Genomic Insights of Alnus-Infective Frankia Strains Reveal Unique Genetic Features and New Evidence on Their Host-Restricted Lifestyle
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Frankia Genomes from Databases
2.2. Genome Sequencing of a New Sp+ Frankia Strain Infective of Alnus cordata
2.3. Comparative Genome Analyses between Frankia Strains
3. Results and Discussion
3.1. Genome Sequencing of a New Alnus cordata-Infective Sp+ Frankia Strain
3.2. Identification of Candidate Molecules Responsible for Host Specificity in Cluster Ia
3.2.1. Specific Core Genome of Frankia Belonging to Cluster Ia
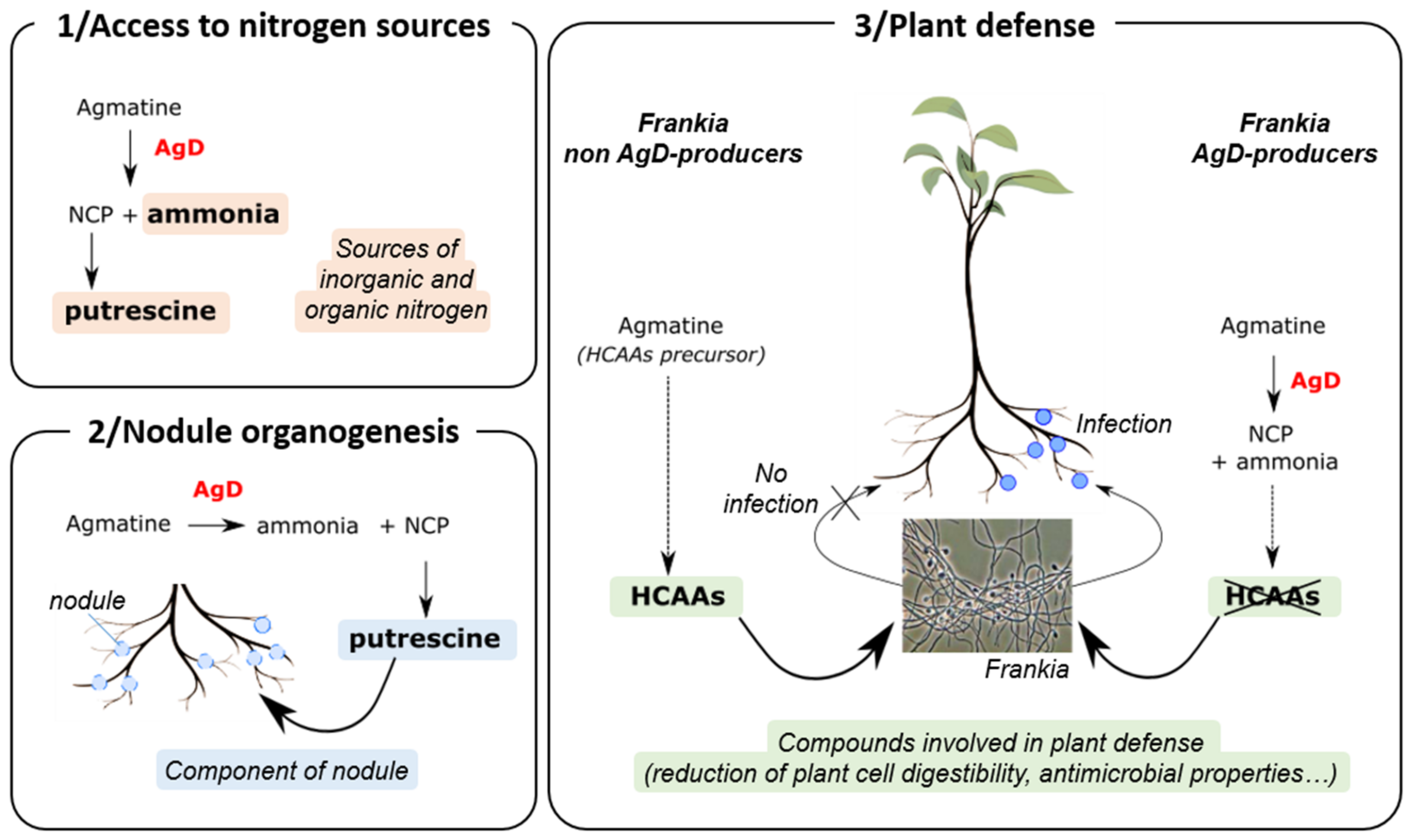
3.2.2. Agmatine Deiminase
3.3. What Genome Comparison Tells Us about Sp+ Alnus-Infective Frankia Strains
3.3.1. The Loss of Transcription-Associated Protein Sequences in Sp+ Frankia Genomes
3.3.2. A Reduced Secretome in Sp+ Frankia Strains
3.3.3. The Potential Loss of Saprophytic Functions in Sp+ Frankia Strains
3.3.4. The Loss of Genetic and Functional Redundancy in Sp+ Genomes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baker, D.D. Relationships among pure cultured strains of Frankia based on host specificity. Physiol. Plant. 1987, 70, 245–248. [Google Scholar] [CrossRef]
- Bosco, M.; Fernandez, M.; Simonet, P.; Materassi, R.; Normand, P. Evidence that some Frankia sp. strains are able to cross boundaries between Alnus and Elaeagnus Host Specificity Groups. Appl. Environ. Microbiol. 1992, 58, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- De Ley, J.; Rassel, A. DNA base composition, flagellation and taxonomy of the genus Rhizobium. J. Gen. Microbiol. 1965, 41, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kundu, B.; Dogra, R. Molecular mechanism of host specificity in Legume-Rhizobium symbiosis. Biotechnol. Adv. 1993, 11, 741–779. [Google Scholar] [CrossRef]
- Zakhia, F.; de Lajudie, P. Taxonomy of Rhizobia. Agronomie 2001, 21, 569–576. [Google Scholar] [CrossRef]
- Krieg, N.R.; Holt, J.G. Bergey’s Manual of Systematic Bacteriology; Yi Hsien Publishing Co.: Taipei, Taiwan, 1984; ISBN 0-683-04108-8. [Google Scholar]
- Normand, P.; Orso, S.; Cournoyer, B.; Jeannin, P.; Chapelon, C.; Dawson, J.; Evtushenko, L.; Misra, A.K. Molecular phylogeny of the genus Frankia and related genera and emendation of the family Frankiaceae. Int. J. Syst. Evol. Microbiol. 1996, 46, 1–9. [Google Scholar] [CrossRef]
- Pozzi, A.C.; Bautista-Guerrero, H.H.; Abby, S.S.; Herrera-Belaroussi, A.; Abrouk, D.; Normand, P.; Menu, F.; Fernandez, M.P. Robust Frankia Phylogeny, species delineation and intraspecies diversity based on Multi-Locus Sequence Analysis (MLSA) and Single-Locus Strain Typing (SLST) adapted to a large sample size. Syst. Appl. Microbiol. 2018, 41, 311–323. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Wibberg, D.; Vigil-Stenman, T.; Berckx, F.; Battenberg, K.; Demchenko, K.N.; Blom, J.; Fernandez, M.P.; Yamanaka, T.; Berry, A.M.; et al. Frankia-enriched metagenomes from the earliest diverging symbiotic Frankia Cluster: They come in teams. Genome Biol. Evol. 2019, 11, 2273–2291. [Google Scholar] [CrossRef]
- Benson, D.R.; Silvester, W.B. Biology of Frankia strains, Actinomycete symbionts of Actinorhizal plants. Microbiol. Mol. Biol. Rev. 1993, 57, 293–319. [Google Scholar] [CrossRef]
- Dawson, J.O. Ecology of actinorhizal plants. In Nitrogen-Fixing Actinorhizal Symbioses-Nitrogen Fixation Research: Origins and Progress; Pawlowski, K., Newton, W.E., Eds.; Springer: New York, NY, USA, 2008; Volume 6, pp. 199–234. [Google Scholar]
- Cotin-Galvan, L.; Pozzi, A.C.; Schwob, G.; Fournier, P.; Fernandez, M.P.; Herrera-Belaroussi, A. In-Planta Sporulation Capacity enhances infectivity and rhizospheric competitiveness of Frankia strains. Microbes Environ. 2016, 31, 11–18. [Google Scholar] [CrossRef]
- Schwintzer, C.R. Spore-Positive and Spore-Negative nodules. In The Biology of Frankia and Actinorhizal Plants; Academic Press: Cambridge, MA, USA, 1990; pp. 177–193. [Google Scholar]
- Weber, A.; Nurmiaho-Lassila, E.; Sundman, V. Features of the intrageneric Alnus-Frankia specificity. Physiol. Plant. 1987, 70, 289–296. [Google Scholar] [CrossRef]
