Novel Insights into Mitochondrial DNA: Mitochondrial Microproteins and mtDNA Variants Modulate Athletic Performance and Age-Related Diseases
Abstract
:1. Introduction
2. Nuclear Genome Encoded Genetic Polymorphism in Athletes
2.1. Athletic Performance-Related SNPs
2.2. Genome-Wide Association Study (GWAS) for Athletic Performance
2.3. Sports Injuries-Related SNPs
3. Mitochondrial Genome Encoded Variants in Athletes
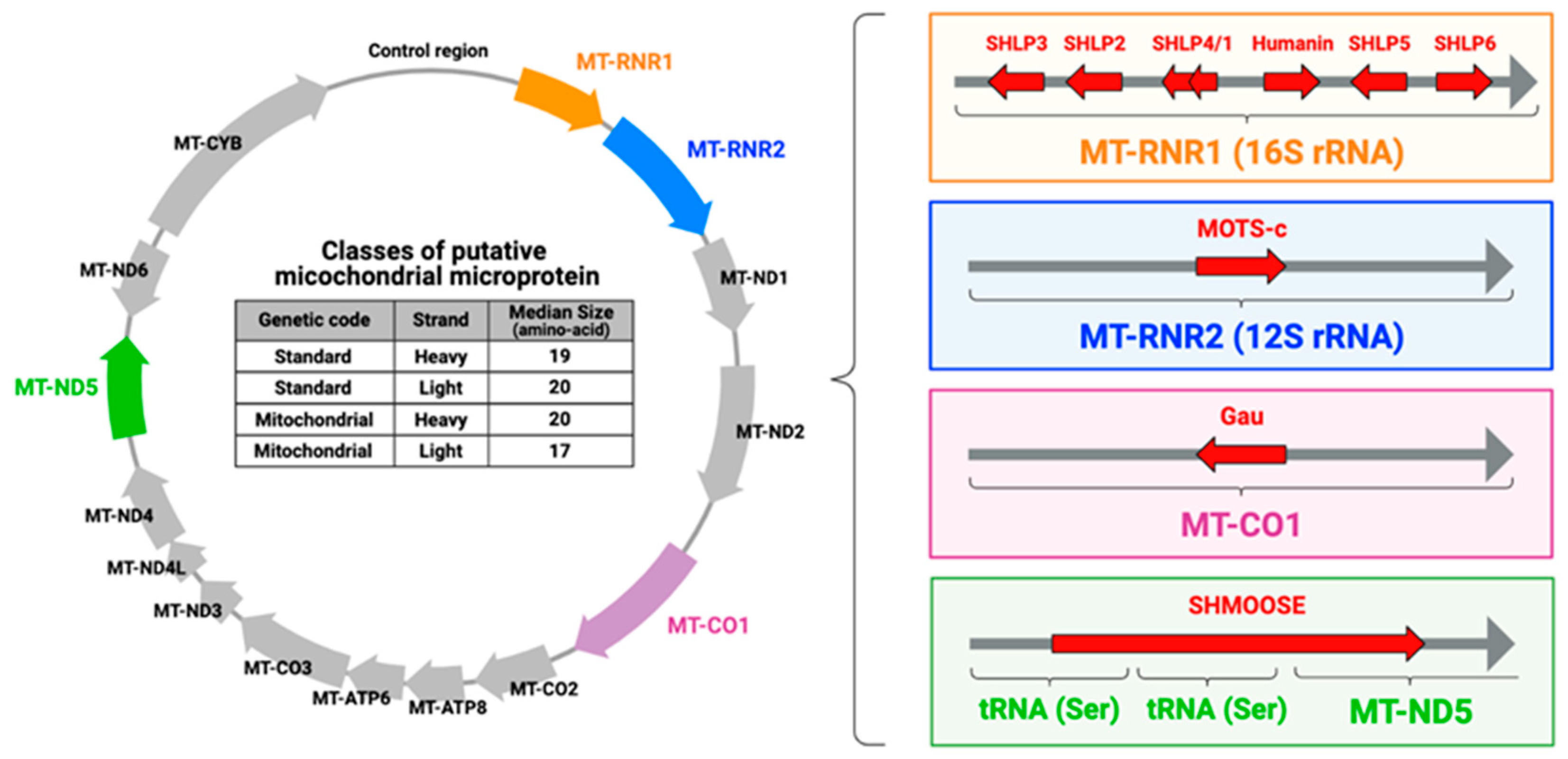
4. Mitochondrial Microproteins: Mitochondrial-Derived Peptides (MDPs)
4.1. MOTS-c
4.1.1. MOTS-c as a Metabolic Regulator
4.1.2. MOTS-c and Exercise-Related Phenotypes
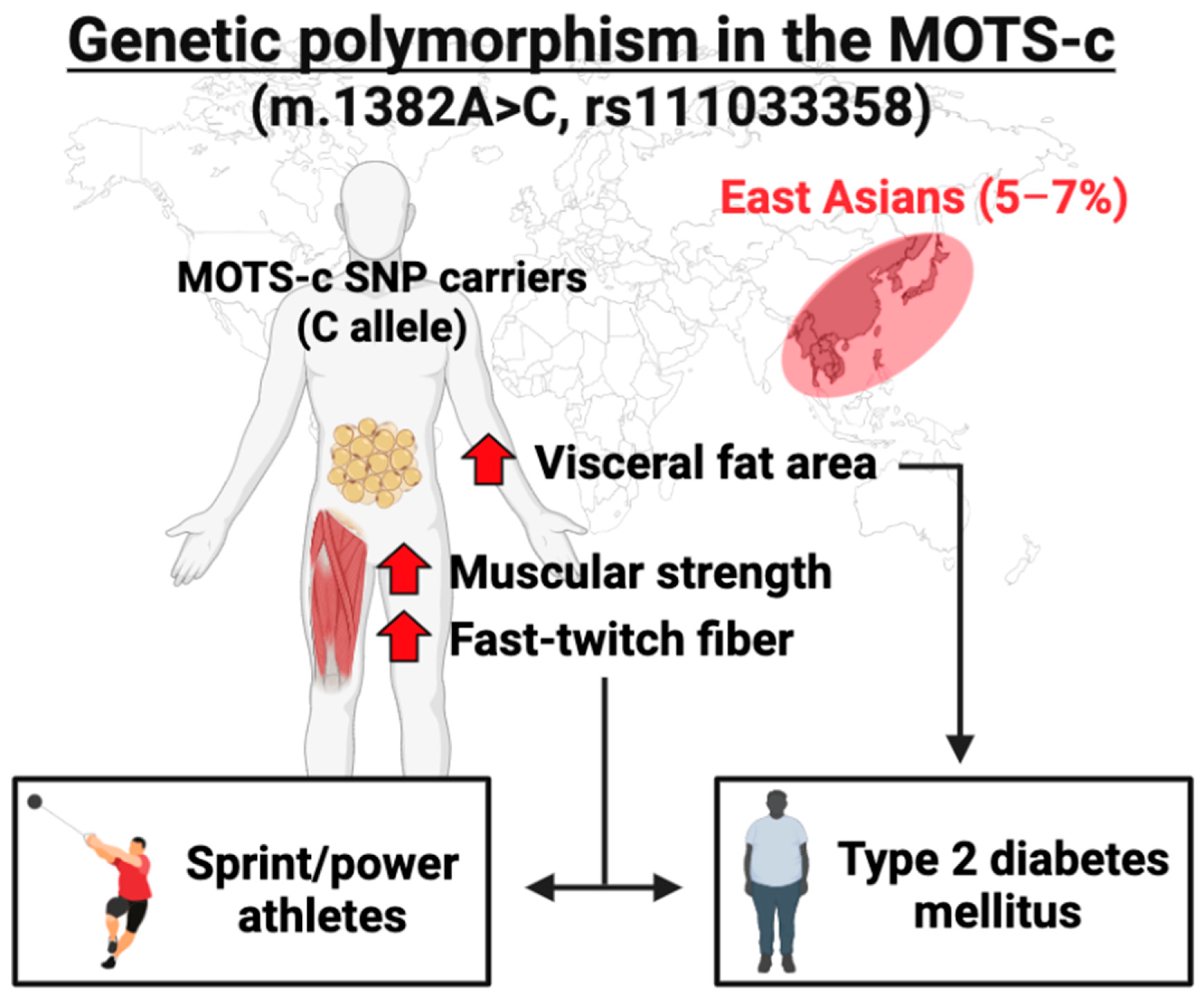
4.1.3. Genetic Polymorphism in the MOTS-c Coding Region
| Stimulation | Term | Observation | Tissue | Reference | |
|---|---|---|---|---|---|
| MOTS-c | MOTS-c | Chronic | Improves insulin resistance | Mouse muscle | Lee et al. [16] |
| MOTS-c | Chronic | Improves glucose metabolism | Mouse muscle | Zempo and Kim et al. [21] | |
| K14Q MOTS-c | Chronic | No difference | Mouse muscle | Zempo and Kim et al. [21] | |
| MOTS-c | Acute | Increases exercise endurance | Mouse muscle | Hyatt. [103] | |
| MOTS-c | Chronic | Increases exercise endurance | Mouse muscle | Reynolds et al. [100] | |
| MOTS-c | Chronic | Prevents muscle wasting | Mouse muscle | Reynolds et al. [100] | |
| MOTS-c | Chronic | Improves muscle strength | Mouse muscle | Reynolds et al. [100] | |
| MOTS-c | Chronic | Supresses myostatin expression | Mouse muscle | Kumagai et al. [99] | |
| MOTS-c | Chronic | Prevents muscle wasting | Mouse muscle | Kumagai et al. [99] | |
| MOTS-c antibody | Chronic | Increases myosin heavy chain-fast | Mouse muscle | Kumagai et al. [22] | |
| AEx | Chronic | Increases MOTS-c | Mouse hypothalamus | Kang et al. [104] | |
| AEx | Chronic | Increases MOTS-c | Rat muscle | Hyatt. [103] | |
| HIIT | Acute | Increases MOTS-c | Human muscle and blood | Reynolds et al. [100] | |
| AEx | Acute | No difference | Human blood and muscle | von Walden et al. [101] | |
| REx | Acute | No difference | Human blood and muscle | von Walden et al. [101] | |
| AEx and REx | Chronic | Increases MOTS-c | Human blood | Dieli-Conwright et al. [102] | |
| AEx | Chronic | No difference | Human blood | Ramanjaneya et al. [105] | |
| Humanin | Humanin | Chronic | Improves rotarod performance | Mouse muscle | Kim et al. [112] |
| Contraction | Acute | Increases humanin | Isolated mouse muscle | Woodhead et al. [113] | |
| HIIT | Acute | Increases humanin | Human muscle and blood | Woodhead et al. [113] | |
| AEx | Acute | Increases humanin | Human blood | von Walden et al. [101] | |
| REx | Acute | No difference | Human blood and muscle | von Walden et al. [101] | |
| REx | Chronic | Increases humanin in muscle | Human muscle and blood | Gidlund et al. [114] | |
| HIIT | Chronic | No difference | Human muscle and blood | Woodhead et al. [113] | |
| AEx | Chronic | No difference | Human muscle and blood | Gidlund et al. [114] | |
| SHLP2 | HIIT | Acute | No difference | Human blood | Woodhead et al. [113] |
| HIIT | Chronic | No difference | Human blood | Woodhead et al. [113] | |
| SHLP6 | HIIT | Acute | Increases SHLP6 | Human blood | Woodhead et al. [113] |
| HIIT | Chronic | No difference | Human blood | Woodhead et al. [113] |
4.2. Humanin
4.2.1. Biological Functions of Humanin
4.2.2. Humanin and Exercise-Related Phenotypes
4.2.3. Genetic Polymorphism in the Humanin Coding Region
4.3. SHLPs
4.4. SHMOOSE
4.5. Gau
5. Implication of Sports Genetics in Age-Related Diseases
6. Perspective: Future Directions of mtDNA and Microproteins
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Moor, M.H.; Spector, T.D.; Cherkas, L.F.; Falchi, M.; Hottenga, J.J.; Boomsma, D.I.; De Geus, E.J. Genome-wide linkage scan for athlete status in 700 British female DZ twin pairs. Twin Res. Hum. Genet. 2007, 10, 812–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komi, P.V.; Viitasalo, J.H.; Havu, M.; Thorstensson, A.; Sjodin, B.; Karlsson, J. Skeletal muscle fibres and muscle enzyme activities in monozygous and dizygous twins of both sexes. Acta Physiol. Scand. 1977, 100, 385–392. [Google Scholar] [CrossRef]
- Simoneau, J.A.; Bouchard, C. Genetic determinism of fiber type proportion in human skeletal muscle. FASEB J. 1995, 9, 1091–1095. [Google Scholar] [CrossRef]
- Zempo, H.; Miyamoto-Mikami, E.; Kikuchi, N.; Fuku, N.; Miyachi, M.; Murakami, H. Heritability estimates of muscle strength-related phenotypes: A systematic review and meta-analysis. Scand. J. Med. Sci. Sports 2017, 27, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto-Mikami, E.; Zempo, H.; Fuku, N.; Kikuchi, N.; Miyachi, M.; Murakami, H. Heritability estimates of endurance-related phenotypes: A systematic review and meta-analysis. Scand. J. Med. Sci. Sport. 2018, 28, 834–845. [Google Scholar] [CrossRef]
