History of Polish Canidae (Carnivora, Mammalia) and Their Biochronological Implications on the Eurasian Background
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. General Remarks
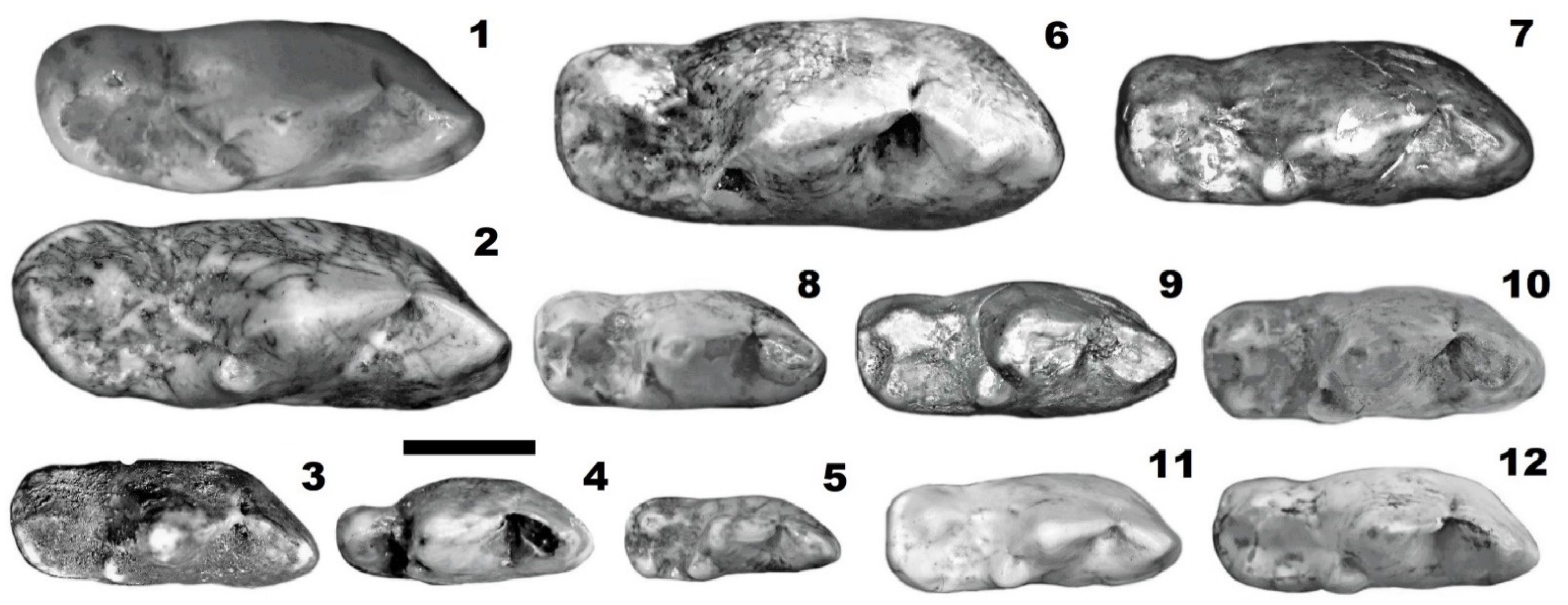
3.2. Neogene
3.3. Quaternary
| Species | Site | Bone | Lab. Code | %C | %N | 14C Age | Cal. BP 95.4% | Source |
|---|---|---|---|---|---|---|---|---|
| C. a. europaeus | Radochowska Cave | maxilla | MAMS-48222 * | 45.7 | 40.480 ± 380 | 44.310–42.960 | [31] | |
| V. vulpes | Obłazowa 2 | mandible | OxA-3696 | 33.430 ± 1230 | 38.885–37,675 | [86] | ||
| V. vulpes | Cave 4 on Mt Birów | mandible | Poz-27279 | 9.9 | 3.5 | 27.980 ± 220 | 32.048–31.681 | [69] |
| C. l. spelaeus | Niedźwiedzia Cave | costae | Poz-143821 | 10.0 | 1.8 | 26.640 ± 290 | 31.078–30.858 | new |
| V. lagopus | Deszczowa Cave, l. VIIa | radius | Poz-26126 | 8.8 | 2.9 | 24.620 ± 200 | 29.038–28.736 | [63] |
| V. vulpes | Deszczowa Cave, l. VIIa | humerus | Poz-23437 | 7.3 | 1.5 | 24.470 ± 260 | 28.824–28.628 | [87] |
| V. lagopus | Kraków-Spadzista | maxilla | Poz-28734 | 8.0 | 2.8 | 24.640 ± 160 | 29.061–28.753 | [88] |
| V. lagopus | Obłazowa Cave, l. 7 | femur | Poz-22686 | 13.1 | 3.0 | 23.500 ± 230 | 27.827–27.627 | [87] |
| V. lagopus | Kraków-Spadzista | mandible | Poz-28733 | 6.6 | 1.9 | 23.150 ± 190 | 27.590–27.274 | [88] |
| V. lagopus | Deszczowa Cave, l. VIIIa | humerus | Poz-25075 | 10.2 | 3.3 | 22.530 ± 160 | 27.091–26.868 | [63] |
| V. vulpes | Deszczowa Cave, l. VIIIa | tibia | Poz-22666 | 7.0 | 1.2 | 20.280 ± 130 | 24.535–24,188 | [87] |
| V. lagopus | Kraków-Spadzista | mandible | Poz-51377 *** | 178.70 ± 110 | 21.906–21,456 | [89] | ||
| V. lagopus | Jasna Strzegowska Cave | mandible | Poz-24205 | 16.7 | 5.1 | 14.400 ± 80 | 17.784–17,375 | new |
| V. lagopus | Nad Potoczkiem Cave | mandible | Poz-23646 | 16.6 | 4.2 | 13.560 ± 70 | 16.487–16,267 | new |
| V. lagopus | Wilczyce | Tooth ** | OxA-16728 | 13.180 ± 60 | 15.928–15,710 | [74] | ||
| V. lagopus | Krucza Skała Shelter, l. 2-4 | mandible | Poz-27245 | 7.1 | 2.1 | 12.970 ± 60 | 15.634–15,364 | [63] |
| V. lagopus | Krucza Skała Shelter, l. 1 | mandible | Poz-27246 | 7.7 | 2.2 | 12.920 ± 60 | 15.577–15,316 | [73] |
| V. lagopus | Cave 4 on Mt Birów | mandible | Poz-27244 | 10.3 | 3.7 | 12.590 ± 60 | 15.114–14,926 | [69] |
