing the actin cytoskeleton. J. Cell Sci. 2011, 124, 679–683. [Google Scholar] [CrossRef]
- Sarasa-Renedo, A.; Tunç-Civelek, V.; Chiquet, M. Role of RhoA/ROCK-dependent actin contractility in the induction of tenascin-C by cyclic tensile strain. Exp. Cell Res. 2006, 312, 1361–1370. [Google Scholar] [CrossRef]
- Woods, A.; Wang, G.; Beier, F. RhoA/ROCK signaling regulates Sox9 expression and actin organization during chondrogenesis. J. Biol. Chem. 2005, 280, 11626–11634. [Google Scholar] [CrossRef]
- Da Silva, J.S.; Medina, M.; Zuliani, C.; Di Nardo, A.; Witke, W.; Dotti, C.G. RhoA/ROCK regulation of neuritogenesis via profilin IIa-mediated control of actin stability. J. Cell Biol. 2003, 162, 1267–1279. [Google Scholar] [CrossRef]
- Amano, M.; Fukata, Y.; Kaibuchi, K. Regulation and functions of Rho-associated kinase. Exp. Cell Res. 2000, 261, 44–51. [Google Scholar] [CrossRef]
- Yang, L.; Tang, L.; Dai, F.; Meng, G.; Yin, R.; Xu, X.; Yao, W. Raf-1/CK2 and RhoA/ROCK signaling promote TNF-α-mediated endothelial apoptosis via regulating vimentin cytoskeleton. Toxicology 2017, 389, 74–84. [Google Scholar] [CrossRef]
- Lei, S.; Tian, Y.P.; Xiao, W.D.; Li, S.; Rao, X.C.; Zhang, J.L.; Yang, J.; Hu, X.M.; Chen, W. ROCK is Involved in Vimentin Phosphorylation and Rearrangement Induced by Dengue Virus. Cell Biochem. Biophys. 2013, 67, 1333–1342. [Google Scholar] [CrossRef]
- Hirose, M.; Ishizaki, T.; Watanabe, N.; Uehata, M.; Kranenburg, O.; Moolenaar, W.H.; Matsumura, F.; Maekawa, M.; Bito, H.; Narumiya, S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)- regulated neurite remodeling in neuroblastoma N1E-115 cells. J. Cell Biol. 1998, 141, 1625–1636. [Google Scholar] [CrossRef]
- Becker, K.N.; Pettee, K.M.; Sugrue, A.; Reinard, K.A.; Schroeder, J.L.; Eisenmann, K.M. The Cytoskeleton Effectors Rho-Kinase (ROCK) and Mammalian Diaphanous-Related (mDia) Formin Have Dynamic Roles in Tumor Microtube Formation in Invasive Glioblastoma Cells. Cells 2022, 11, 1559. [Google Scholar] [CrossRef]
- Heng, Y.W.; Lim, H.H.; Mina, T.; Utomo, P.; Zhong, S.; Lim, C.T.; Koh, C.G. TPPP acts downstream of RhoA-ROCK-LIMK2 to regulate astral microtubule organization and spindle orientation. J. Cell Sci. 2012, 125, 1579–1590. [Google Scholar] [CrossRef]
- Fonseca, A.V.; Freund, D.; Bornhäuser, M.; Corbeil, D. Polarization and migration of hematopoietic stem and progenitor cells rely on the RhoA/ROCK I pathway and an active reorganization of the microtubule network. J. Biol. Chem. 2010, 285, 31661–31671. [Google Scholar] [CrossRef] [PubMed]
- Takesono, A.; Heasman, S.J.; Wojciak-Stothard, B.; Garg, R.; Ridley, A.J. Microtubules regulate migratory polarity through Rho/ ROCK signaling in T cells. PLoS ONE 2010, 5, e8774. [Google Scholar] [CrossRef]
- Han, D.; Sun, J.; Fan, D.; Zhang, C.; Du, S.; Zhang, W. Simvastatin ameliorates oxygen glucose deprivation/reoxygenation-induced pulmonary endothelial barrier dysfunction by restoring cell-cell junctions and actin cytoskeleton dynamics via the PI3K/Akt signaling pathway. Am. J. Transl. Res. 2020, 12, 5586–5596. [Google Scholar] [PubMed]
- Lien, E.C.; Dibble, C.C.; Toker, A. PI3K signaling in cancer: Beyond AKT. Curr. Opin. Cell Biol. 2017, 45, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, N.; Roy, B.C.; Zhu, Y.; Wang, Y.; Kiyama, R. Kank regulates RhoA-dependent formation of actin stress fibers and cell migration via 14-3-3 in PI3K-Akt signaling. J. Cell Biol. 2008, 181, 537–549. [Google Scholar] [CrossRef]
- Qian, Y.; Zhong, X.; Flynn, D.C.; Zheng, J.Z.; Qiao, M.; Wu, C.; Dedhar, S.; Shi, X.; Jiang, B.H. ILK mediates actin filament rearrangements and cell migration and invasion through PI3K/Akt/Rac1 signaling. Oncogene 2005, 24, 3154–3165. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Corum, L.; Meng, Q.; Blenis, J.; Zheng, J.Z.; Shi, X.; Flynn, D.C.; Jiang, B.H. PI3K induced actin filament remodeling through Akt and p70S6K1: Implication of essential role in cell migration. Am. J. Physiol. Cell Physiol. 2004, 286, C153–C163. [Google Scholar] [CrossRef]
- Krasilnikov, M.A. Phosphatidylinositol-3 kinase dependent pathways: The role in control of cell growth, survival, and malignant transformation. Biochemistry 2000, 65, 59–67. [Google Scholar]
- Roux, A.; Loranger, A.; Lavoie, J.N.; Marceau, N. Keratin 8/18 regulation of insulin receptor signaling and trafficking in hepatocytes through a concerted phosphoinositide-dependent Akt and Rab5 modulation. FASEB J. 2017, 31, 3555–3573. [Google Scholar] [CrossRef]
- Wang, R.C.; Wei, Y.; An, Z.; Zou, Z.; Xiao, G.; Bhagat, G.; White, M.; Reichelt, J.; Levine, B. Akt-mediated regulation of autophagy and tumorigenesis through Beclin 1 phosphorylation. Science 2012, 338, 956–959. [Google Scholar] [CrossRef]
- Kong, L.; Schäfer, G.; Bu, H.; Zhang, Y.; Zhang, Y.; Klocker, H. Lamin A/C protein is overexpressed in tissue-invading prostate cancer and promotes prostate cancer cell growth, migration and invasion through the PI3K/AKT/PTEN pathway. Carcinogenesis 2012, 33, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.H.; Yang, C.C.; Lin, S.C.; Cheng, C.C.; Lin, S.H.; Liu, C.J.; Chang, K.W. Areca nut extract upregulates vimentin by activating PI3K/AKT signaling in oral carcinoma. J. Oral Pathol. Med. 2011, 40, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, S.; Nag, D.; Ganguli, A.; Das, A.; Ghosh Dastidar, D.; Chakrabarti, G. Theaflavin and epigallocatechin-3-gallate synergistically induce apoptosis through inhibition of PI3K/Akt signaling upon depolymerizing microtubules in HeLa cells. J. Cell. Biochem. 2019, 120, 5987–6003. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.F.; Liu, X.; Gao, M.; Zhang, Y.N.; Liu, J. Endoplasmic reticulum stress induces autophagy and apoptosis while inhibiting proliferation and drug resistance in multiple myeloma through the PI3K/Akt/mTOR signaling pathway. Oncotarget 2017, 8, 61093–61106. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef]