- Kurdali, F.; Domenach, A.-M.; Fernandez, M.D.L.P.; Capellano, A.; Moiroud, A. Compatibility of Frankiae Spore Positive and Spore Negative inocula with Alnus glutinosa and Alnus incana. Soil Sci. Plant Nutr. 1988, 34, 451–459. [Google Scholar] [CrossRef]
- Markham, J.H. Variability of nitrogen-fixing Frankia on Alnus species. Botany 2008, 86, 501–510. [Google Scholar] [CrossRef]
- Van Dijk, C. Spore Formation and endophyte diversity in root nodules of Alnus Glutinosa (L.) Vill. New Phytol. 1978, 81, 601–615. [Google Scholar] [CrossRef]
- Torrey, J.G. Endophyte sporulation in root nodules of Actinorhizal plants. Physiol. Plant. 1987, 70, 279–288. [Google Scholar] [CrossRef]
- Schwob, G.; Roy, M.; Pozzi, A.; Herrera-Belaroussi, A.; Fernandez, M. In Planta Sporulation of Frankia Spp. as a Determinant of Alder-Symbiont Interactions. Appl. Environ. Microbiol. 2018, 84, e01737-18. [Google Scholar] [CrossRef]
- Bethencourt, L.; Vautrin, F.; Taib, N.; Dubost, A.; Castro-Garcia, L.; Imbaud, O.; Abrouk, D.; Fournier, P.; Briolay, J.; Nguyen, A.; et al. Draft genome sequences for three unisolated Alnus-infective Frankia Sp+ strains, AgTrS, AiOr and AvVan, the first sequenced Frankia strains able to sporulate in-planta. J. Genom. 2019, 7, 50–55. [Google Scholar] [CrossRef]
- Pozzi, A.C.M.; Herrera-Belaroussi, A.; Schwob, G.; Bautista-Guerrero, H.H.; Bethencourt, L.; Fournier, P.; Dubost, A.; Abrouk, D.; Normand, P.; Fernandez, M.P. Proposal of ‘Candidatus Frankia alpina’, the uncultured symbiont of Alnus alnobetula and A. incana that forms spore-containing nitrogen-fixing root nodules. Int. J. Syst. Evol. Microbiol. 2020, 70, 5453–5459. [Google Scholar] [CrossRef]
- Herrera-Belaroussi, A.; Normand, P.; Pawlowski, K.; Fernandez, M.P.; Wibberg, D.; Kalinowski, J.; Brachmann, A.; Berckx, F.; Lee, N.; Blom, J.; et al. Candidatus Frankia nodulisporulans sp. nov., an Alnus glutinosa-infective Frankia species unable to grow in pure culture and able to sporulate in-planta. Syst. Appl. Microbiol. 2020, 43, 126134. [Google Scholar] [CrossRef]
- Cérémonie, H.; Debellé, F.; Fernandez, M.P. Structural and functional comparison of Frankia root hair deforming factor and Rhizobia Nod factor. Can. J. Bot. 1999, 77, 1293–1301. [Google Scholar]
- Normand, P.; Lapierre, P.; Tisa, L.S.; Gogarten, J.P.; Alloisio, N.; Bagnarol, E.; Bassi, C.A.; Berry, A.M.; Bickhart, D.M.; Choisne, N.; et al. Genome characteristics of facultatively symbiotic Frankia Sp. strains reflect Host Range and Host Plant Biogeography. Genome Res. 2007, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Franche, C.; Lindström, K.; Elmerich, C. Nitrogen-fixing bacteria associated with Leguminous and non-Leguminous plants. Plant Soil 2009, 321, 35–59. [Google Scholar] [CrossRef]
- Persson, T.; Battenberg, K.; Demina, I.V.; Vigil-Stenman, T.; Vanden Heuvel, B.; Pujic, P.; Facciotti, M.T.; Wilbanks, E.G.; O’Brien, A.; Fournier, P.; et al. Candidatus Frankia datiscae Dg1, the Actinobacterial microsymbiont of Datisca glomerata, expresses the Canonical Nod genes NodABC in symbiosis with its host plant. PLoS ONE 2015, 10, e0127630. [Google Scholar] [CrossRef] [PubMed]
- Udwary, D.W.; Gontang, E.A.; Jones, A.C.; Jones, C.S.; Schultz, A.W.; Winter, J.M.; Yang, J.Y.; Beauchemin, N.; Capson, T.L.; Clark, B.R.; et al. Significant natural product biosynthetic potential of Actinorhizal symbionts of the genus Frankia, as revealed by comparative genomic and proteomic analyses. Appl. Environ. Microbiol. 2011, 77, 3617–3625. [Google Scholar] [CrossRef] [PubMed]
- Deicke, M.; Mohr, J.F.; Roy, S.; Herzsprung, P.; Bellenger, J.-P.; Wichard, T. Metallophore profiling of Nitrogen-Fixing Frankia Spp. to understand metal management in the rhizosphere of Actinorhizal plants. Metallomics 2019, 11, 810–821. [Google Scholar] [CrossRef]
- Nouioui, I.; Cortés-Albayay, C.; Carro, L.; Castro, J.F.; Gtari, M.; Ghodhbane-Gtari, F.; Klenk, H.-P.; Tisa, L.S.; Sangal, V.; Goodfellow, M. Genomic insights into Plant-Growth-Promoting potentialities of the genus Frankia. Front. Microbiol. 2019, 10, 1457. [Google Scholar] [CrossRef]