| V. lagopus | Puchacza Skała Shelter | mandible | Poz-27260 | 9.5 | 2.4 | 123.00 ± 70 | 14.328–14,132 | new |
| V. lagopus | Wilczyce | bone | Ua-15723 | 11.890 ± 105 | 13.797–13,747 | [74] |
4. Discussion
4.1. Large Canids: Genus Eucyon, Lycaon, Canis and Cuon
4.2. Small Canids: Genus Nyctereutes and Vulpes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brugal, J.P.; Boudadi-Maligne, M. Quaternary small to large canids in Europe: Taxonomic status and biochronological contribution. Quat. Int. 2011, 243, 171–182. [Google Scholar] [CrossRef]
- Azzaroli, A. Quaternary mammals and the ‘End Villafranchian’ dispersal event—A turning point in the history of Eurasia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1983, 44, 117–138. [Google Scholar] [CrossRef]
- Rook, L.; Torre, D. The “wolf-event” in Western Europe and the beginning of the Late Villafranchian. Neues Jahrb. Geol. Palaontol. Monatsh. 1996, 8, 495–501. [Google Scholar] [CrossRef]
- Sardella, R.; Palombo, M.P. The Pliocene-Pleistocene boundary: Which significance for the so called "wolf event"? Evidences from Western Europe. Quaternaire 2007, 18, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Meloro, C.; Sansalone, G. Palaeoecological significance of the “wolf event” as revealed by skull ecometrics of the canid guilds. Quat. Sci. Rev. 2022, 281, 107419. [Google Scholar] [CrossRef]
- Monguillon, A. Canis sp. Les grands mammifères fossiles du Velay. Les collections paléontologiques du Plio-Pléistocene du Musée Crozatier, Le Puy-en-Velay. Ann. Amis Mus. Crozatier 2005, 13–14, 42–43. [Google Scholar]
- Lacombat, F. New data on the Early Villafranchian fauna from Vialette (Haute-Loire, France). Collection of the Musee Crozatier (Le Puy-en-Velay, Haute-Loire). Stratigraphy, paleontology and paleoenvironment of Pliocene-Pleistocene of Transbaikalia and interregional correlations. In Proceedings of the International Symposium, Ulan-Ude, Russia, 28 August–3 September 2006; Volume 56. [Google Scholar]
- Lacombat, F.; Abbazzi, L.; Ferretti, M.P.; Martínez-Navarro, B.; Martínez, P.E.; Palombo, M.R.; Rook, L.; Turner, A.; Valli, A.M.F. New data on the Early Villafranchian fauna from Vialette (Haute-Loire, France) based on the collection of the Crozatier Museum (Le Puy-en-Velay, Haute-Loire, France). Quat. Int. 2008, 179, 64–71. [Google Scholar] [CrossRef]