- Onishi, K.; Higuchi, M.; Asakura, T.; Masuyama, N.; Gotoh, Y. The PI3K-Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells 2007, 12, 535–546. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Hosokawa, Y.; Watanabe, K.; Tanimura, S.; Ozaki, K.I.; Kohno, M. Blockade of the phosphatidylinositol-3-kinase-Akt signaling pathway enhances the induction of apoptosis by microtubule-destabilizing agents in tumor cells in which the pathway is constitutively activated. Mol. Cancer Ther. 2007, 6, 1133–1142. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Q.; Jiang, X.; Wang, S.; Zhou, X.; Lu, Y.; Huang, X.; Duan, H.; Zhang, T.; Ge, H.; et al. Actin Alpha 2 Downregulation Inhibits Neural Stem Cell Proliferation and Differentiation into Neurons through Canonical Wnt/β-Catenin Signaling Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 7486726. [Google Scholar] [CrossRef]
- Galli, C.; Piemontese, M.; Lumetti, S.; Ravanetti, F.; MacAluso, G.M.; Passeri, G. Actin cytoskeleton controls activation of Wnt/β-catenin signaling in mesenchymal cells on implant surfaces with different topographies. Acta Biomater. 2012, 8, 2963–2968. [Google Scholar] [CrossRef]
- Lehmann, M.; Hu, Q.; Hu, Y.; Hafner, K.; Costa, R.; van den Berg, A.; Königshoff, M. Chronic WNT/β-catenin signaling induces cellular senescence in lung epithelial cells. Cell. Signal. 2020, 70, 109588. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, X.; Zhao, Z.; Zhang, F.; Deng, T. The Wnt/β-catenin signaling pathway affects the distribution of cytoskeletal proteins in Aβ treated PC12 cells. J. Integr. Neurosci. 2019, 18, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Bermeo, S.; Vidal, C.; Zhou, H.; Duque, G. Lamin A/C Acts as an Essential Factor in Mesenchymal Stem Cell Differentiation Through the Regulation of the Dynamics of the Wnt/β-Catenin Pathway. J. Cell. Biochem. 2015, 116, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.P.; Mirza, S.; Sharma, G.; Prashad, R.; DattaGupta, S.; Rath, G.; Ralhan, R. Epigenetic alterations of CDH1 and APC genes: Relationship with activation of Wnt/β-catenin Pathway in invasive ductal carcinoma of breast. Life Sci. 2008, 83, 318–325. [Google Scholar] [CrossRef]
- Bierie, B.; Nozawa, M.; Renou, J.P.; Shillingford, J.M.; Morgan, F.; Oka, T.; Taketo, M.M.; Cardiff, R.D.; Miyoshi, K.; Wagner, K.U.; et al. Activation of β-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene 2003, 22, 3875–3887. [Google Scholar] [CrossRef] [PubMed]
- Puri, D.; Ponniah, K.; Biswas, K.; Basu, A.; Dey, S.; Lundquist, E.A.; Ghosh-Roy, A. Wnt signaling establishes the microtubule polarity in neurons through regulation of kinesin-13. J. Cell Biol. 2021, 220, e202005080. [Google Scholar] [CrossRef] [PubMed]
- Ou, D.; Chen, L.; He, J.; Rong, Z.; Gao, J.; Li, Z.; Liu, L.; Tang, F.; Li, J.; Deng, Y.; et al. CDK11 negatively regulates Wnt/β-catenin signaling in the endosomal compartment by affecting microtubule stability. Cancer Biol. Med. 2020, 17, 328–342. [Google Scholar] [CrossRef]
- Huang, P.; Senga, T.; Hamaguchi, M. A novel role of phospho-β-catenin in microtubule regrowth at centrosome. Oncogene 2007, 26, 4357–4371. [Google Scholar] [CrossRef]
- Ciani, L.; Krylova, O.; Smalley, M.J.; Dale, T.C.; Salinas, P.C. A divergent canonical WNT-signaling pathway regulates microtubule dynamics: Dishevelled signals locally to stabilize microtubules. J. Cell Biol. 2004, 164, 243–253. [Google Scholar] [CrossRef]
- Peifer, M.; Polakis, P. Wnt signaling in oncogenesis and embryogenesis—A look outside the nucleus. Science 2000, 287, 1606–1609. [Google Scholar] [CrossRef]
- Hajka, D.; Budziak, B.; Pietras, Ł.; Duda, P.; McCubrey, J.A.; Gizak, A. GSK3 as a Regulator of Cytoskeleton Architecture: Consequences for Health and Disease. Cells 2021, 10, 2092. [Google Scholar] [CrossRef]
- Yoshino, Y.; Suzuki, M.; Takahashi, H.; Ishioka, C. Inhibition of invasion by glycogen synthase kinase-3 beta inhibitors through dysregulation of actin re-organisation via down-regulation of WAVE2. Biochem. Biophys. Res. Commun. 2015, 464, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Noritake, J.; Kakeno, M.; Matsui, T.; Harada, T.; Wang, S.; Itoh, N.; Sato, K.; Matsuzawa, K.; Iwamatsu, A.; et al. Phosphorylation of CLASP2 by GSK-3β regulates its interaction with IQGAP1, EB1 and microtubules. J. Cell Sci. 2009, 122, 2969–2979. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Rodriguez, M.; Kim, L. Glycogen synthase kinase 3 in the world of cell migration. Dev. Growth Differ. 2009, 51, 735–742. [Google Scholar] [CrossRef]
- Vaidya, R.J.; Ray, R.M.; Johnson, L.R. Akt-mediated GSK-3β inhibition prevents migration of polyamine-depleted intestinal epithelial cells via Rac1. Cell. Mol. Life Sci. 2006, 63, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Lagas, S.; Roy, A.; Kumar, H. Cytoskeleton saga: Its regulation in normal physiology and modulation in neurodegenerative disorders. Eur. J. Pharmacol. 2022, 925, 175001. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Kan, D.; Chen, Y.Y.; Han, S.K.; Lu, K.S.; Chien, C.L. Suppression of extensive neurofilament phosphorylation rescues α-internexin/peripherin-overexpressing PC12 cells from neuronal cell death. PLoS ONE 2012, 7, e43883. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.M.; Yang, C.H.; Cho, S.K.; Lee, J.W.; Cho, M. Functional regulation of Slug/Snail2 is dependent on GSK-3β-mediated phosphorylation. FEBS J. 2012, 279, 2929–2939. [Google Scholar] [CrossRef]