- Carlos-Shanley, C.; Guerra, T.; Hahn, D. Draft genomes of non-nitrogen-fixing Frankia strains. J. Genom. 2021, 9, 68–75. [Google Scholar] [CrossRef]
- Beauchemin, N.; Gtari, M.; Ghodhbane-Gtari, F.; Furnholm, T.; Sen, A.; Wall, L.; Tisa, L.S. What can the genome of an infective ineffective (Fix-) Frankia strain (EuI1c) that is able to form nodules with its host plant tell us about Actinorhizal symbiosis and Frankia evolution. In Proceedings of the 112th General Meeting of the American Society for Microbiology, San Francisco, CA, USA, 16–19 June 2012. [Google Scholar]
- Tisa, L.S.; Beauchemin, N.; Gtari, M.; Sen, A.; Wall, L.G. What Stories Can the Frankia Genomes Start to Tell Us? J. Biosci. 2013, 38, 719–726. [Google Scholar] [CrossRef]
- Tisa, L.S.; Oshone, R.; Sarkar, I.; Ktari, A.; Sen, A.; Gtari, M. Genomic approaches toward understanding the Actinorhizal symbiosis: An update on the status of the Frankia genomes. Symbiosis 2016, 70, 5–16. [Google Scholar] [CrossRef]
- Pozzi, A.C.; Roy, M.; Nagati, M.; Schwob, G.; Manzi, S.; Gardes, M.; Moreau, P.-A.; Fernandez, M.P. Patterns of diversity, endemism and specialization in the root symbiont communities of Alder species on the island of Corsica. New Phytol. 2018, 219, 336–349. [Google Scholar] [CrossRef]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Vallenet, D.; Calteau, A.; Cruveiller, S.; Gachet, M.; Lajus, A.; Josso, A.; Mercier, J.; Renaux, A.; Rollin, J.; Rouy, Z.; et al. MicroScope in 2017: An expanding and evolving integrated resource for community expertise of microbial genomes. Nucleic Acids Res. 2017, 45, D517–D528. [Google Scholar] [CrossRef]
- Goris, J.; Konstantinidis, K.T.; Klappenbach, J.A.; Coenye, T.; Vandamme, P.; Tiedje, J.M. DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int. J. Syst. Evol. Microbiol. 2007, 57, 81–91. [Google Scholar] [CrossRef]
- Blom, J.; Kreis, J.; Spänig, S.; Juhre, T.; Bertelli, C.; Ernst, C.; Goesmann, A. EDGAR 2.0: An enhanced software platform for comparative gene content analyses. Nucleic Acids Res. 2016, 44, W22–W28. [Google Scholar] [CrossRef]
- Penel, S.; Arigon, A.-M.; Dufayard, J.-F.; Sertier, A.-S.; Daubin, V.; Duret, L.; Gouy, M.; Perrière, G. Databases of homologous gene families for comparative genomics. BMC Bioinform. 2009, 10, S3. [Google Scholar] [CrossRef]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden Markov Model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Annika, C.M.; Justice, N.B.; Bowen, B.P.; Baran, R.; Thomas, B.C.; Northern, T.R.; Banfield, J.F. Metabolites associated with adaptation of microorganisms to an acidophilic, metal-rich environment identified by Stable-Isotope-enabled metabolomics. mBio 2013, 4, e00484-12. [Google Scholar] [CrossRef]
- Tan, Y.; Shan, Y.; Zheng, R.; Liu, R.; Sun, C. Characterization of a Deep-Sea Actinobacterium Strain Uncovers Its Prominent Capability of Utilizing Taurine and Polyvinyl Alcohol. Front. Microbiol. 2022, 13, 868728. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI Search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.; Dreyton, C.J.; Flick, H.; Causey, C.P.; Thompson, P.R. Mechanistic studies of Agmatine Deiminase from multiple bacterial species. Biochemistry 2010, 49, 9413–9423. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, C.T.; Tonin, G.S.; Sutcliffe, A. Poly Amines of Frankia in relation to nitrogen nutrition. Soil Biol. Biochem. 1994, 26, 577–581. [Google Scholar] [CrossRef]
- Smith, T.A. Homospermidine in Rhizobium and Legume root nodules. Phytochemistry 1977, 16, 278–279. [Google Scholar] [CrossRef]
- Chatterjee, S.; Choudhuri, M.; Ghosh, B. Changes in Polyamine contents during root and nodule growth of Phaseolus Mungo. Phytochemistry 1983, 22, 1553–1556. [Google Scholar] [CrossRef]
- Tonin, G.; Wheeler, C.; Crozier, A. Effect of changes in Nitrogen nutrition on the polyamine content of Alnus glutinosa. Plant Cell Environ. 1991, 14, 415–421. [Google Scholar] [CrossRef]