- Magnusson, K.; Turkiewicz, A.; Hughes, V.; Frobell, R.; Englund, M. High genetic contribution to anterior cruciate ligament rupture: Heritability ~69%. Br. J. Sport. Med. 2021, 55, 385–389. [Google Scholar] [CrossRef]
- Gayagay, G.; Yu, B.; Hambly, B.; Boston, T.; Hahn, A.; Celermajer, D.S.; Trent, R.J. Elite endurance athletes and the ACE I allele—The role of genes in athletic performance. Hum. Genet. 1998, 103, 48–50. [Google Scholar] [CrossRef]
- Montgomery, H.E.; Marshall, R.; Hemingway, H.; Myerson, S.; Clarkson, P.; Dollery, C.; Hayward, M.; Holliman, D.E.; Jubb, M.; World, M.; et al. Human gene for physical performance. Nature 1998, 393, 221–222. [Google Scholar] [CrossRef]
- Yang, N.; MacArthur, D.G.; Gulbin, J.P.; Hahn, A.G.; Beggs, A.H.; Easteal, S.; North, K. ACTN3 genotype is associated with human elite athletic performance. Am. J. Hum. Genet. 2003, 73, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Ahmetov, I.I.; Hall, E.C.R.; Semenova, E.A.; Pranckeviciene, E.; Gineviciene, V. Advances in sports genomics. Adv. Clin. Chem. 2022, 107, 215–263. [Google Scholar] [CrossRef]
- Basrai, M.A.; Hieter, P.; Boeke, J.D. Small open reading frames: Beautiful needles in the haystack. Genome Res. 1997, 7, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Saghatelian, A.; Couso, J.P. Discovery and characterization of smORF-encoded bioactive polypeptides. Nat. Chem. Biol. 2015, 11, 909–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, Y.; Niikura, T.; Tajima, H.; Yasukawa, T.; Sudo, H.; Ito, Y.; Kita, Y.; Kawasumi, M.; Kouyama, K.; Doyu, M.; et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc. Natl. Acad. Sci. USA 2001, 98, 6336–6341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, B.; Zhai, D.; Cabezas, E.; Welsh, K.; Nouraini, S.; Satterthwait, A.C.; Reed, J.C. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature 2003, 423, 456–461. [Google Scholar] [CrossRef]
- Ikonen, M.; Liu, B.; Hashimoto, Y.; Ma, L.; Lee, K.W.; Niikura, T.; Nishimoto, I.; Cohen, P. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc. Natl. Acad. Sci. USA 2003, 100, 13042–13047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobb, L.J.; Lee, C.; Xiao, J.; Yen, K.; Wong, R.G.; Nakamura, H.K.; Mehta, H.H.; Gao, Q.; Ashur, C.; Huffman, D.M.; et al. Naturally occurring mitochondrial-derived peptides are age-dependent regulators of apoptosis, insulin sensitivity, and inflammatory markers. Aging 2016, 8, 796–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, B.; Kim, S.J.; Mehta, H.H.; Cao, K.; Kumagai, H.; Thumaty, N.; Leelaprachakul, N.; Jiao, H.; Vaughan, J.; Diedrich, J.; et al. Mitochondrial DNA variation in Alzheimer’s disease reveals a unique microprotein called SHMOOSE. Mol. Psychiatry 2022. [Google Scholar] [CrossRef]
- Faure, E.; Delaye, L.; Tribolo, S.; Levasseur, A.; Seligmann, H.; Barthelemy, R.M. Probable presence of an ubiquitous cryptic mitochondrial gene on the antisense strand of the cytochrome oxidase I gene. Biol. Direct. 2011, 6, 56. [Google Scholar] [CrossRef] [Green Version]
- Yen, K.; Wan, J.; Mehta, H.H.; Miller, B.; Christensen, A.; Levine, M.E.; Salomon, M.P.; Brandhorst, S.; Xiao, J.; Kim, S.J.; et al. Humanin Prevents Age-Related Cognitive Decline in Mice and is Associated with Improved Cognitive Age in Humans. Sci. Rep. 2018, 8, 14212. [Google Scholar] [CrossRef]
- Zempo, H.; Kim, S.J.; Fuku, N.; Nishida, Y.; Higaki, Y.; Wan, J.; Yen, K.; Miller, B.; Vicinanza, R.; Miyamoto-Mikami, E.; et al. A pro-diabetogenic mtDNA polymorphism in the mitochondrial-derived peptide, MOTS-c. Aging 2021, 13, 1692–1717. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Natsume, T.; Kim, S.J.; Tobina, T.; Miyamoto-Mikami, E.; Shiose, K.; Ichinoseki-Sekine, N.; Kakigi, R.; Tsuzuki, T.; Miller, B.; et al. The MOTS-c K14Q polymorphism in the mtDNA is associated with muscle fiber composition and muscular performance. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130048. [Google Scholar] [CrossRef]
- North, K.N.; Yang, N.; Wattanasirichaigoon, D.; Mills, M.; Easteal, S.; Beggs, A.H. A common nonsense mutation results in α-actinin-3 deficiency in the general population. Nat. Genet. 1999, 21, 353–354. [Google Scholar] [CrossRef] [PubMed]
- Vincent, B.; De Bock, K.; Ramaekers, M.; Van den Eede, E.; Van Leemputte, M.; Hespel, P.; Thomis, M.A. ACTN3 (R577X) genotype is associated with fiber type distribution. Physiol. Genom. 2007, 32, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, H.; Tobina, T.; Ichinoseki-Sekine, N.; Kakigi, R.; Tsuzuki, T.; Zempo, H.; Shiose, K.; Yoshimura, E.; Kumahara, H.; Ayabe, M.; et al. Role of selected polymorphisms in determining muscle fiber composition in Japanese men and women. J. Appl. Physiol. 2018, 124, 1377–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zempo, H.; Tanabe, K.; Murakami, H.; Iemitsu, M.; Maeda, S.; Kuno, S. Age differences in the relation between ACTN3 R577X polymorphism and thigh-muscle cross-sectional area in women. Genet. Test Mol. Biomark. 2011, 15, 639–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niemi, A.K.; Majamaa, K. Mitochondrial DNA and ACTN3 genotypes in Finnish elite endurance and sprint athletes. Eur. J. Hum. Genet. 2005, 13, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Ahmetov, I.I.; Druzhevskaya, A.M.; Astratenkova, I.V.; Popov, D.V.; Vinogradova, O.L.; Rogozkin, V.A. The ACTN3 R577X polymorphism in Russian endurance athletes. Br. J. Sports Med. 2010, 44, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Mikami, E.; Fuku, N.; Murakami, H.; Tsuchie, H.; Takahashi, H.; Ohiwa, N.; Tanaka, H.; Pitsiladis, Y.P.; Higuchi, M.; Miyachi, M.; et al. ACTN3 R577X genotype is associated with sprinting in elite Japanese athletes. Int. J. Sports Med. 2014, 35, 172–177. [Google Scholar] [CrossRef]