- Sasaki, T.; Taoka, M.; Ishiguro, K.; Uchida, A.; Saito, T.; Toshiaki Isobe, D.; Hisanaga, S.I. In vivo and in vitro phosphorylation at Ser-493 in the glutamate (E)-segment of neurofilament-H subunit by glycogen synthase kinase 3β. J. Biol. Chem. 2002, 277, 36032–36039. [Google Scholar] [CrossRef]
- Guidato, S.; Tsai, L.H.; Woodgett, J.; Miller, C.C.J. Differential cellular phosphorylation of neurofilament heavy side-arms by glycogen synthase kinase-3 and cydin-dependent kinase-5. J. Neurochem. 1996, 66, 1698–1706. [Google Scholar] [CrossRef]
- Fumoto, K.; Hoogenraad, C.C.; Kikuchi, A. GSK-3β-regulated interaction of BICD with dynein is involved in microtubule anchorage at centrosome. EMBO J. 2006, 25, 5670–5682. [Google Scholar] [CrossRef]
- Grimes, C.A.; Jope, R.S. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Prog. Neurobiol. 2001, 65, 391–426. [Google Scholar] [CrossRef]
- Joe, S.Y.; Yang, S.G.; Lee, J.H.; Park, H.J.; Koo, D.B. Stabilization of F-Actin Cytoskeleton by Paclitaxel Improves the Blastocyst Developmental Competence through P38 MAPK Activity in Porcine Embryos. Biomedicines 2022, 10, 1867. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Patel, K.; Harding, P.; Sorokin, A.; Glass Ii, W.F. Regulation of TGF-β1/MAPK-mediated PAI-1 gene expression by the actin cytoskeleton in human mesangial cells. Exp. Cell Res. 2007, 313, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Jin, E.; Ghazizadeh, M.; Kawanami, O. Activation of PAR4 induces a distinct actin fiber formation via p38 MAPK in human lung endothelial cells. J. Histochem. Cytochem. 2005, 53, 1121–1129. [Google Scholar] [CrossRef]
- Paliga, A.J.M.; Natale, D.R.; Watson, A.J. p38 mitogen-activated protein kinase (MAPK) first regulates filamentous actin at the 8-16-cell stage during preimplantation development. Biol. Cell 2005, 97, 629–640. [Google Scholar] [CrossRef]
- Tania, M.; Khan, M.A.; Fu, J. Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumour Biol. 2014, 35, 7335–7342. [Google Scholar] [CrossRef]
- Wang, P.B.; Chen, Y.; Ding, G.R.; Du, H.W.; Fan, H.Y. Keratin 18 induces proliferation, migration, and invasion in gastric cancer via the MAPK signalling pathway. Clin. Exp. Pharmacol. Physiol. 2021, 48, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wöll, S.; Windoffer, R.; Leube, R.E. p38 MAPK-dependent shaping of the keratin cytoskeleton in cultured cells. J. Cell Biol. 2007, 177, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Schechter, R.; Yanovitch, T.; Abboud, M.; Johnson III, G.; Gaskins, J. Effects of brain endogenous insulin on neurofilament and MAPK in fetal rat neuron cell cultures. Brain Res. 1998, 808, 270–278. [Google Scholar] [CrossRef]
- Cheng, T.J.; Lai, Y.K. Identification of mitogen-activated protein kinase-activated protein kinase-2 as a vimentin kinase activated by okadaic acid in 9L rat brain tumor cells. J. Cell. Biochem. 1998, 71, 169–181. [Google Scholar] [CrossRef]
- Li, L.; Hu, J.; He, T.; Zhang, Q.; Yang, X.; Lan, X.; Zhang, D.; Mei, H.; Chen, B.; Huang, Y. P38/MAPK contributes to endothelial barrier dysfunction via MAP4 phosphorylation-dependent microtubule disassembly in inflammation-induced acute lung injury. Sci. Rep. 2015, 5, 8895. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Chu, Z.G.; Han, J.; Dang, Y.M.; Yan, H.; Zhang, Q.; Liang, G.P.; Huang, Y.S. The p38/MAPK pathway regulates microtubule polymerization through phosphorylation of MAP4 and Op18 in hypoxic cells. Cell. Mol. Life Sci. 2010, 67, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, J.H.; Kim, N.H. Inactivation of MAPK affects centrosome assembly, but not actin filament assembly, in mouse oocytes maturing in vitro. Mol. Reprod. Dev. 2007, 74, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Chambers, T.C. Role of mitogen-activated protein kinases in the response of tumor cells to chemotherapy. Drug Resist. Updates 2001, 4, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Reszka, A.A.; Seger, R.; Diltz, C.D.; Krebs, E.G.; Fischer, E.H. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc. Natl. Acad. Sci. USA 1995, 92, 8881–8885. [Google Scholar] [CrossRef] [PubMed]
- Butt, E.; Gambaryan, S.; Göttfert, N.; Galler, A.; Marcus, K.; Meyer, H.E. Actin binding of human LIM and SH3 protein is regulated by cGMP- and cAMP-dependent protein kinase phosphorylation on serine 146. J. Biol. Chem. 2003, 278, 15601–15607. [Google Scholar] [CrossRef]
- Butt, E.; Immler, D.; Meyer, H.E.; Kotlyarov, A.; Laaß, K.; Gaestel, M. Heat shock protein 27 is a substrate of cGMP-dependent protein kinase in intact human platelets. Phosphorylation-induced actin polymerization caused by HSP27 mutants. J. Biol. Chem. 2001, 276, 7108–7113. [Google Scholar] [CrossRef]
- Sandau, K.B.; Gantner, F.; Brüne, B. Nitric oxide-induced F-actin disassembly is mediated via cGMP, cAMP, and protein kinase A activation in rat mesangial cells. Exp. Cell Res. 2001, 271, 329–336. [Google Scholar] [CrossRef]
- Pryzwansky, K.B.; Wyatt, T.A.; Lincoln, T.M. Cyclic guanosine monophosphate-dependent protein kinase is targeted to intermediate filaments and phosphorylates vimentin in A23187-stimulated human neutrophils. Blood 1995, 85, 222–230. [Google Scholar] [CrossRef]