- Muroi, A.; Ishihara, A.; Tanaka, C.; Ishizuka, A.; Takabayashi, J.; Miyoshi, H.; Nishioka, T. Accumulation of Hydroxycinnamic Acid Amides induced by pathogen infection and identification of Agmatine Coumaroyltransferase in Arabidopsis thaliana. Planta 2009, 230, 517–527. [Google Scholar] [CrossRef]
- Facchini, P.J.; Hagel, J.; Zulak, K.G. Hydroxycinnamic Acid Amide metabolism: Physiology and biochemistry. Can. J. Bot. 2002, 80, 577–589. [Google Scholar] [CrossRef]
- Roumani, M.; Duval, R.E.; Ropars, A.; Risler, A.; Robin, C.; Larbat, R. Phenolamides: Plant specialized metabolites with a wide range of promising pharmacological and health-promoting interests. Biomed. Pharmacother. 2020, 131, 110762. [Google Scholar] [CrossRef]
- Mayama, S.; Tani, T.; Matsuura, Y.; Ueno, T.; Fukami, H. The production of Phytoalexins by Oat in response to Crown Rust, Puccinia coronata f. sp. avenae. Physiol. Plant Pathol. 1981, 19, 217–226. [Google Scholar] [CrossRef]
- Mayama, S.; Matsuura, Y.; Iida, H.; Tani, T. The role of Avenalumin in the Resistance of Oat to Crown Rust, Puccinia coronata f. sp. avenae. Physiol. Plant Pathol. 1982, 20, 189–199. [Google Scholar] [CrossRef]
- Miyagawa, H.; Ishihara, A.; Nishimoto, T.; Ueno, T.; Mayama, S. induction of Avenanthramides in Oat leaves inoculated with Crown Rust fungus, Puccinia coronata f. sp. avenae. Biosci. Biotechnol. Biochem. 1995, 59, 2305–2306. [Google Scholar] [CrossRef]
- Pozzi, A.C.; Bautista-Guerrero, H.H.; Nouioui, I.; Cotin-Galvan, L.; Pepin, R.; Fournier, P.; Menu, F.; Fernandez, M.P.; Herrera-Belaroussi, A. In-planta Sporulation Phenotype: A Major life history trait to understand the evolution of Alnus-infective Frankia strains. Environ. Microbiol. 2015, 17, 3125–3138. [Google Scholar] [CrossRef]
- Galan-Vasquez, E.; Sanchez-Osorio, I.; Martinez-Antonio, A. Transcription factors exhibit differential conservation in bacteria with reduced genomes. PLoS ONE 2016, 11, e0146901. [Google Scholar] [CrossRef]
- Miravet-Verde, S.; Lloréns-Rico, V.; Serrano, L. Alternative transcriptional regulation in genome-reduced bacteria. Curr. Opin. Microbiol. 2017, 39, 89–95. [Google Scholar] [CrossRef]
- Mastronunzio, J.E.; Tisa, L.S.; Normand, P.; Benson, D.R. Comparative secretome analysis suggests low plant cell wall degrading capacity in Frankia Symbionts. BMC Genom. 2008, 9, 47. [Google Scholar] [CrossRef]
- Mastronunzio, J.; Huang, Y.; Benson, D. Diminished exoproteome of Frankia spp. in culture and symbiosis. Appl. Environ. Microbiol. 2009, 75, 6721–6728. [Google Scholar] [CrossRef]
- Capyk, J.K.; D’Angelo, I.; Strynadka, N.C.; Eltis, L.D. Characterization of 3-Ketosteroid 9α-Hydroxylase, a rieske oxygenase in the Cholesterol Degradation Pathway of Mycobacterium tuberculosis. J. Biol. Chem. 2009, 284, 9937–9946. [Google Scholar] [CrossRef]
- Rohman, A.; Dijkstra, B.W. The Role and Mechanism of Microbial 3-Ketosteroid Δ1-Dehydrogenases in steroid breakdown. J. Steroid Biochem. Mol. Biol. 2019, 191, 105366. [Google Scholar] [CrossRef]
- Holert, J.; Cardenas, E.; Bergstrand, L.H.; Zaikova, E.; Hahn, A.S.; Hallam, S.J.; Mohn, W.W. Metagenomes reveal global distribution of bacterial steroid catabolism in natural, engineered, and host environments. mBio 2018, 9, e02345-17. [Google Scholar] [CrossRef]
- Marques, M.A.M.; Berrêdo-Pinho, M.; Rosa, T.L.; Pujari, V.; Lemes, R.M.; Lery, L.M.; Silva, C.A.M.; Guimarães, A.C.R.; Atella, G.C.; Wheat, W.H.; et al. The essential role of Cholesterol metabolism in the intracellular survival of Mycobacterium leprae is not coupled to central carbon metabolism and energy production. J. Bacteriol. 2015, 197, 3698–3707. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Solhtalab, M.; Thongsomboon, W.; Aristilde, L. Strategies of organic Phosphorus recycling by soil bacteria: Acquisition, metabolism, and regulation. Environ. Microbiol. Rep. 2022, 14, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R. Hydrogenases and efficiency of nitrogen fixation in Aerobes. Nature 1976, 262, 173. [Google Scholar] [CrossRef]
- Leul, M.; Normand, P.; Sellstedt, A. The organization, regulation and phylogeny of uptake Hydrogenase genes in Frankia. Physiol. Plant. 2007, 130, 464–470. [Google Scholar] [CrossRef]