- Kikuchi, N.; Miyamoto-Mikami, E.; Murakami, H.; Nakamura, T.; Min, S.K.; Mizuno, M.; Naito, H.; Miyachi, M.; Nakazato, K.; Fuku, N. ACTN3 R577X genotype and athletic performance in a large cohort of Japanese athletes. Eur. J. Sport Sci. 2016, 16, 694–701. [Google Scholar] [CrossRef]
- Coelho, D.B.; Pimenta, E.M.; Rosse, I.C.; de Castro, B.M.; Becker, L.K.; de Oliveira, E.C.; Carvalho, M.R.S.; Garcia, E.S. Evidence for a Role of ACTN3 R577X Polymorphism in Football Player’s Career Progression. Int. J. Sports Med. 2018, 39, 1088–1093. [Google Scholar] [CrossRef] [Green Version]
- Akazawa, N.; Ohiwa, N.; Shimizu, K.; Suzuki, N.; Kumagai, H.; Fuku, N.; Suzuki, Y. The association of ACTN3 R577X polymorphism with sports specificity in Japanese elite athletes. Biol. Sport 2022, 39, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Massidda, M.; Vona, G.; Calo, C.M. Association between the ACTN3 R577X polymorphism and artistic gymnastic performance in Italy. Genet. Test Mol. Biomark. 2009, 13, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Kaneko, T.; Shintake, Y.; Miyamoto-Mikami, E.; Tomita, H.; Fukuo, M.; Kawai, W.; Harada, M.; Kikuchi, N.; Kamiya, N.; et al. Genetic polymorphisms related to muscular strength and flexibility are associated with artistic gymnastic performance in the Japanese population. Eur. J. Sport Sci. 2022. [Google Scholar] [CrossRef]
- Myerson, S.; Hemingway, H.; Budget, R.; Martin, J.; Humphries, S.; Montgomery, H. Human angiotensin I-converting enzyme gene and endurance performance. J. Appl. Physiol. 1999, 87, 1313–1316. [Google Scholar] [CrossRef] [Green Version]
- Nazarov, I.B.; Woods, D.R.; Montgomery, H.E.; Shneider, O.V.; Kazakov, V.I.; Tomilin, N.V.; Rogozkin, V.A. The angiotensin converting enzyme I/D polymorphism in Russian athletes. Eur. J. Hum. Genet. 2001, 9, 797–801. [Google Scholar] [CrossRef]
- Ma, F.; Yang, Y.; Li, X.; Zhou, F.; Gao, C.; Li, M.; Gao, L. The association of sport performance with ACE and ACTN3 genetic polymorphisms: A systematic review and meta-analysis. PLoS ONE 2013, 8, e54685. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Cho, J.Y.; Jeon, J.Y.; Koh, Y.G.; Kim, Y.M.; Kim, H.J.; Park, M.; Um, H.S.; Kim, C. ACE DD genotype is unfavorable to Korean short-term muscle power athletes. Int. J. Sports Med. 2010, 31, 65–71. [Google Scholar] [CrossRef]
- Tobina, T.; Michishita, R.; Yamasawa, F.; Zhang, B.; Sasaki, H.; Tanaka, H.; Saku, K.; Kiyonaga, A. Association between the angiotensin I-converting enzyme gene insertion/deletion polymorphism and endurance running speed in Japanese runners. J. Physiol. Sci. 2010, 60, 325–330. [Google Scholar] [CrossRef]
- Wang, G.; Mikami, E.; Chiu, L.L.; Perini, A.D.E.; Deason, M.; Fuku, N.; Miyachi, M.; Kaneoka, K.; Murakami, H.; Tanaka, M.; et al. Association analysis of ACE and ACTN3 in elite Caucasian and East Asian swimmers. Med. Sci. Sports Exerc. 2013, 45, 892–900. [Google Scholar] [CrossRef]
- Pickering, C.; Suraci, B.; Semenova, E.A.; Boulygina, E.A.; Kostryukova, E.S.; Kulemin, N.A.; Borisov, O.V.; Khabibova, S.A.; Larin, A.K.; Pavlenko, A.V.; et al. A Genome-Wide Association Study of Sprint Performance in Elite Youth Football Players. J. Strength Cond. Res. 2019, 33, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, J.; Semenova, E.A.; Zempo, H.; Martins, G.L.; Lancha Junior, A.H.; Miyamoto-Mikami, E.; Kumagai, H.; Tobina, T.; Shiose, K.; Kakigi, R.; et al. Are Genome-Wide Association Study Identified Single-Nucleotide Polymorphisms Associated With Sprint Athletic Status? A Replication Study With 3 Different Cohorts. Int. J. Sports Physiol. Perform. 2020, 16, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ahmetov, I.; Kulemin, N.; Popov, D.; Naumov, V.; Akimov, E.; Bravy, Y.; Egorova, E.; Galeeva, A.; Generozov, E.; Kostryukova, E.; et al. Genome-wide association study identifies three novel genetic markers associated with elite endurance performance. Biol. Sport 2015, 32, 3–9. [Google Scholar] [CrossRef]
- Rankinen, T.; Fuku, N.; Wolfarth, B.; Wang, G.; Sarzynski, M.A.; Alexeev, D.G.; Ahmetov, I.I.; Boulay, M.R.; Cieszczyk, P.; Eynon, N.; et al. No Evidence of a Common DNA Variant Profile Specific to World Class Endurance Athletes. PLoS ONE 2016, 11, e0147330. [Google Scholar] [CrossRef]