- MacMillan-Crow, L.A.; Lincoln, T.M. High-affinity binding and localization of the cyclic GMP-dependent protein kinase with the intermediate filament protein vimentin. Biochemistry 1994, 33, 8035–8043. [Google Scholar] [CrossRef]
- Wyatt, T.A.; Lincoln, T.M.; Pryzwansky, K.B. Regulation of human neutrophil degranulation by LY-83583 and L-arginine: Role of cGMP-dependent protein kinase. Am. J. Physiol. Cell Physiol. 1993, 265, C201–C211. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, T.A.; Lincoln, T.M.; Pryzwansky, K.B. Vimentin is transiently co-localized with and phosphorylated by cyclic GMP-dependent protein kinase in formyl-peptide-stimulated neutrophils. J. Biol. Chem. 1991, 266, 21274–21280. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Nguyen, M.; Garrison, A.K.; Zhao, Z.; Wang, Z.; Sutherland, C.; Ma, L. CNP/cGMP signaling regulates axon branching and growth by modulating microtubule polymerization. Dev. Neurobiol. 2013, 73, 673–687. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Xing, D.; Li, P.; Hilgers, R.H.; Hage, F.G.; Oparil, S.; Chen, Y.F. cGMP inhibits TGF-beta signaling by sequestering Smad3 with cytosolic beta2-tubulin in pulmonary artery smooth muscle cells. Mol. Endocrinol. 2011, 25, 1794–1803. [Google Scholar] [CrossRef]
- Guo, J.; Wenk, M.R.; Pellegrini, L.; Onofri, F.; Benfenati, F.; De Camilli, P. Phosphatidylinositol 4-kinase type IIα is responsible for the phosphatidylinositol 4-kinase activity associated with synaptic vesicles. Proc. Natl. Acad. Sci. USA 2003, 100, 3995–4000. [Google Scholar] [CrossRef]
- Michalczyk, I.; Sikorski, A.F.; Kotula, L.; Junghans, R.P.; Dubielecka, P.M. The emerging role of protein kinase Cθ in cytoskeletal signaling. J. Leukoc. Biol. 2013, 93, 319–327. [Google Scholar] [CrossRef]
- Liao, J.K.; Seto, M.; Noma, K. Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 2007, 50, 17–24. [Google Scholar] [CrossRef]
- Apodaca, G. Endocytic traffic in polarized epithelial cells: Role of the actin and microtubule cytoskeleton. Traffic 2001, 2, 149–159. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho proteins: Linking signaling with membrane trafficking. Traffic 2001, 2, 303–310. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Burridge, K.; Wennerberg, K. Rho and Rac take center stage. Cell 2004, 116, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.L.; Hall, A. Rho GTPases and their effector proteins. Biochem. J. 2000, 348 Pt 2, 241–255. [Google Scholar] [CrossRef]
- Narumiya, S.; Tanji, M.; Ishizaki, T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009, 28, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Wittinghofer, A.; Vetter, I.R. Structure-function relationships of the G domain, a canonical switch motif. Annu. Rev. Biochem. 2011, 80, 943–971. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y. Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 2001, 26, 724–732. [Google Scholar] [CrossRef]
- Hodge, R.G.; Ridley, A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016, 17, 496–510. [Google Scholar] [CrossRef]
- Jiu, Y.; Peränen, J.; Schaible, N.; Cheng, F.; Eriksson, J.E.; Krishnan, R.; Lappalainen, P. Vimentin intermediate filaments control actin stress fiber assembly through GEF-H1 and RhoA. J. Cell Sci. 2017, 130, 892–902. [Google Scholar] [CrossRef]
- Inaba, H.; Yamakawa, D.; Tomono, Y.; Enomoto, A.; Mii, S.; Kasahara, K.; Goto, H.; Inagaki, M. Regulation of keratin 5/14 intermediate filaments by CDK1, Aurora-B, and Rho-kinase. Biochem. Biophys. Res. Commun. 2018, 498, 544–550. [Google Scholar] [CrossRef]
- Dharmawardhane, S. Rho family GTPases in cancer. Cancers 2021, 13, 1271. [Google Scholar] [CrossRef]
- Ellenbroek, S.I.J.; Collard, J.G. Rho GTPases: Functions and association with cancer. Clin. Exp. Metastasis 2007, 24, 657–672. [Google Scholar] [CrossRef]
- El-Sibai, M.; Pertz, O.; Pang, H.; Yip, S.C.; Lorenz, M.; Symons, M.; Condeelis, J.S.; Hahn, K.M.; Backer, J.M. RhoA/ROCK-mediated switching between Cdc42- and Rac1-dependent protrusion in MTLn3 carcinoma cells. Exp. Cell Res. 2008, 314, 1540–1552. [Google Scholar] [CrossRef] [PubMed]
- Bustelo, X.R.; Sauzeau, V.; Berenjeno, I.M. GTP-binding proteins of the Rho/Rac family: Regulation, effectors and functions in vivo. Bioessays 2007, 29, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Pinyol, R.; Haeckel, A.; Ritter, A.; Qualmann, B.; Kessels, M.M. Regulation of N-WASP and the Arp2/3 complex by Abp1 controls neuronal morphology. PLoS ONE 2007, 2, e400. [Google Scholar] [CrossRef] [PubMed]
- Koka, S.; Neudauer, C.L.; Li, X.; Lewis, R.E.; McCarthy, J.B.; Westendorf, J.J. The formin-homology-domain-containing protein FHOD1 enhances cell migration. J. Cell Sci. 2003, 116, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Royal, I.; Lamarche-Vane, N.; Lamorte, L.; Kaibuchi, K.; Park, M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell 2000, 11, 1709–1725. [Google Scholar] [CrossRef]
- Watanabe, N.; Madaule, P.; Reid, T.; Ishizaki, T.; Watanabe, G.; Kakizuka, A.; Saito, Y.; Nakao, K.; Jockusch, B.M.; Narumiya, S. p140mDia, a mammalian homolog of Drosophila diaphanous, is a target protein for Rho small GTPase and is a ligand for profilin. EMBO J. 1997, 16, 3044–3056. [Google Scholar] [CrossRef]
- ROCK1, Rho Associated Coiled-Coil Containing Protein Kinase 1 [Homo Sapiens (Human)]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2022. Available online: https://www.ncbi.nlm.nih.gov/gene/60932022 (accessed on 20 December 2022).