- Leul, M.; Normand, P.; Sellstedt, A. The phylogeny of uptake Hydrogenases in Frankia. Int. Microbiol. 2009, 12, 23–28. [Google Scholar]
- Corda, D.; Mosca, M.G.; Ohshima, N.; Grauso, L.; Yanaka, N.; Mariggiò, S. The emerging physiological roles of the Glycerophosphodiesterase family. FEBS J. 2014, 281, 998–1016. [Google Scholar] [CrossRef]
- De Carvalho, C.C.; Caramujo, M.J. The various roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The Carbohydrate-Active Enzyme Database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.; Zhang, L.; Wang, J.; Wu, C. Glycosyltransferase GT1 Family: Phylogenetic distribution, substrates coverage, and representative structural features. Comput. Struct. Biotechnol. J. 2020, 18, 1383–1390. [Google Scholar] [CrossRef]
- Bolam, D.N.; Roberts, S.; Proctor, M.R.; Turkenburg, J.P.; Dodson, E.J.; Martinez-Fleites, C.; Yang, M.; Davis, B.G.; Davies, G.J.; Gilbert, H.J. The Crystal Structure of Two Macrolide Glycosyltransferases Provides a Blueprint for Host Cell Antibiotic Immunity. Proc. Natl. Acad. Sci. USA 2007, 104, 5336–5341. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Zhang, P.; Wang, Y.; Li, Z.; Li, X.; Wang, R.; Shang, Z.; Yan, J.; He, H.; et al. Bacillus licheniformis escapes from Myxococcus xanthus predation by deactivating Myxovirescin a through enzymatic glucosylation. Environ. Microbiol. 2019, 21, 4755–4772. [Google Scholar] [CrossRef] [PubMed]
- Yakovlieva, L.; Fülleborn, J.A.; Walvoort, M.T. Opportunities and challenges of bacterial Glycosylation for the development of novel antibacterial strategies. Front. Microbiol. 2021, 12, 745702. [Google Scholar] [CrossRef] [PubMed]
- Bethencourt, L.; Boubakri, H.; Taib, N.; Normand, P.; Armengaud, J.; Fournier, P.; Brochier-Armanet, C.; Herrera-Belaroussi, A. Comparative genomics and proteogenomics highlight key molecular players involved in Frankia sporulation. Res. Microbiol. 2019, 170, 202–213. [Google Scholar] [CrossRef] [PubMed]
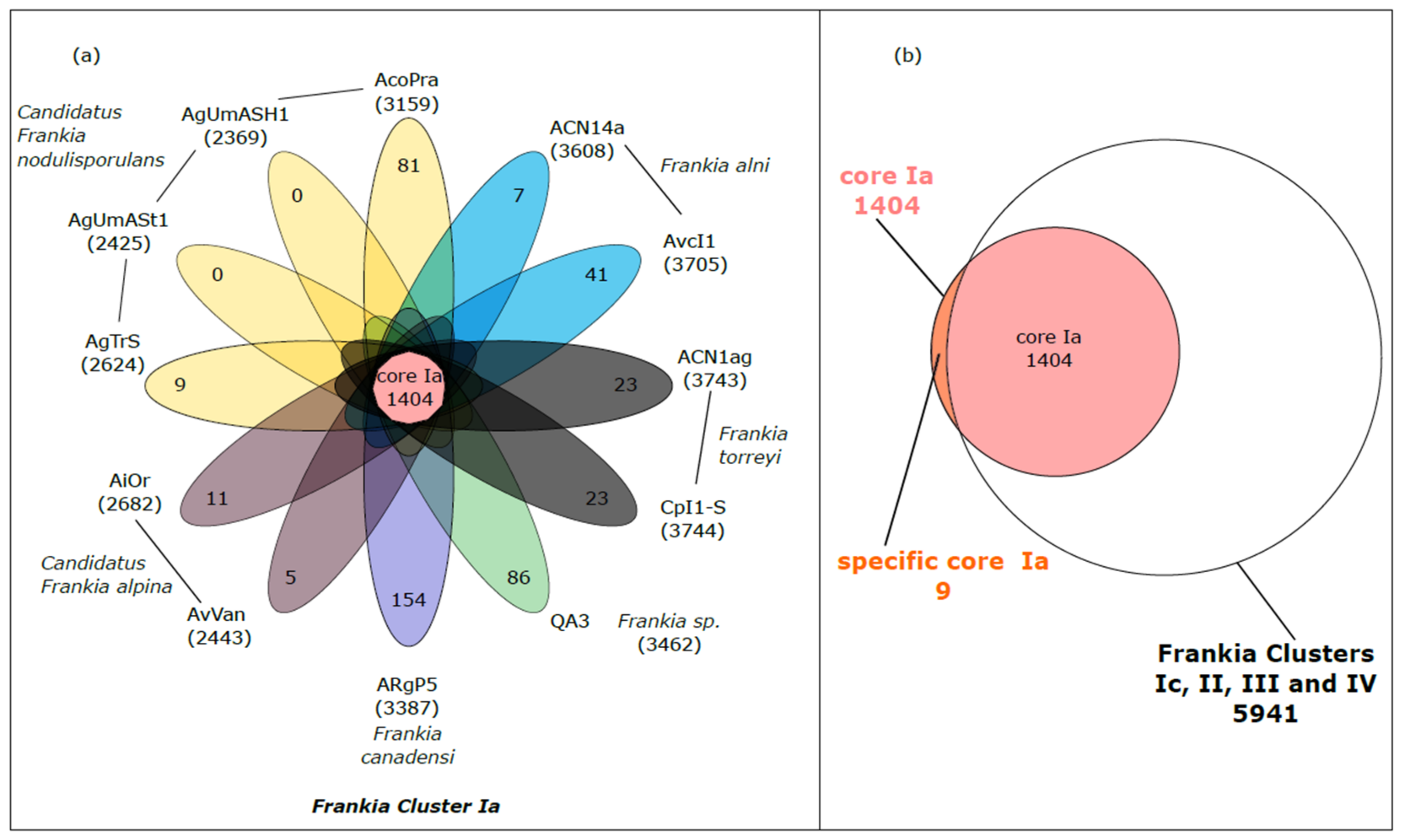
| Frankia Strain | Cluster | Genome Size (pb) | Number of Contig | CheckM Completeness (%) | GC% | Total Number of CDS * | Accession Number |
|---|---|---|---|---|---|---|---|
| Candidatus Frankia nodulisporulans AgTrS | Ia | 4,943,752 | 612 | 98.09 | 71.61 | 5178 | NZ_CADCWS010000612.1 |
| Candidatus Frankia nodulisporulans AgUmASt1 | Ia | 4,311,763 | 304 | 98.63 | 71.34 | 3665 | CADDZU010000001 |
| Candidatus Frankia nodulisporulans AgUmASH1 | Ia | 4,285,763 | 231 | 97.54 | 71.23 | 3652 | CADDZW010000001 |
| Candidatus Frankia alpina AiOr | Ia | 5,571,616 | 669 | 99.38 | 71.57 | 6192 | GCA_902806485 |
| Candidatus Frankia alpina AvVan | Ia | 5,009,155 | 1233 | 98.10 | 71.34 | 5157 | GCA_004803575 |
| Frankia alni ACN14a | Ia | 7,497,934 | 1 | 100 | 72.83 | 6714 | NC_008278.1 |
| Frankia alni AvcI1 | Ia | 7,741,902 | 77 | 99.65 | 72.60 | 7255 | LJFZ01000001.1 |
| Frankia sp. QA3 | Ia | 7,590,853 | 120 | 100 | 72.59 | 7307 | CM001489.1 |
| Frankia torreyi CpI1-S | Ia | 7,639,958 | 153 | 99.38 | 72.43 | 7201 | JYFN00000000.1 |
| Frankia torreyi ACN1ag | Ia | 7,521,047 | 108 | 99.37 | 72,50 | 5687 | LJPA01000001.1 |
| Frankia canadensi ARgP5 | Ia | 7,730,285 | 568 | 99.73 | 72.39 | 7500 | OESX01000001 |
| Frankia casuarinae CcI3 | Ic | 5,433,628 | 1 | 99.59 | 70.08 | 5593 | CP000249.1 |
| Frankia casuarinae CcI6 | Ic | 5,592,323 | 138 | 99.59 | 69.99 | 5837 | GCA_000503735.2 |
| Frankia casuarinae Thr | Ic | 5,298,125 | 184 | _ | _ | 4654 | NZ_JENI00000000.1 |
| Frankia casuarinae Allo2 | Ic | 5,352,110 | 110 | _ | 70.00 | 4368 | GCA_000733325.1 |
| Frankia casuarinae BR | Ic | 5,227,240 | 180 | _ | _ | 6478 | NZ_LRTJ00000000.1 |
| Frankia casuarinae CeD | Ic | 5,004,600 | 120 | _ | 70.10 | 3937 | GCA_000732115.1 |
| Frankia casuarinae KB5 | Ic | 5,455,564 | 420 | 97.40 | 70.10 | 4915 | NZ_MRUJ00000000.1 |
| Candidatus Frankia datiscae Dg1 | II | 5,341,139 | 1 | 98.36 | 70.04 | 5472 | CP002801 |
| Frankia coriaria BMG5.1 | II | 5,806,763 | 116 | 95.31 | 70.24 | 6487 | JWIO00000000 |