- Al-Khelaifi, F.; Yousri, N.A.; Diboun, I.; Semenova, E.A.; Kostryukova, E.S.; Kulemin, N.A.; Borisov, O.V.; Andryushchenko, L.B.; Larin, A.K.; Generozov, E.V.; et al. Genome-Wide Association Study Reveals a Novel Association Between MYBPC3 Gene Polymorphism, Endurance Athlete Status, Aerobic Capacity and Steroid Metabolism. Front. Genet. 2020, 11, 595. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.R.; Voisin, S.; Lea, R.A.; Yan, X.; Benton, M.C.; Papadimitriou, I.D.; Jacques, M.; Haupt, L.M.; Ashton, K.J.; Eynon, N.; et al. Investigating the influence of mtDNA and nuclear encoded mitochondrial variants on high intensity interval training outcomes. Sci. Rep. 2020, 10, 11089. [Google Scholar] [CrossRef]
- Eirale, C.; Tol, J.L.; Farooq, A.; Smiley, F.; Chalabi, H. Low injury rate strongly correlates with team success in Qatari professional football. Br. J. Sports Med. 2013, 47, 807–808. [Google Scholar] [CrossRef] [Green Version]
- Hagglund, M.; Walden, M.; Magnusson, H.; Kristenson, K.; Bengtsson, H.; Ekstrand, J. Injuries affect team performance negatively in professional football: An 11-year follow-up of the UEFA Champions League injury study. Br. J. Sports Med. 2013, 47, 738–742. [Google Scholar] [CrossRef] [Green Version]
- Collins, M. Genetic risk factors for soft-tissue injuries 101: A practical summary to help clinicians understand the role of genetics and ‘personalised medicine’. Br. J. Sports Med. 2010, 44, 915–917. [Google Scholar] [CrossRef]
- Vlahovich, N.; Fricker, P.A.; Brown, M.A.; Hughes, D. Ethics of genetic testing and research in sport: A position statement from the Australian Institute of Sport. Br. J. Sports Med. 2017, 51, 5–11. [Google Scholar] [CrossRef]
- Pruna, R.; Artells, R.; Ribas, J.; Montoro, B.; Cos, F.; Munoz, C.; Rodas, G.; Maffulli, N. Single nucleotide polymorphisms associated with non-contact soft tissue injuries in elite professional soccer players: Influence on degree of injury and recovery time. BMC Musculoskelet. Disord. 2013, 14, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massidda, M.; Bachis, V.; Corrias, L.; Piras, F.; Scorcu, M.; Calo, C.M. Influence of the COL5A1 rs12722 on musculoskeletal injuries in professional soccer players. J. Sports Med. Phys. Fitness 2015, 55, 1348–1353. [Google Scholar] [PubMed]
- Massidda, M.; Corrias, L.; Bachis, V.; Cugia, P.; Piras, F.; Scorcu, M.; Calo, C.M. Vitamin D receptor gene polymorphisms and musculoskeletal injuries in professional football players. Exp. Ther. Med. 2015, 9, 1974–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massidda, M.; Eynon, N.; Bachis, V.; Corrias, L.; Culigioni, C.; Piras, F.; Cugia, P.; Scorcu, M.; Calo, C.M. Influence of the MCT1 rs1049434 on Indirect Muscle Disorders/Injuries in Elite Football Players. Sports Med. Open 2015, 1, 33. [Google Scholar] [CrossRef] [Green Version]
- Pruna, R.; Ribas, J.; Montoro, J.B.; Artells, R. The impact of single nucleotide polymorphisms on patterns of non-contact musculoskeletal soft tissue injuries in a football player population according to ethnicity. Med. Clin. Barc. 2015, 144, 105–110. [Google Scholar] [CrossRef]
- Myosotis, M.; Sarah, V.; Claudia, C.; Francesco, P.; Paolo, C.; Xu, Y.; Nir, E.; Calo, C.M. ACTN3 R577X Polymorphism Is Associated With the Incidence and Severity of Injuries in Professional Football Players. Clin. J. Sport Med. 2017, 29, 57–61. [Google Scholar] [CrossRef]
- Pruna, R.; Artells, R.; Lundblad, M.; Maffulli, N. Genetic biomarkers in non-contact muscle injuries in elite soccer players. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3311–3318. [Google Scholar] [CrossRef]
- Clos, E.; Pruna, R.; Lundblad, M.; Artells, R.; Esquirol Caussa, J. ACTN3 single nucleotide polymorphism is associated with non-contact musculoskeletal soft-tissue injury incidence in elite professional football players. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 4055–4061. [Google Scholar] [CrossRef]
- Kumagai, H.; Miyamoto-Mikami, E.; Hirata, K.; Kikuchi, N.; Kamiya, N.; Hoshikawa, S.; Zempo, H.; Naito, H.; Miyamoto, N.; Fuku, N. ESR1 rs2234693 Polymorphism Is Associated with Muscle Injury and Muscle Stiffness. Med. Sci. Sports Exerc. 2019, 51, 19–26. [Google Scholar] [CrossRef]
- Miyamoto-Mikami, E.; Kumagai, H.; Tanisawa, K.; Taga, Y.; Hirata, K.; Kikuchi, N.; Kamiya, N.; Kawakami, R.; Midorikawa, T.; Kawamura, T.; et al. Female Athletes Genetically Susceptible to Fatigue Fracture Are Resistant to Muscle Injury: Potential Role of COL1A1 Variant. Med. Sci. Sports Exerc. 2021, 53, 1855–1864. [Google Scholar] [CrossRef]
- Miyamoto-Mikami, E.; Kumagai, H.; Kikuchi, N.; Kamiya, N.; Miyamoto, N.; Fuku, N. eQTL variants in COL22A1 are associated with muscle injury in athletes. Physiol. Genomics. 2020, 52, 588–589. [Google Scholar] [CrossRef] [PubMed]