- ROCK2, Rho Associated Coiled-Coil Containing Protein Kinase 1 [Homo Sapiens (Human)]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2022. Available online: https://www.ncbi.nlm.nih.gov/gene/9475 (accessed on 20 December 2022).
- Totsukawa, G.; Yamakita, Y.; Yamashiro, S.; Hartshorne, D.J.; Sasaki, Y.; Matsumura, F. Distinct roles of ROCK (Rho-kinase) and MLCK in spatial regulation of MLC phosphorylation for assembly of stress fibers and focal adhesions in 3T3 fibroblasts. J. Cell Biol. 2000, 150, 797–806. [Google Scholar] [CrossRef]
- Ohashi, K.; Nagata, K.; Maekawa, M.; Ishizaki, T.; Narumiya, S.; Mizuno, K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J. Biol. Chem. 2000, 275, 3577–3582. [Google Scholar] [CrossRef]
- Qiao, Y.N.; He, W.Q.; Chen, C.P.; Zhang, C.H.; Zhao, W.; Wang, P.; Zhang, L.; Wu, Y.Z.; Yang, X.; Peng, Y.J.; et al. Myosin phosphatase target subunit 1 (MYPT1) regulates the contraction and relaxation of vascular smooth muscle and maintains blood pressure. J. Biol. Chem. 2014, 289, 22512–22523. [Google Scholar] [CrossRef]
- Lee, J.H.; Katakai, T.; Hara, T.; Gonda, H.; Sugai, M.; Shimizu, A. Roles of p-ERM and Rho-ROCK signaling in lymphocyte polarity and uropod formation. J. Cell Biol. 2004, 167, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Fukata, Y.; Oshiro, N.; Kinoshita, N.; Kawano, Y.; Matsuoka, Y.; Bennett, V.; Matsuura, Y.; Kaibuchi, K. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J. Cell Biol. 1999, 145, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Shibukawa, Y.; Yamazaki, N.; Daimon, E.; Wada, Y. Rock-dependent calponin 3 phosphorylation regulates myoblast fusion. Exp. Cell Res. 2013, 319, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Ikenoya, M.; Hidaka, H.; Hosoya, T.; Suzuki, M.; Yamamoto, N.; Sasaki, Y. Inhibition of rho-kinase-induced myristoylated alanine-rich C kinase substrate (MARCKS) phosphorylation in human neuronal cells by H-1152, a novel and specific Rho-kinase inhibitor. J. Neurochem. 2002, 81, 9–16. [Google Scholar] [CrossRef]
- Izawa, T.; Fukata, Y.; Kimura, T.; Iwamatsu, A.; Dohi, K.; Kaibuchi, K. Elongation factor-1 alpha is a novel substrate of rho-associated kinase. Biochem. Biophys. Res. Commun. 2000, 278, 72–78. [Google Scholar] [CrossRef]
- Vahebi, S.; Kobayashi, T.; Warren, C.M.; de Tombe, P.P.; Solaro, R.J. Functional effects of rho-kinase-dependent phosphorylation of specific sites on cardiac troponin. Circ. Res. 2005, 96, 740–747. [Google Scholar] [CrossRef]
- Shao, J.; Welch, W.J.; Diprospero, N.A.; Diamond, M.I. Phosphorylation of profilin by ROCK1 regulates polyglutamine aggregation. Mol. Cell. Biol. 2008, 28, 5196–5208. [Google Scholar] [CrossRef]
- Amano, M.; Ito, M.; Kimura, K.; Fukata, Y.; Chihara, K.; Nakano, T.; Matsuura, Y.; Kaibuchi, K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 1996, 271, 20246–20249. [Google Scholar] [CrossRef]
- Shi, J.; Wu, X.; Surma, M.; Vemula, S.; Zhang, L.; Yang, Y.; Kapur, R.; Wei, L. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell Death Dis. 2013, 4, e483. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.-H.; Yang, Z.-J.; Xie, B.; Zhong, Y.-S. The effect of ROCK-1 activity change on the adhesive and invasive ability of Y79 retinoblastoma cells. BMC Cancer 2014, 14, 89. [Google Scholar] [CrossRef]
- Rochelle, T.; Daubon, T.; Van Troys, M.; Harnois, T.; Waterschoot, D.; Ampe, C.; Roy, L.; Bourmeyster, N.; Constantin, B. p210bcr-abl induces amoeboid motility by recruiting ADF/destrin through RhoA/ROCK1. FASEB J. 2013, 27, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Amano, M.; Nagata, K.; Inagaki, N.; Nakamura, H.; Saya, H.; Kaibuchi, K.; Inagaki, M. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. J. Cell Biol. 1998, 143, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.; Nakamura, Y.; Goto, H.; Wada, Y.; Sakoda, S.; Kaibuchi, K.; Inagaki, M.; Takeda, M. Domain- and site-specific phosphorylation of bovine NF-L by Rho-associated kinase. Biochem. Biophys. Res. Commun. 1998, 245, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Kosako, H.; Inagaki, M. Regulation of intermediate filament organization during cytokinesis: Possible roles of Rho-associated kinase. Microsc. Res. Tech. 2000, 49, 173–182. [Google Scholar] [CrossRef]
- Bordeleau, F.; Myrand Lapierre, M.E.; Sheng, Y.; Marceau, N. Keratin 8/18 regulation of cell stiffness-extracellular matrix interplay through modulation of Rho-mediated actin cytoskeleton dynamics. PLoS ONE 2012, 7, e38780. [Google Scholar] [CrossRef] [PubMed]
- Sin, W.C.; Chen, X.Q.; Leung, T.; Lim, L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol. Cell. Biol. 1998, 18, 6325–6339. [Google Scholar] [CrossRef]
- Amano, M.; Kaneko, T.; Maeda, A.; Nakayama, M.; Ito, M.; Yamauchi, T.; Goto, H.; Fukata, Y.; Oshiro, N.; Shinohara, A.; et al. Identification of Tau and MAP2 as novel substrates of Rho-kinase and myosin phosphatase. J. Neurochem. 2003, 87, 780–790. [Google Scholar] [CrossRef]
- Arimura, N.; Ménager, C.; Kawano, Y.; Yoshimura, T.; Kawabata, S.; Hattori, A.; Fukata, Y.; Amano, M.; Goshima, Y.; Inagaki, M.; et al. Phosphorylation by Rho kinase regulates CRMP-2 activity in growth cones. Mol. Cell. Biol. 2005, 25, 9973–9984. [Google Scholar] [CrossRef]