| Candidatus Frankia californiensis Dg2 | II | 6,180,138 | 2742 | 89.39 | 67.99 | 7838 | FLUV00000000 |
| Frankia meridionalis Cppng1 | II | 4,858,260 | 1 | _ | 68,10 | 4968 | PRJEB19438 |
| Frankia discariae BCU110501 | III | 7,907,741 | 200 | 100 | 72.39 | 7567 | ARDT00000000 |
| Frankia sp. EAN1pec | III | 8,982,042 | 1 | 100 | 71.15 | 9063 | NC_009921.1 |
| Frankia sp. EUN1f | III | 9,392,240 | 396 | 98.37 | 70.81 | 9728 | ADGX01000001.1 |
| Frankia elaeagni BMG5.12 | III | 7,602,436 | 136 | 98.62 | 71.67 | 6977 | ARFH00000000 |
| Frankia irregularis G2 | III | 9,538,404 | 83 | 99.46 | 70.95 | 8663 | FAOZ00000000 |
| Frankia soli NRRL B-16219 | III | 8,032,739 | 289 | _ | 71.70 | 7114 | MN238860.1 |
| Frankia inefficax EuI1c | IV | 8,815,781 | 1 | 100 | 72.31 | 8099 | CP002299.1 |
| Frankia sp. DC12 | IV | 6,884,336 | 12 | 100 | 71.93 | 6630 | KQ031391.1 |
| Frankia saprophytica CN3 | IV | 9,978,692 | 2 | 98.35 | 71.81 | 9262 | AGJN00000000 |
| Frankia asymbiotica M16386 | IV | 9,453,064 | 174 | 100 | 71.97 | 8884 | MOMC00000000 |
| Frankia sp. QA3 | Candidatus Frankia Alpina AiOr | Candidatus Frankia Alpina AvVan | Frankia alni ACN14a | Frankia alni AvcI1 | Frankia torreyi ACN1ag | Frankia torreyi CpI1 | Frankia canadensis ARgP5 | Frankia sp. AcoPra | Candidatus Frankia Nodulisporulans AgTrs | |
|---|---|---|---|---|---|---|---|---|---|---|
| Frankia sp. QA3 | 100.0 | 90.5 | 91.1 | 89.9 | 89.8 | 90.8 | 90.7 | 79.9 | 78.0 | 78.9 |
| Candidatus Frankia alpina AiOr | 90.1 | 100.0 | 99.3 | 88.3 | 88.3 | 89.3 | 89.2 | 79.6 | 77.7 | 78.8 |
| Candidatus Frankia alpina AvVan | 90.6 | 99.3 | 100.0 | 88.8 | 88.8 | 89.6 | 89.6 | 80.5 | 78.7 | 79.4 |
| Frankia alni ACN14a | 90.0 | 88.9 | 89.5 | 100.0 | 99.7 | 92.1 | 92.1 | 79.6 | 77.8 | 78.9 |
| Frankia alni AvcI1 | 89.9 | 88.8 | 89.4 | 99.7 | 100.0 | 92.0 | 92.0 | 79.4 | 77.7 | 78.9 |
| Frankia torreyi ACN1ag | 90.8 | 89.8 | 90.3 | 92.1 | 92.0 | 100.0 | 99.9 | 79.5 | 77.7 | 78.8 |
| Frankia torreyi CpI1 | 90.9 | 89.8 | 90.3 | 92.2 | 92.0 | 99.9 | 100.0 | 79.4 | 77.7 | 78.8 |
| Frankia canadensis ARgP5 | 79.9 | 80.4 | 81.3 | 79.3 | 79.4 | 79.6 | 79.4 | 100.0 | 78.5 | 79.4 |
| Frankia sp. AcoPra | 77.7 | 78.0 | 78.9 | 77.5 | 77.3 | 77.3 | 77.3 | 78.3 | 100.0 | 98.0 |
| Candidatus Frankia nodulisporulans AgTrs | 78.0 | 78.6 | 79.3 | 78.0 | 77.8 | 77.8 | 77.7 | 78.5 | 97.9 | 100.0 |
| Product | Localization | EC Number | Pathway | Frankia alni ACN14a | |||
|---|---|---|---|---|---|---|---|
| N° Accession | Begin | End | Length (pb) | ||||
| Flavodoxin domain-containing protein | FRAAL2448 | 2,667,169 | 2,667,918 | 750 | |||
| Putative signal peptide | SP | FRAAL6541 | 7,118,052 | 7,118,477 | 426 | ||
| Hypothetical protein | FRAAL4761 | 5,156,216 | 5,157,130 | 915 | |||
| Hypothetical protein | SP | FRAAL1649 | 1,769,411 | 1,769,734 | 324 | ||
| Hypothetical protein | FRAAL0667 | 728,649 | 729,065 | 417 | |||
| Agmatine deiminase | EC:3.5.3.12 | arginine catabolism | FRAAL0164 | 158,747 | 159,802 | 1056 | |
| Putative esterase/acetylhydrolase domains-containing protein | SP | FRAAL0169 | 163,780 | 164,418 | 639 | ||
| Hypothetical integral membrane protein | TM | FRAAL4245 | 4,608,083 | 4,608,523 | 441 | ||
| Sulfite exporter TauE/SafE family protein | TM | FRAAL4244 | 4,607,181 | 4,608,086 | 906 | ||
| Species | Strain | AcoPra | AgTrS | AgUmASt1 | AgUmASH1 | ACN14a | AvcI1 | CpI1-S | ACN1ag | AvVan | AiOr | ARgP5 | QA3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Candidatus Frankia nodulisporulans | AcoPra | ||||||||||||
| AgTrS | 98.82 | ||||||||||||
| AgUmASt1 | 98.82 | 100 | |||||||||||
| AgUmASH1 | 98.82 | 100 | 100 | ||||||||||
| Frankia alni | ACN14a | 79.01 | 79.17 | 79.17 | 79.17 | ||||||||
| AvcI1 | 78.4 | 78.57 | 78.57 | 78.57 | 99.43 | ||||||||
| Frankia torreyi | CpI1-S | 79.32 | 79.46 | 79.46 | 79.46 | 93.16 | 92.59 | ||||||
| ACN1ag | 79.63 | 79.76 | 79.76 | 79.76 | 93.45 | 92.88 | 99.72 | ||||||
| Candidatus Frankia alpina | AvVan | 79.32 | 79.46 | 79.46 | 79.46 | 90.88 | 90.31 | 90.03 | 90.31 | ||||