- Sotnikova, M.V.; Rook, L. Dispersal of the Canini (Mammalia, Canidae: Caninae) across Eurasia during the Late Miocene to Early Pleistocene. Quat. Int. 2010, 212, 86–97. [Google Scholar] [CrossRef]
- Ossowski, G. Sprawozdanie z badań geologiczno-antropologicznych dokonanych w 1879 w jaskiniach okolic Krakowa. Zbiór Wiad. Antrop. Kraj. 1880, 4, 35–57. [Google Scholar]
- Ossowski, G. Drugie sprawozdanie z badań geologiczno-antropologicznych w jaskiniach okolic Krakowa w r. 1880. Zbiór Wiad. Antrop. Kraj. 1881, 5, 18–46. [Google Scholar]
- Ossowski, G. O szczątkach fauny dyluwialnej znalezionych w namule jaskiń wąwozu mnikowskiego. Spraw. Kom. Fizjogr. 1883, 17, 91–103. [Google Scholar]
- Ossowski, G. Czwarte sprawozdanie z badań antropologiczno-archeologicznych w jaskiniach okolic Krakowa w 1882. Zbiór Wiad. Antrop. Kraj. 1883, 7, 66–88. [Google Scholar]
- Ossowski, G. Jaskinie okolic Ojcowa pod względem paleo-etnologicznym. Pam. PAU Wyd. Mat. Przyr. 1885, 11, 1–60. [Google Scholar]
- Ossowski, G. Sprawozdanie z badań paleo-etnologicznych w jaskiniach okolic Ojcowa w r. 1886. Zbiór Wiad. Antrop. Kraj. 1887, 11, 12–32. [Google Scholar]
- Ossowski, G. Fouillos de la Caverne de Wierzchowska-Gorna en Pologne. Antiqua 1887, 2, 37–47. [Google Scholar]
- Römer, F. Die Knochenhöhlen von Ojców in Polen. Palaeontographica 1883, 29, 193–235. [Google Scholar]
- Kiernik, E. Materiały do paleozoologii dyluwialnych ssaków ziem polskich. Cz. 5. Szczątki wilka z dyluwium warstw ziem polskich. Roz. Wydz. Mat. Przyrod. PAU III 1913, 13, 491–541. [Google Scholar]
- Kiernik, E. Materiały do paleozoologii dyluwialnych ssaków ziem polskich. Cz. 5. Familia: Canidae; Sectio: Lupinae. Spraw. Czynn. Pos. PAU Kraków 1913, 18, 15. [Google Scholar]
- Stach, J. Nyctereutes (Canidae) w pliocenie Polski. Acta Geol. Pol. 1954, 4, 191–206. [Google Scholar]
- Kowalski, K. Polskie badania nad systematyką, zoogeografią i faunistyką kręgowców w latach 1953-1956. Przegl. Zool. 1957, 1, 228–236. [Google Scholar]
- Kowalski, K. Katalog Ssaków Plejstocenu Polski; PWN: Warszawa, Poland, 1959. [Google Scholar]
- Kowalski, K. Les Mammiferes Fossiles des Remplissages Karstiques de Pologne—Prohlemes Paleobiologiques et Stratigraphiques. In Livre du Centenaire Emile G. Racovitza, 1868–1968; Orghidan, T., Racovitza, G., Eds.; Académie de la République Socialiste de Roumanie: Bucharest, Romania, 1970; pp. 491–498. [Google Scholar]