- Amano, M.; Tsumura, Y.; Taki, K.; Harada, H.; Mori, K.; Nishioka, T.; Kato, K.; Suzuki, T.; Nishioka, Y.; Iwamatsu, A.; et al. A proteomic approach for comprehensively screening substrates of protein kinases such as Rho-kinase. PLoS ONE 2010, 5, e8704. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, X.P. Dysfunction of microtubule-associated proteins of MAP2/tau family in Prion disease. Prion 2012, 6, 334–338. [Google Scholar] [CrossRef]
- Lin, P.C.; Chan, P.M.; Hall, C.; Manser, E. Collapsin response mediator proteins (CRMPs) are a new class of microtubule-associated protein (MAP) that selectively interacts with assembled microtubules via a taxol-sensitive binding interaction. J. Biol. Chem. 2011, 286, 41466–41478. [Google Scholar] [CrossRef] [PubMed]
- Schofield, A.V.; Steel, R.; Bernard, O. Rho-associated coiled-coil kinase (ROCK) protein controls microtubule dynamics in a novel signaling pathway that regulates cell migration. J. Biol. Chem. 2012, 287, 43620–43629. [Google Scholar] [CrossRef]
- Huang, C.H.; Cheng, J.C.; Chen, J.C.; Tseng, C.P. Evaluation of the role of Disabled-2 in nerve growth factor-mediated neurite outgrowth and cellular signalling. Cell. Signal. 2007, 19, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- OuYang, C.; Xie, Y.; Fu, Q.; Xu, G. SYNPO2 suppresses hypoxia-induced proliferation and migration of colorectal cancer cells by regulating YAP-KLF5 axis. Tissue Cell 2021, 73, 101598. [Google Scholar] [CrossRef] [PubMed]
- Kai, F.; Tanner, K.; King, C.; Duncan, R. Myopodin isoforms alter the chemokinetic response of PC3 cells in response to different migration stimuli via differential effects on Rho-ROCK signaling pathways. Carcinogenesis 2012, 33, 2100–2107. [Google Scholar] [CrossRef]
- Maldonado, H.; Calderon, C.; Burgos-Bravo, F.; Kobler, O.; Zuschratter, W.; Ramirez, O.; Härtel, S.; Schneider, P.; Quest, A.F.; Herrera-Molina, R.; et al. Astrocyte-to-neuron communication through integrin-engaged Thy-1/CBP/Csk/Src complex triggers neurite retraction via the RhoA/ROCK pathway. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 243–254. [Google Scholar] [CrossRef]
- Fusella, F.; Seclì, L.; Busso, E.; Krepelova, A.; Moiso, E.; Rocca, S.; Conti, L.; Annaratone, L.; Rubinetto, C.; Mello-Grand, M.; et al. The IKK/NF-κB signaling pathway requires Morgana to drive breast cancer metastasis. Nat. Commun. 2017, 8, 1636. [Google Scholar] [CrossRef]
- Barry, D.M.; Koo, Y.; Norden, P.R.; Wylie, L.A.; Xu, K.; Wichaidit, C.; Azizoglu, D.B.; Zheng, Y.; Cobb, M.H.; Davis, G.E.; et al. Rasip1-Mediated Rho GTPase Signaling Regulates Blood Vessel Tubulogenesis via Nonmuscle Myosin II. Circ. Res. 2016, 119, 810–826. [Google Scholar] [CrossRef]
- de Kreuk, B.J.; Gingras, A.R.; Knight, J.D.; Liu, J.J.; Gingras, A.C.; Ginsberg, M.H. Heart of glass anchors Rasip1 at endothelial cell-cell junctions to support vascular integrity. Elife 2016, 5, e11394. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Kasa, M.; Amano, M.; Kaibuchi, K.; Hakoshima, T. Molecular Mechanism for the Regulation of Rho-Kinase by Dimerization and Its Inhibition by Fasudil. Structure 2006, 14, 589–600. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhong, Y. Rho/Rho-associated kinase pathway in glaucoma (Review). Int. J. Oncol. 2013, 43, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; LoGrasso, P.V.; Defert, O.; Li, R. Rho Kinase (ROCK) Inhibitors and Their Therapeutic Potential. J. Med. Chem. 2016, 59, 2269–2300. [Google Scholar] [CrossRef]
- Liu, Y.; Gray, N.S. Rational design of inhibitors that bind to inactive kinase conformations. Nat. Chem. Biol. 2006, 2, 358–364. [Google Scholar] [CrossRef]
- Zhang, T.; Inesta-Vaquera, F.; Niepel, M.; Zhang, J.; Ficarro, S.B.; Machleidt, T.; Xie, T.; Marto, J.A.; Kim, N.; Sim, T.; et al. Discovery of potent and selective covalent inhibitors of JNK. Chem. Biol. 2012, 19, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Parker, L.; Birdsong, O.C.; Ronquillo, Y.C.; Hofstedt, D.; Shah, T.J.; Gomez, A.T.; Hoopes, P.C.S. Use of Rho kinase Inhibitors in Ophthalmology: A Review of the Literature. Med. Hypothesis Discov. Innov. Ophthalmol. 2018, 7, 101–111. [Google Scholar] [PubMed]
- Zhao, J.; Zhou, D.; Guo, J.; Ren, Z.; Zhou, L.; Wang, S.; Xu, B.; Wang, R. Effect of fasudil hydrochloride, a protein kinase inhibitor, on cerebral vasospasm and delayed cerebral ischemic symptoms after aneurysmal subarachnoid hemorrhage. Neurol. Med. Chir. 2006, 46, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Satoh, S.; Ueda, Y.; Koyanagi, M.; Kadokami, T.; Sugano, M.; Yoshikawa, Y.; Makino, N. Chronic inhibition of Rho kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. J. Mol. Cell. Cardiol. 2003, 35, 59–70. [Google Scholar] [CrossRef]
- Rikitake, Y.; Oyama, N.; Wang, C.Y.; Noma, K.; Satoh, M.; Kim, H.H.; Liao, J.K. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/- haploinsufficient mice. Circulation 2005, 112, 2959–2965. [Google Scholar] [CrossRef]
- Hannan, J.L.; Albersen, M.; Kutlu, O.; Gratzke, C.; Stief, C.G.; Burnett, A.L.; Lysiak, J.J.; Hedlund, P.; Bivalacqua, T.J. Inhibition of Rho-kinase improves erectile function, increases nitric oxide signaling and decreases penile apoptosis in a rat model of cavernous nerve injury. J. Urol. 2013, 189, 1155–1161. [Google Scholar] [CrossRef]