| AiOr | 79.94 | 80.06 | 80.06 | 80.06 | 90.88 | 90.31 | 90.03 | 90.31 | 98.86 | ||||
| Frankia canadensi | ARgP5 | 77.23 | 77.15 | 77.15 | 77.15 | 80.12 | 79.54 | 79.83 | 80.12 | 78.1 | 78.1 | ||
| Frankia sp. | QA3 | 80.56 | 80.36 | 80.36 | 80.36 | 91.45 | 90.88 | 90.88 | 91.17 | 93.45 | 93.45 | 80.98 |
| COG | N° Accession in Frankia alni ACN14a | Gene Name | Product | Genetic or Functional Paralog * | Localization # | |
|---|---|---|---|---|---|---|
| Several Copies | ||||||
| C | Energy production and conversion | FRAAL2393 | hupL1 | Uptake hydrogenase large subunit | FRAAL1829 | |
| FRAAL2391 | hupD1 | Hydrogenase maturation protein | FRAAL1828 | |||
| FRAAL2392 | hupS1 | Uptake hydrogenase small subunit precursor | FRAAL1830 | SP | ||
| FRAAL3522 | Putative Formyl-CoA transferase | FRAAL4675 | ||||
| FRAAL3876 | Putative acyl-CoA transferases/carnitine dehydratase | FRAAL4764 | ||||
| FRAAL2565 | Putative polyketide oxygenase/hydroxylase | FRAAL4792, FRAAL2325, FRAAL3051, FRAAL3395 | ||||
| FRAAL3041 | Putative Dihydrolipoamide acyltransferases | FRAAL5152 | ||||
| E | Amino acid transport and metabolism | FRAAL6516 | Putative membrane protein | FRAAL1256 | TM | |
| I | Lipid transport and metabolism | FRAAL2505 | atoD | Acetoacetyl-CoA transferase | FRAAL2504, FRAAL3148, FRAAL3149 | |
| FRAAL4765 | Putative enoyl-CoA hydratase | FRAAL2509, FRAAL2514, FRAAL3092, FRAAL3517, FRAAL3973, FRAAL5910, FRAAL6774 | ||||
| FRAAL1660 | Putative Acyl-CoA dehydrogenase | FRAAL6459 | ||||
| J | Translation, ribosomal structure and biogenesis | FRAAL4260 | Putative glutamyl-tRNA(Gln) amidotransferase, subunit A | FRAAL0363, FRAAL3665, FRAAL6013, FRAAL6173 | ||
| K | Transcription | FRAAL2359 | Putative tetR family transcriptional regulator | FRAAL4751 | ||
| FRAAL1892 | Putative HTH-type transcriptional regulator | FRAAL4821 | ||||
| FRAAL6046 | Transcriptional regulator (MerR-family) | FRAAL6751 | ||||
| FRAAL1282 | Putative merR family transcriptional regulator | FRAAL6823 | ||||
| L | Replication, recombination and repair | FRAAL5342 | Hypothetical protein; putative DNA helicase IIhomolog | FRAAL0267 | ||
| FRAAL6137 | Putative ribosylglycoyhydrolase | FRAAL0303, FRAAL5802, FRAAL6736 | ||||
| P | Inorganic ion transport and metabolism | FRAAL1452 | Putative ABC transporter, permease protein | FRAAL1453, FRAAL1557 | TM | |
| Q | Secondary metabolites biosynthesis, transport and catabolism | FRAAL3901 | Putative Phytoene dehydrogenase | FRAAL2168 | ||
| R | General function prediction only | FRAAL0277 | surE | Acid phosphatase SurE, survival protein. | FRAAL6200 | SP |
| T | Signal transduction mechanisms | FRAAL3898 | Hypothetical protein | FRAAL6520 | ||
| NI | FRAAL6489 | Hypothetical protein | FRAAL1398 | TM | ||
| FRAAL1769 | Hypothetical protein | FRAAL5611 | ||||
| Single copy | ||||||
| C | Energy production and conversion | FRAAL1457 | Putative Xanthine dehydrogenase | |||
| FRAAL4787 | Putative N-glycosyltransferase | |||||
| FRAAL3448 | glpQ | Glycerophosphoryl diester phosphodiesterase | SP | |||
| D | Cell cycle control, cell division, chromosome partitioning | FRAAL2959 | ATP/GTP binding protein | TM | ||
| E | Amino acid transport and metabolism | FRAAL5354 | Hypothetical protein | |||
| FRAAL4450 | Putative Monomeric sarcosine oxidase (MSOX) | |||||
| FRAAL1891 | Putative sarcosine oxidase subunit β | |||||
| FRAAL4839 | ABC peptide transporter | SP | ||||
| F | Nucleotide transport and metabolism | FRAAL3674 | Uridine kinase | |||
| G | Carbohydrate transport and metabolism | FRAAL0592 | Putative ROK family transcriptional regulator | |||
| H | Coenzyme transport and metabolism | FRAAL6157 | Conserved hypothetical protein; putative Pantothenate kinase | |||
| I | Lipid transport and metabolism | FRAAL2810 | Hypothetical protein | |||
| K | Transcription | FRAAL0335 | Putative LuxR family transcriptional regulator | |||
| FRAAL1455 | Hypothetical protein | |||||
| FRAAL1658 | Putative two-component system response regulator | |||||
| FRAAL2338 | Hypothetical protein | |||||
| FRAAL2354 | Putative DNA-binding protein | |||||