- Massidda, M.; Myamoto-Mikami, E.; Kumagai, H.; Ikeda, H.; Shimasaki, Y.; Yoshimura, M.; Cugia, P.; Piras, F.; Scorcu, M.; Kikuchi, N.; et al. Association between the ACE I/D polymorphism and muscle injuries in Italian and Japanese elite football players. J. Sports Sci. 2020, 38, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Miyamoto-Mikami, E.; Takaragawa, M.; Kuriki, K.; Goto, C.; Shibata, K.; Yamada, N.; Hosono, A.; Fuku, M.; Suzuki, S.; et al. Genetic polymorphisms in CYP19A1 and ESR1 are associated with serum CK activity after prolonged running in men. J. Appl. Physiol. 2022, 132, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Larruskain, J.; Celorrio, D.; Barrio, I.; Odriozola, A.; Gil, S.M.; Fernandez-Lopez, J.R.; Nozal, R.; Ortuzar, I.; Lekue, J.A.; Aznar, J.M. Genetic Variants and Hamstring Injury in Soccer: An Association and Validation Study. Med. Sci. Sports Exerc. 2018, 50, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Mokone, G.G.; Schwellnus, M.P.; Noakes, T.D.; Collins, M. The COL5A1 gene and Achilles tendon pathology. Scand. J. Med. Sci. Sports 2006, 16, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Posthumus, M.; September, A.V.; Keegan, M.; O’Cuinneagain, D.; Van der Merwe, W.; Schwellnus, M.P.; Collins, M. Genetic risk factors for anterior cruciate ligament ruptures: COL1A1 gene variant. Br. J. Sports Med. 2009, 43, 352–356. [Google Scholar] [CrossRef] [Green Version]
- Posthumus, M.; September, A.V.; O’Cuinneagain, D.; van der Merwe, W.; Schwellnus, M.P.; Collins, M. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. Am. J. Sports Med. 2009, 37, 2234–2240. [Google Scholar] [CrossRef]
- September, A.V.; Cook, J.; Handley, C.J.; van der Merwe, L.; Schwellnus, M.P.; Collins, M. Variants within the COL5A1 gene are associated with Achilles tendinopathy in two populations. Br. J. Sports Med. 2009, 43, 357–365. [Google Scholar] [CrossRef]
- Abrahams, Y.; Laguette, M.J.; Prince, S.; Collins, M. Polymorphisms within the COL5A1 3’-UTR that alters mRNA structure and the MIR608 gene are associated with Achilles tendinopathy. Ann. Hum. Genet. 2013, 77, 204–214. [Google Scholar] [CrossRef]
- Alvarez-Romero, J.; Laguette, M.-J.N.; Seale, K.; Jacques, M.; Voisin, S.; Hiam, D.; Feller, J.A.; Tirosh, O.; Miyamoto-Mikami, E.; Kumagai, H.; et al. Genetic variants within the COL5A1 gene are associated with ligament injuries in physically active populations from Australia, South Africa, and Japan. Eur. J. Sport Sci. 2021, 1–21. [Google Scholar] [CrossRef]
- Varley, I.; Hughes, D.C.; Greeves, J.P.; Stellingwerff, T.; Ranson, C.; Fraser, W.D.; Sale, C. The association of novel polymorphisms with stress fracture injury in Elite Athletes: Further insights from the SFEA cohort. J. Sci. Med. Sport 2018, 21, 564–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, H.; Miyamoto-Mikami, E.; Kikuchi, N.; Kamiya, N.; Zempo, H.; Fuku, N. A rs936306 C/T Polymorphism in the CYP19A1 Is Associated With Stress Fractures. J. Strength Cond. Res. 2022, 36, 2322–2325. [Google Scholar] [CrossRef]
- Varley, I.; Greeves, J.P.; Sale, C.; Friedman, E.; Moran, D.S.; Yanovich, R.; Wilson, P.J.; Gartland, A.; Hughes, D.C.; Stellingwerff, T.; et al. Functional polymorphisms in the P2X7 receptor gene are associated with stress fracture injury. Purinergic Signal 2016, 12, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varley, I.; Hughes, D.C.; Greeves, J.P.; Stellingwerff, T.; Ranson, C.; Fraser, W.D.; Sale, C. RANK/RANKL/OPG pathway: Genetic associations with stress fracture period prevalence in elite athletes. Bone 2015, 71, 131–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holloszy, J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967, 242, 2278–2282. [Google Scholar] [CrossRef] [PubMed]
- Holloszy, J.O.; Coyle, E.F. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J. Appl. Physiol. Respir Environ. Exerc. Physiol. 1984, 56, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Larsson, N.-G.; Wang, J.; Wilhelmsson, H.; Oldfors, A.; Rustin, P.; Lewandoski, M.; Barsh, G.S.; Clayton, D.A. Mitochondrial transcription factor A is necessary for mtDNA maintance and embryogenesis in mice. Nat. Genet. 1998, 18, 231–236. [Google Scholar] [CrossRef]
- Moreno-Loshuertos, R.; Acin-Perez, R.; Fernandez-Silva, P.; Movilla, N.; Perez-Martos, A.; Rodriguez de Cordoba, S.; Gallardo, M.E.; Enriquez, J.A. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nat. Genet. 2006, 38, 1261–1268. [Google Scholar] [CrossRef]
- Dionne, F.T.; Turcotte, L.; Thibault, M.C.; Boulay, M.R.; Skinner, J.S.; Bouchard, C. Mitochondrial DNA sequence polymorphism, VO2max, and response to endurance training. Med. Sci. Sports Exerc. 1991, 23, 177–185. [Google Scholar] [CrossRef]
- Vellers, H.L.; Verhein, K.C.; Burkholder, A.B.; Lee, J.; Kim, Y.; Lightfoot, J.T.; Shi, M.; Weinberg, C.R.; Sarzynski, M.A.; Bouchard, C.; et al. Association between Mitochondrial DNA Sequence Variants and V O2 max Trainability. Med. Sci. Sports Exerc. 2020, 52, 2303–2309. [Google Scholar] [CrossRef]