- Wingard, C.J.; Johnson, J.A.; Holmes, A.; Prikosh, A. Improved erectile function after Rho-kinase inhibition in a rat castrate model of erectile dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1572–R1579. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, Y.I.; Cho, S.J.; Lee, M.K.; Kook, M.C.; Lee, J.H.; Lee, S.S.; Ashktorab, H.; Smoot, D.T.; Ryu, K.W.; et al. MicroRNA 135a suppresses lymph node metastasis through down-regulation of ROCK1 in early gastric cancer. PLoS ONE 2014, 9, e85205. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Zhang, J.; Yuan, T.; Ma, B. MiR-145 inhibits osteosarcoma cells proliferation and invasion by targeting ROCK1. Tumour Biol. 2014, 35, 7645–7650. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.; Wang, Y.; Luo, J.; Yu, W. MicroRNA-148a suppresses epithelial-to-mesenchymal transition by targeting ROCK1 in non-small cell lung cancer cells. Mol. Cell. Biochem. 2013, 380, 277–282. [Google Scholar] [CrossRef]
- Cascione, M.; De Matteis, V.; Toma, C.C.; Pellegrino, P.; Leporatti, S.; Rinaldi, R. Morphomechanical and structural changes induced by ROCK inhibitor in breast cancer cells. Exp. Cell Res. 2017, 360, 303–309. [Google Scholar] [CrossRef]
- Ueno, K.; Hirata, H.; Shahryari, V.; Chen, Y.; Zaman, M.S.; Singh, K.; Tabatabai, Z.L.; Hinoda, Y.; Dahiya, R. Tumour suppressor microRNA-584 directly targets oncogene Rock-1 and decreases invasion ability in human clear cell renal cell carcinoma. Br. J. Cancer 2011, 104, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Tashiro, H.; Miyata, Y.; Ushitora, Y.; Fudaba, Y.; Kobayashi, T.; Arihiro, K.; Okajima, M.; Asahara, T. Rho-associated kinase inhibitor reduces tumor recurrence after liver transplantation in a rat hepatoma model. Am. J. Transplant. 2007, 7, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Kroiss, A.; Vincent, S.; Decaussin-Petrucci, M.; Meugnier, E.; Viallet, J.; Ruffion, A.; Chalmel, F.; Samarut, J.; Allioli, N. Androgen-regulated microRNA-135a decreases prostate cancer cell migration and invasion through downregulating ROCK1 and ROCK2. Oncogene 2015, 34, 2846–2855. [Google Scholar] [CrossRef]
- Patel, R.A.; Forinash, K.D.; Pireddu, R.; Sun, Y.; Sun, N.; Martin, M.P.; Schönbrunn, E.; Lawrence, N.J.; Sebti, S.M. RKI-1447 is a potent inhibitor of the Rho-associated ROCK kinases with anti-invasive and antitumor activities in breast cancer. Cancer Res. 2012, 72, 5025–5034. [Google Scholar] [CrossRef]
- McLeod, R.; Kumar, R.; Papadatos-Pastos, D.; Mateo, J.; Brown, J.S.; Garces, A.H.I.; Ruddle, R.; Decordova, S.; Jueliger, S.; Ferraldeschi, R.; et al. First-in-Human Study of AT13148, a Dual ROCK-AKT Inhibitor in Patients with Solid Tumors. Clin. Cancer Res. 2020, 26, 4777–4784. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Riento, K.; Keep, N.; Morris, J.D.; Ridley, A.J. N-terminus-mediated dimerization of ROCK-I is required for RhoE binding and actin reorganization. Biochem. J. 2008, 411, 407–414. [Google Scholar] [CrossRef]
- Jacobs, M.; Hayakawa, K.; Swenson, L.; Bellon, S.; Fleming, M.; Taslimi, P.; Doran, J. The structure of dimeric ROCK I reveals the mechanism for ligand selectivity. J. Biol. Chem. 2006, 281, 260–268. [Google Scholar] [CrossRef]
- Gu, Z.; Yan, T.; Yan, F. Rational design and improvement of the dimerization-disrupting peptide selectivity between ROCK-I and ROCK-II kinase isoforms in cerebrovascular diseases. J. Mol. Recognit. 2020, 33, e2835. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Ridley, A.J.; Lutz, S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front. Pharmacol. 2015, 6, 276. [Google Scholar] [CrossRef] [PubMed]
- Couzens, A.L.; Saridakis, V.; Scheid, M.P. The hydrophobic motif of ROCK2 requires association with the N-terminal extension for kinase activity. Biochem. J. 2009, 419, 141–148. [Google Scholar] [CrossRef] [PubMed]

| Cytoskeleton Component | Functions | References |
|---|---|---|
| Microfilament | ||
| Actin | Generation of forces in cellular contraction, endo- and exocytosis, secretion, vesicle transfer, cell division and cell integrity | Jiang et al., 2021 [20]; Falahzadeh et al., 2015 [21]; Gordon-Alonso et al., 2010 [22]; Blessing et al., 2004 [23]; Halpain 2003 [24]; Lanier and Gertler, 2000 [25]; Pollard et al., 2000 [26]; Schmidt and Hall, 1998 [27]; Bretscher, 1993 [28]. |
| Intermediate filament | ||
| Type I (Acidic Keratins) | Expressed in epithelial cells, form the structural framework of cells and contribute to mechanical resilience | Gül et al., 2022 [29]; Jacob et al., 2018 [30]; Saitoh et al., 2016 [31]; Schweizer et al., 2006 [32]. |
| Type II (Basic Keratins) | Expressed in epithelial cells, they also maintain the stability of the cell nucleus and mechanical stability of the whole cell | Honda et al., 2014 [33]; Infante et al., 2011 [34]; Moll et al., 2008 [35]; Bowden et al., 1984 [36]. |
| Type III (Vimentin) | Regulate cell mechanics and coordinate mechanosensing, transduction, signaling pathways, motility and inflammatory responses | Ridge et al., 2022 [37]; Yue et al., 2016 [38]; Kidd et al., 2014 [39]; Menko et al., 2014 [40]; Herrmann et al., 2007 [41]; Wang et al., 2006 [42] |
| Type IV (Neurofilaments) | Provide structural support for axons and regulate axon radial growth | Didonna and Opal, 2019 [43]; Yuan andNixon, 2016 [44]; Yuan et al., 2012 [45]; Lin and Schlaepfer, 2006 [46]; Hoffman1988 [47] |
| Type V (Nuclear Lamins) | Attach chromatin domains to the nuclear periphery and localize some nuclear membrane proteins | Khadija et al., 2015 [48]; Carmosino et al., 2014 [49]; Burke and Stewart, 2013 [50]; Lopez-Soler et al., 2001 [51]; Parnaik, 2008 [52] |