- Bigaj, J. Szczątki Canidae z plejstocenu Polski. Folia Quat. 1963, 13, 1–18. [Google Scholar]
- Czyżewska, T. Nyctereutes sinensis Schlosser (Canidae, Mammalia) from the Pliocene breccia in Węże (Poland). Acta Zool. Crac. 1969, 14, 441–450. [Google Scholar]
- Czyżewska, T. Natural endocranial casts of the Canidae from Węże I near Działoszyn (Poland). Acta Zool. Crac. 1981, 25, 251–260. [Google Scholar]
- Ivanoff, D.V.; Wolsan, M.; Marciszak, A. Brainy stuff of long-gone dogs: A reappraisal of the supposed Canis endocranial cast from the Pliocene of Poland. Sci. Nat. 2014, 101, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Wolsan, M. Drapieżne—Carnivora. Fol. Quat. 1989, 59–60, 177–197. [Google Scholar]
- Wolsan, M. Lower Pleistocene carnivores of Poland. Quarterpäläntologie 1990, 8, 277–280. [Google Scholar]
- Wolsan, M. Evolution des carnivores quaternaires en Europe centrale dans leur contexte stratigraphique et paleoclimatique. L’Anthropologie 1993, 97, 203–222. [Google Scholar]
- Marciszak, A.; Kropczyk, A.; Lipecki, G. The first record of Cuon alpinus (Pallas, 1811) from Poland and the possible impact of other large canids on the evolution of the species. J. Quat. Sci. 2021, 36, 1101–1121. [Google Scholar] [CrossRef]
- Steininger, F.F.; Bernor, R.L.; Fahlbusch, V. European Neogene marine/continental chronologic correlations. In European Neogene Mammal Chronology; Lindsay, E.H., Fahlbusch, V., Mein, P., Eds.; NATO ASI Series: Brussels, Belgium, 1990; Volume 180, pp. 15–46. [Google Scholar]
- Gibbard, P.L.; Head, M.J. IUGS ratification of the Quaternary system/period and the Pleistocene series/epoch with a base at 2.58 ma. Quaternaire 2009, 20, 411–412. [Google Scholar] [CrossRef] [Green Version]
- Gibbard, P.L.; Head, M.J.; Walker, M.J. Formal ratification of the Quaternary system/period and the Pleistocene series/epoch with a base at 2.58 ma. J. Quat. Sci. 2010, 25, 96–102. [Google Scholar] [CrossRef]
- Cohen, K.M.; Harper, D.A.T.; Gibbard, P.L.; Fan, J.H. International Chronostratigraphic Chart (V2018/08). International Commission on Stratigraphy. 2018. Available online: http://www.stratigraphy.org/index.php/ics-charttimescale (accessed on 6 January 2023).