- Miller, B.; Arpawong, T.; Jiao, H.; Kim, S.-J.; Yen, K.; Mehta, H.; Wan, J.; Carpten, J.; Cohen, P. Comparing the Utility of Mitochondrial and Nuclear DNA to Adjust for Genetic Ancestry in Association Studies. Cells 2019, 8, 306. [Google Scholar] [CrossRef] [Green Version]
- Miller, B.; Torres, M.; Jiang, X.; McKean-Cowdin, R.; Nousome, D.; Kim, S.-J.; Mehta, H.H.; Yen, K.; Cohen, P.; Varma, R. A Mitochondrial Genome-Wide Association Study of Cataract in a Latino Population. Transl. Vis. Sci. Technol. 2020, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Choteau, S.A.; Wagner, A.; Pierre, P.; Spinelli, L.; Brun, C. MetamORF: A repository of unique short open reading frames identified by both experimental and computational approaches for gene and metagene analyses. Database 2021, 2021, baab032. [Google Scholar] [CrossRef]
- Mudge, J.M.; Ruiz-Orera, J.; Prensner, J.R.; Brunet, M.A.; Calvet, F.; Jungreis, I.; Gonzalez, J.M.; Magrane, M.; Martinez, T.F.; Schulz, J.F.; et al. Standardized annotation of translated open reading frames. Nat. Biotechnol. 2022, 40, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Martinez, T.F.; Chu, Q.; Donaldson, C.; Tan, D.; Shokhirev, M.N.; Saghatelian, A. Accurate annotation of human protein-coding small open reading frames. Nat. Chem. Biol. 2020, 16, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Neph, S.; Dinger, M.E.; Crawford, J.; Smith, M.A.; Shearwood, A.M.; Haugen, E.; Bracken, C.P.; Rackham, O.; Stamatoyannopoulos, J.A.; et al. The human mitochondrial transcriptome. Cell 2011, 146, 645–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-J.; Miller, B.; Kumagai, H.; Silverstein, A.R.; Flores, M.; Yen, K. Mitochondrial-derived peptides in aging and age-related diseases. GeroScience 2020, 43, 1113–1121. [Google Scholar] [CrossRef]
- Miller, B.; Kim, S.J.; Kumagai, H.; Mehta, H.H.; Xiang, W.; Liu, J.; Yen, K.; Cohen, P. Peptides derived from small mitochondrial open reading frames: Genomic, biological, and therapeutic implications. Exp. Cell Res. 2020, 393, 112056. [Google Scholar] [CrossRef]
- Miller, B.; Kim, S.J.; Kumagai, H.; Yen, K.; Cohen, P. Mitochondria-derived peptides in aging and healthspan. J. Clin. Investig. 2022, 132, e158449. [Google Scholar] [CrossRef]
- Kim, S.J.; Xiao, J.; Wan, J.; Cohen, P.; Yen, K. Mitochondrially derived peptides as novel regulators of metabolism. J. Physiol. 2017, 595, 6613–6621. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Woodhead, J.S.T.; Hedges, C.P.; Zeng, N.; Wan, J.; Kumagai, H.; Lee, C.; Cohen, P.; Cameron-Smith, D.; Mitchell, C.J.; et al. Increased expression of the mitochondrial derived peptide, MOTS-c, in skeletal muscle of healthy aging men is associated with myofiber composition. Aging 2020, 12, 5244–5258. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tang, S.; Xue, C.; Liu, Y.; Wang, J.; Zhang, W.; Luo, W.; Chen, J. Mitochondrial-Derived Peptide MOTS-c Increases Adipose Thermogenic Activation to Promote Cold Adaptation. Int. J. Mol. Sci. 2019, 20, 2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.; Wei, M.; Zhai, Y.; Li, Q.; Ye, Z.; Wang, L.; Luo, W.; Chen, J.; Lu, Z. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J. Mol. Med. 2019, 97, 473–485. [Google Scholar] [CrossRef]
- Kim, S.J.; Miller, B.; Kumagai, H.; Yen, K.; Cohen, P. MOTS-c: An equal opportunity insulin sensitizer. J. Mol. Med. 2019, 97, 487–490. [Google Scholar] [CrossRef]
- Merry, T.L.; Chan, A.; Woodhead, J.S.T.; Reynolds, J.C.; Kumaga, H.; Kim, S.J.; Lee, C. Mitochondrial-derived peptides in energy metabolism. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E659–E666. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; de Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; Newman, A.B. Decreased muscle strength and quality in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes 2006, 55, 1813–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; Kuller, L.H.; Broudeau, R.; Kammerer, C.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: The health, aging, and body composition study. Diabetes Care 2007, 30, 1507–1512. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, H.; Coelho, A.R.; Wan, J.; Mehta, H.; Yen, K.; Huang, A.; Zempo, H.; Fuku, N.; Maeda, S.; Oliveira, P.J.; et al. MOTS-c reduces myostatin and muscle atrophy signaling. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E680–E690. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Lai, R.W.; Woodhead, J.S.T.; Joly, J.H.; Mitchell, C.J.; Cameron-Smith, D.; Lu, R.; Cohen, P.; Graham, N.A.; Benayoun, B.A.; et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat. Commun. 2021, 12, 470. [Google Scholar] [CrossRef]