| Type VI (Phakinin and Filensin) | Lens fiber and maintenance of lens transparency | Oka et al., 2008 [53]; Pittenger et al., 2007 [54]; Blankenship et al., 2001 [55]; Goulielmos et al., 1996 [56]; Georgatos et al., 1994 [57] |
| Microtubule | ||
| Tubulin | Maintain the structure of the cell, transport secretory vesicles, organelles and macromolecular assemblies and assembly of mitotic spindle | Hlavaty and Lechler, 2021 [58]; Chang and Gu, 2020 [59]; Matis 2020 [60]; Logan and Menko, 2019 [61]; Cirillo et al., 2017 [62]; Ganguly et al., 2012 [63]; van der Vaart et al., 2009 [64]; Kulić et al., 2008 [65] |
| Pathway | Function | Cytoskeleton Components Affected | Authors, Year |
|---|---|---|---|
| Rho-kinase/ROCK | Major signaling pathway that regulates the cytoskeleton and cell polarity | Actin | Shi and Wei, 2022 [120]; Tang et al., 2018 [121]; Schofield and Bernard, 2013 [122]; Sit and Manser, 2011 [123]; Sarasa-Renedo et al., 2006 [124]; Woods et al., 2005 [125]; McBeath et al., 2004 [10]; Da Silva et al., 2003 [126]; Amano et al., 2000 [127]; Maekawa et al., 1999 [11] |
| Intermediate filaments | Tang et al., 2018 [121]; Yang et al., 2017 [128]; Lei et al., 2013 [129]; Schofield et al., 2013 [122]; Amano et al., 2010 [12]; Hirose et al., 1998 [130] | ||
| Microtubules | Becker et al., 2022 [131]; Schofield et al., 2013 [122]; Heng et al., 2012 [132]; Fonseca et al., 2010 [133]; Takesono et al., 2010 [134]; Gao et al., 2004 [14] | ||
| PI3K/AKT | Roles in the assembly of actin filaments, polymerization of microtubules and intermediate filaments | Actin | Han et al., 2020 [135]; Lien et al., 2017 [136]; Kakinuma et al., 2008 [137]; Qian et al., 2005 [138]; Qian et al., 2004 [139]; Krasilnikov 2000 [140] |
| Intermediate filaments | Deng et al., 2022 [117]; Roux et al., 2017 [141]; Wang et al., 2012 [142]; Kong et al., 2012 [143]; Tseng et al., 2011 [144] | ||
| Microtubules | Chakrabarty et al., 2019 [145]; Fu et al., 2017 [146]; Kitagishi et al., 2014 [147]; Onishi et al., 2007 [148]; Fujiwara et al., 2007 [149]; | ||
| Wnt/β-catenin | Cell proliferation, differentiation, survival and adhesion (cytoskeleton reorganization) and regulation of microtubule stability. Wnt ligands stabilize β-catenin whereas β-catenin helps link cadherin adhesion molecules to cytoskeleton | Actin | Zhang et al., 2022 [150]; Roarty et al., 2017 [115]; Galli et al., 2012 [151]; Lai et al., 2009 [110]; James et al., 2008 [114] |
| Intermediate filaments | Lehmann et al., 2020 [152]; Tian et al., 2019 [153]; Bermeo et al., 2015 [154]; Prasad et al., 2008 [155]; Bierie et al., 2003 [156] | ||
| Microtubule | Puri et al., 2021 [157]; Ou et al., 2020 [158]; Huang et al., 2007 [159]; Ciani et al., 2004 [160]; Peifer et al., 2000 [161] | ||
| Gsk-3β | Inhibition of Wnt signaling pathway. Responsible for actin branching, regulation of intermediate filaments and controlling microtubule dynamics | Actin | Hajka et al., 2021 [162]; Yoshino et al., 2015 [163]; Watanabe et al., 2009 [164]; Sun et al., 2009 [165]; Vaidya et al., 2006 [166] |
| Intermediate filaments | Sen et al., 2022 [167]; Lee et al., 2012 [168]; Kim et al., 2012 [169]; Sasaki et al., 2002 [170]; Guidato et al., 1996 [171] | ||
| Microtubules | Sen et al., 2022 [167]; Hajka et al., 2021 [162]; Beurel et al., 2015 [116]; Watanabe et al., 2009 [164]; Fumoto et al., 2006 [172]; Grimes and Jope, 2001 [173] | ||
| MAPK | Cytoskeleton remodelling, downregulation of vimentin and affects the polymerization and stability of microtubules | Actin | Joe et al., 2022 [174]; Hoffman et al., 2017 [111]; Yang et al., 2007 [175]; Fujiwara et al., 2005 [176]; Paliga et al., 2005 [177] |
| Intermediate filaments | Tania et al., 2014 [178]; Wang et al., 2021 [179]; Wöll et al., 2007 [180]; Schechter et al., 1998 [181]; Cheng and Lai, 1998 [182] | ||
| Microtubules | Li et al., 2015 [183]; Hu et al., 2010 [184]; Lee et al., 2007 [185]; Fan and Chambers, 2001 [186]; Reszka et al., 1995 [187]; | ||
| cGMP kinase | Inhibition of actin cytoskeleton organization, phosphorylation of vimentin and modulating microtubules and their associated proteins | Actin | Zou et al., 2018 [113]; Butt et al., 2003 [188]; Butt et al., 2001 [189]; Sandau et al., 2001 [190]; Sauzeau et al., 2000 [112] |
| Intermediate filaments | Pryzwansky et al., 1995 [191]; MacMillan-Crow and Lincoln, 1994 [192]; Wyatt et al., 1993 [193]; Wyatt et al., 1991 [194] | ||
| Microtubules | Xia et al., 2013 [195]; Gong et al., 2011 [196] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, G.; Cannon, R.D.; Coates, D.E.; Mei, L. Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components. Genes 2023, 14, 272. https://doi.org/10.3390/genes14020272
Guan G, Cannon RD, Coates DE, Mei L. Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components. Genes. 2023; 14(2):272. https://doi.org/10.3390/genes14020272
Chicago/Turabian StyleGuan, Guangzhao, Richard D. Cannon, Dawn E. Coates, and Li Mei. 2023. "Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components" Genes 14, no. 2: 272. https://doi.org/10.3390/genes14020272
APA StyleGuan, G., Cannon, R. D., Coates, D. E., & Mei, L. (2023). Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components. Genes, 14(2), 272. https://doi.org/10.3390/genes14020272