| FRAAL3054 | Hypothetical protein | |||||
| FRAAL3611 | Putative MarR family transcriptional regulator | |||||
| FRAAL3970 | Putative repressor | |||||
| FRAAL3977 | Putative TetR-family transcriptional regulator | |||||
| FRAAL4738 | Putative LuxR-family transcriptional regulator | |||||
| L | Replication, recombination and repair | FRAAL0558 | Conserved hypothetical protein; putative DNA-glycosylase domain | |||
| L | Replication, recombination and repair | FRAAL4221 | Hypothetical protein | |||
| O | Posttranslational modification, protein turnover, chaperones | FRAAL1895 | Putative heat shock protein 16 | |||
| FRAAL2394 | Thioredoxin-like protein | |||||
| FRAAL5033 | Putative alkaline serine protease | SP | ||||
| P | Inorganic ion transport and metabolism | FRAAL3036 | Hypothetical protein | |||
| FRAAL3387 | Cyclohexanone monooxygenase | SP | ||||
| FRAAL3502 | Hypothetical protein; putative Rieske [2Fe-2S] domain | |||||
| R | General function prediction only | FRAAL0327 | Putative amidohydrolase | |||
| FRAAL5340 | Hypothetical protein | |||||
| FRAAL3906 | Putative integral membrane transport protein | TM | ||||
| FRAAL3907 | Putative ABC-type uncharacterized transport system | TM | ||||
| S | Function unknown | FRAAL1385 | Hypothetical protein | |||
| FRAAL3029 | Hypothetical protein | |||||
| FRAAL1789 | Hypothetical protein | TM | ||||
| T | Signal transduction mechanisms | FRAAL1745 | Tellurium resistance protein terE | |||
| U | Intracellular trafficking, secretion, and vesicular transport | FRAAL4430 | Putative signal peptide | SP | ||
| NI | FRAAL0290 | Hypothetical protein | ||||
| FRAAL1186 | Hypothetical protein | |||||
| FRAAL6274 | Hypothetical protein | |||||
| FRAAL6706 | Hypothetical protein | |||||
| FRAAL3025 | gvpA | Gas vesicle synthesis-like protein | ||||
| FRAAL3026 | gvpF | Gas vesicle protein F | ||||
| FRAAL1685 | Putative IMP dehydrogenase/ GMP reductase domain | |||||
| FRAAL1686 | Putative P-loop containing nucleotide triphosphate hydrolase domain | |||||
| FRAAL2305 | Hypothetical protein | |||||
| FRAAL2306 | Hypothetical protein | |||||
| FRAAL2795 | Hypothetical protein | |||||
| FRAAL3310 | Hypothetical protein | |||||
| FRAAL3311 | Hypothetical protein | |||||
| FRAAL3894 | Hypothetical protein | |||||
| FRAAL4437 | Hypothetical protein | |||||
| FRAAL4895 | Hypothetical protein | |||||
| FRAAL4893 | Putative N-acetylmuramoyl-L-alanine amidase domains | SP | ||||
| FRAAL0360 | Putative signal peptide | SP | ||||
| FRAAL5030 | Putative signal peptide | SP | ||||
| FRAAL5032 | Putative signal peptide | SP | ||||
| FRAAL4294 | Putative signal peptide | SP | ||||
| FRAAL4721 | Putative signal peptide | SP | ||||
| FRAAL5515 | Putative lipoprotein | SP | ||||
| FRAAL6270 | Putative signal peptide | TM | ||||
| FRAAL3669 | Hypothetical protein | TM | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim Tiam, S.; Boubakri, H.; Bethencourt, L.; Abrouk, D.; Fournier, P.; Herrera-Belaroussi, A. Genomic Insights of Alnus-Infective Frankia Strains Reveal Unique Genetic Features and New Evidence on Their Host-Restricted Lifestyle. Genes 2023, 14, 530. https://doi.org/10.3390/genes14020530
Kim Tiam S, Boubakri H, Bethencourt L, Abrouk D, Fournier P, Herrera-Belaroussi A. Genomic Insights of Alnus-Infective Frankia Strains Reveal Unique Genetic Features and New Evidence on Their Host-Restricted Lifestyle. Genes. 2023; 14(2):530. https://doi.org/10.3390/genes14020530
Chicago/Turabian StyleKim Tiam, Sandra, Hasna Boubakri, Lorine Bethencourt, Danis Abrouk, Pascale Fournier, and Aude Herrera-Belaroussi. 2023. "Genomic Insights of Alnus-Infective Frankia Strains Reveal Unique Genetic Features and New Evidence on Their Host-Restricted Lifestyle" Genes 14, no. 2: 530. https://doi.org/10.3390/genes14020530
APA StyleKim Tiam, S., Boubakri, H., Bethencourt, L., Abrouk, D., Fournier, P., & Herrera-Belaroussi, A. (2023). Genomic Insights of Alnus-Infective Frankia Strains Reveal Unique Genetic Features and New Evidence on Their Host-Restricted Lifestyle. Genes, 14(2), 530. https://doi.org/10.3390/genes14020530