- von Walden, F.; Fernandez-Gonzalo, R.; Norrbom, J.; Emanuelsson, E.B.; Figueiredo, V.C.; Gidlund, E.K.; Norrbrand, L.; Liu, C.; Sandstrom, P.; Hansson, B.; et al. Acute endurance exercise stimulates circulating levels of mitochondrial-derived peptides in humans. J. Appl. Physiol. 2021, 131, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Sami, N.; Norris, M.K.; Wan, J.; Kumagai, H.; Kim, S.-J.; Cohen, P. Effect of aerobic and resistance exercise on the mitochondrial peptide MOTS-c in Hispanic and Non-Hispanic White breast cancer survivors. Sci. Rep. 2021, 11, 16916. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, J.K. MOTS-c increases in skeletal muscle following long-term physical activity and improves acute exercise performance after a single dose. Physiol. Rep. 2022, 10, e15377. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.M.; Min, S.H.; Lee, C.H.; Kim, J.Y.; Lim, H.S.; Choi, M.J.; Jung, S.-B.; Park, J.W.; Kim, S.; Park, C.B.; et al. Mitohormesis in Hypothalamic POMC Neurons Mediates Regular Exercise-Induced High-Turnover Metabolism. Cell Metabolism. 2021, 33, 334–349.e336. [Google Scholar] [CrossRef] [PubMed]
- Ramanjaneya, M.; Jerobin, J.; Bettahi, I.; Bensila, M.; Aye, M.; Siveen, K.S.; Sathyapalan, T.; Skarulis, M.; Abou-Samra, A.B.; Atkin, S.L. Lipids and insulin regulate mitochondrial-derived peptide (MOTS-c) in PCOS and healthy subjects. Clin. Endocrinol. 2019, 91, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Son, J.M.; Benayoun, B.A.; Lee, C. The Mitochondrial-Encoded Peptide MOTS-c Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress. Cell Metabolism. 2018, 28, 516–524.e517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, J.; Chang, X.; Nie, Y.; Shen, Y.; Liang, X.; Peng, Y.; Chang, M. Peripheral Administration of a Cell-Penetrating MOTS-c Analogue Enhances Memory and Attenuates Aβ1–42- or LPS-Induced Memory Impairment through Inhibiting Neuroinflammation. ACS Chem. Neurosci. 2021, 12, 1506–1518. [Google Scholar] [CrossRef]
- Lin, J.; Wu, H.; Tarr, P.T.; Zhang, C.-Y.; Wu, Z.; Boss, O.; Michael, L.F.; Puigserver, P.; Isotani, E.; Olson, E.N.; et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 2002, 418, 797–801. [Google Scholar] [CrossRef]
- Handschin, C.; Chin, S.; Li, P.; Liu, F.; Maratos-Flier, E.; Lebrasseur, N.K.; Yan, Z.; Spiegelman, B.M. Skeletal Muscle Fiber-type Switching, Exercise Intolerance, and Myopathy in PGC-1α Muscle-specific Knock-out Animals. J. Biol. Chem. 2007, 282, 30014–30021. [Google Scholar] [CrossRef] [Green Version]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kai, Y.; Mizukami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H.; et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J. Biol. Chem. 2004, 279, 41114–41123. [Google Scholar] [CrossRef]
- Yang, B.; Yu, Q.; Chang, B.; Guo, Q.; Xu, S.; Yi, X.; Cao, S. MOTS-c interacts synergistically with exercise intervention to regulate PGC-1α expression, attenuate insulin resistance and enhance glucose metabolism in mice via AMPK signaling pathway. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2021, 1867, 166126. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Devgan, A.; Miller, B.; Lee, S.M.; Kumagai, H.; Wilson, K.A.; Wassef, G.; Wong, R.; Mehta, H.H.; Cohen, P.; et al. Humanin-induced autophagy plays important roles in skeletal muscle function and lifespan extension. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130017. [Google Scholar] [CrossRef] [PubMed]
- Woodhead, J.S.T.; D’Souza, R.F.; Hedges, C.P.; Wan, J.; Berridge, M.V.; Cameron-Smith, D.; Cohen, P.; Hickey, A.J.R.; Mitchell, C.J.; Merry, T.L. High-intensity interval exercise increases humanin, a mitochondrial encoded peptide, in the plasma and muscle of men. J. Appl. Physiol. 2020, 128, 1346–1354. [Google Scholar] [CrossRef]
- Gidlund, E.K.; von Walden, F.; Venojarvi, M.; Riserus, U.; Heinonen, O.J.; Norrbom, J.; Sundberg, C.J. Humanin skeletal muscle protein levels increase after resistance training in men with impaired glucose metabolism. Physiol. Rep. 2016, 4, e13063. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Ito, Y.; Niikura, T.; Shao, Z.; Hata, M.; Oyama, F.; Nishimoto, I. Mechanisms of neuroprotection by a novel rescue factor humanin from Swedish mutant amyloid precursor protein. Biochem. Biophys. Res. Commun. 2001, 283, 460–468. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kurita, M.; Aiso, S.; Nishimoto, I.; Matsuoka, M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol. Biol. Cell 2009, 20, 2864–2873. [Google Scholar] [CrossRef] [Green Version]
- Ying, G.; Iribarren, P.; Zhou, Y.; Gong, W.; Zhang, N.; Yu, Z.X.; Le, Y.; Cui, Y.; Wang, J.M. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J. Immunol. 2004, 172, 7078–7085. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Guerrero, N.; Wassef, G.; Xiao, J.; Mehta, H.H.; Cohen, P.; Yen, K. The mitochondrial-derived peptide humanin activates the ERK1/2, AKT, and STAT3 signaling pathways and has age-dependent signaling differences in the hippocampus. Oncotarget 2016, 7, 46899–46912. [Google Scholar] [CrossRef] [Green Version]
- Gong, Z.; Su, K.; Cui, L.; Tas, E.; Zhang, T.; Dong, H.H.; Yakar, S.; Muzumdar, R.H. Central effects of humanin on hepatic triglyceride secretion. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E283–E292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzumdar, R.H.; Huffman, D.M.; Atzmon, G.; Buettner, C.; Cobb, L.J.; Fishman, S.; Budagov, T.; Cui, L.; Einstein, F.H.; Poduval, A.; et al. Humanin: A Novel Central Regulator of Peripheral Insulin Action. PLoS ONE 2009, 4, e6334. [Google Scholar] [CrossRef]
- Kuliawat, R.; Klein, L.; Gong, Z.; Nicoletta-Gentile, M.; Nemkal, A.; Cui, L.; Bastie, C.; Su, K.; Huffman, D.; Surana, M.; et al. Potent humanin analog increases glucose-stimulated insulin secretion through enhanced metabolism in the beta cell. FASEB J. 2013, 27, 4890–4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanjaneya, M.; Bettahi, I.; Jerobin, J.; Chandra, P.; Abi Khalil, C.; Skarulis, M.; Atkin, S.L.; Abou-Samra, A.B. Mitochondrial-Derived Peptides Are Down Regulated in Diabetes Subjects. Front. Endocrinol. 2019, 10, 331. [Google Scholar] [CrossRef] [Green Version]
- Chin, Y.P.; Keni, J.; Wan, J.; Mehta, H.; Anene, F.; Jia, Y.; Lue, Y.H.; Swerdloff, R.; Cobb, L.J.; Wang, C.; et al. Pharmacokinetics and tissue distribution of humanin and its analogues in male rodents. Endocrinology 2013, 154, 3739–3744. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto-Mikami, E.; Fujita, Y.; Murakami, H.; Ito, M.; Miyachi, M.; Kawahara, T.; Fuku, N. CNTFR Genotype and Sprint/power Performance: Case-control Association and Functional Studies. Int. J. Sports Med. 2016, 37, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Nashine, S.; Cohen, P.; Nesburn, A.B.; Kuppermann, B.D.; Kenney, M.C. Characterizing the protective effects of SHLP2, a mitochondrial-derived peptide, in macular degeneration. Sci. Rep. 2018, 8, 15175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, A.K.; Teranishi, K.; Lobo, F.; Isas, J.M.; Xiao, J.; Yen, K.; Cohen, P.; Langen, R. The Mitochondrial-Derived Peptides, HumaninS14G and Small Humanin-like Peptide 2, Exhibit Chaperone-like Activity. Sci. Rep. 2017, 7, 7802. [Google Scholar] [CrossRef] [Green Version]
- Lillioja, S.; Young, A.A.; Culter, C.L.; Ivy, J.L.; Abbott, W.G.; Zawadzki, J.K.; Yki-Jarvinen, H.; Christin, L.; Secomb, T.W.; Bogardus, C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J. Clin. Invest. 1987, 80, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Fisher, G.; Windham, S.T.; Griffin, P.; Warren, J.L.; Gower, B.A.; Hunter, G.R. Associations of human skeletal muscle fiber type and insulin sensitivity, blood lipids, and vascular hemodynamics in a cohort of premenopausal women. Eur. J. Appl. Physiol. 2017, 117, 1413–1422. [Google Scholar] [CrossRef] [Green Version]
- Essen, B.; Jansson, E.; Henriksson, J.; Taylor, A.W.; Saltin, B. Metabolic characteristics of fibre types in human skeletal muscle. Acta Physiol. Scand. 1975, 95, 153–165. [Google Scholar] [CrossRef]
- Gollnick, P.D.; Matoba, H. The muscle fiber composition of skeletal muscle as a predictor of athletic success. Am. J. Sport. Med. 1984, 12, 212–217. [Google Scholar] [CrossRef]
- Tanner, C.J.; Barakat, H.A.; Dohm, G.L.; Pories, W.J.; MacDonald, K.G.; Cunningham, P.R.; Swanson, M.S.; Houmard, J.A. Muscle fiber type is associated with obesity and weight loss. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E1191–E1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, C.A.; McCurry, M.P.; Marino, A.; South, M.A.; Howell, M.E.; Layne, A.S.; Ramsey, M.W.; Stone, M.H. Slow-twitch fiber proportion in skeletal muscle correlates with insulin responsiveness. J. Clin. Endocrinol. Metab. 2013, 98, 2027–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houmard, J.A.; Weidner, M.L.; Koves, T.R.; Hickner, R.C.; Cortright, R.L. Association between muscle fiber composition and blood pressure levels during exercise in men. Am. J. Hypertens. 2000, 13, 586–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernelahti, M.; Tikkanen, H.O.; Karjalainen, J.; Kujala, U.M. Muscle fiber-type distribution as a predictor of blood pressure: A 19-year follow-up study. Hypertension 2005, 45, 1019–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leslie, M. Outsize impact. Science 2019, 366, 296–299. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumagai, H.; Miller, B.; Kim, S.-J.; Leelaprachakul, N.; Kikuchi, N.; Yen, K.; Cohen, P. Novel Insights into Mitochondrial DNA: Mitochondrial Microproteins and mtDNA Variants Modulate Athletic Performance and Age-Related Diseases. Genes 2023, 14, 286. https://doi.org/10.3390/genes14020286
Kumagai H, Miller B, Kim S-J, Leelaprachakul N, Kikuchi N, Yen K, Cohen P. Novel Insights into Mitochondrial DNA: Mitochondrial Microproteins and mtDNA Variants Modulate Athletic Performance and Age-Related Diseases. Genes. 2023; 14(2):286. https://doi.org/10.3390/genes14020286
Chicago/Turabian StyleKumagai, Hiroshi, Brendan Miller, Su-Jeong Kim, Naphada Leelaprachakul, Naoki Kikuchi, Kelvin Yen, and Pinchas Cohen. 2023. "Novel Insights into Mitochondrial DNA: Mitochondrial Microproteins and mtDNA Variants Modulate Athletic Performance and Age-Related Diseases" Genes 14, no. 2: 286. https://doi.org/10.3390/genes14020286