- Lisiecki, L.E.; Raymo, M.E. A Pliocene-Pleistocene stack of 57 globally distributed benthic δ18O records. Paleoceanography 2005, 20, PA1003. [Google Scholar] [CrossRef] [Green Version]
- Mein, P. Updating of MN Zones. In European Neogene Mammal Chronology, European Neogene Mammal Chronology; Lindsay, E.H., Fahlbusch, V., Mein, P., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 73–90. [Google Scholar]
- Guérin, C. Première biozonation du Plistocène europen, principal resultat biostratigraphique de l’tude des Rhinocerotidae (Mammalia, Perissodactyla) du Miocène terminal au Plistocène suprieur d′Europe occidentale. Geobios 1982, 15, 593–598. [Google Scholar] [CrossRef]
- Guérin, C. Biozones of Mammal Units? Methods and Limits in Biochronology. In European Neogene Mammal Chronology, European Neogene Mammal Chronology; Lindsay, E.H., Fahlbusch, V., Mein, P., Eds.; Plenum Press: New York, NY, USA, 1990; pp. 119–130. [Google Scholar]
- Azzaroli, A.; De Giuli, C.; Ficcarelli, G.; Torre, D. Late Pliocene to early Mid-Pleistocene in Eurasia: Faunal succesion and dispersal events. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1988, 66, 77–100. [Google Scholar] [CrossRef]
- Caloi, L.; Palombo, R. Biochronology of large mammals in the Early and Middle Pleistocene of the Italian peninsula. Hystrix 1997, 9, 3–12. [Google Scholar] [CrossRef]
- Gliozzi, E.; Abbazzi, L.; Argenti, A.; Azzaroli, A.; Caloi, L.; Capasso Barbato, L.; Di Stefano, G.; Esu, D.; Ficcarelli, G.; Girotti, O.; et al. Biochronology of selected mammals, molluscs and ostracodes from the Middle Pliocene to the Late Pleistocene in Italy. The state of the art. Riv. Ital. Paleontol. Stratigr. 1997, 103, 369–388. [Google Scholar] [CrossRef]
- Sardella, R.; Caloi, L.; Di Stefano, G.; Palombo, M.R.; Petroni, C.; Abbazzi, L.; Azzaroli, A.; Ficcarelli, G.; Mazza, P.; Mezzabotta, C.; et al. Mammal faunal turnover in Italy from the middle Pliocene to Holocene. Medd. Ned. Inst. Toegep. Geoweten. TNO 1998, 60, 499–512. [Google Scholar]
- Rook, L.; Martinez-Navarro, B. Villafranchian: The long story of a Plio-Pleistocene European large mammal biochronologic unit. Quat. Int. 2010, 219, 134–144. [Google Scholar] [CrossRef]
- Kahlke, R.-D. Untermassfeld—a late Early Pleistocene (Epivillafranchian) fossil site near Meiningen (Thuringia, Germany) and its position in the development of the European mammal fauna. BAR Int. Ser. 2006, 1578, 1–141. [Google Scholar]
- Kahlke, R.D. Late Early Pleistocene European large mammals and the concept on an Epivillafranhian biochron. Cour. Forschungsinst. Senckenberg 2007, 259, 265–278. [Google Scholar]
- Kahlke, R.D. Les communautés de grands mammifères du Pléistocène inférieur terminal et le concept d’un biochrone Épivillafranchien. Quaternaire 2009, 20, 415–427. [Google Scholar] [CrossRef] [Green Version]
- Kahlke, R.D. The origin of Eurasian Mammoth faunas (Mammuthus-Coelodonta Faunal Complex). Quat. Sci. Rev. 2014, 96, 32–49. [Google Scholar] [CrossRef]
- Kahlke, R.-D.; Garcia, N.; Kostopoulos, D.S.; Lacombat, F.; Lister, A.M.; Mazza, P.P.A.; Spassov, N.; Titov, V.V. Western Palearctic paleoenvironment conditions during communities, and implications for hominin dispersal in Europe. Quat. Sci. Rev. 2011, 30, 1368–1395. [Google Scholar] [CrossRef]
- Petronio, C.; Bellucci, L.; Martiinetto, E.; Pandolfi, L.; Salari, L. Biochronology and palaeoenvironmental changes from the Middle Pliocene to the Late Pleistocene in Central Italy. Geodiversitas 2011, 33, 485–517. [Google Scholar] [CrossRef]
- Bellucci, L.; Sardella, R.; Rook, L. Large mammal biochronology framework in Europe at Jaramillo: The Epivillafranchian as a formal biochron. Quat. Int. 2015, 389, 84–89. [Google Scholar] [CrossRef]
- Soria, D.; Aguirre, E. 1976. El canido de Layna: Revision de los Nyctereutes fosiles. Trab. Neogeno-Cuatern. 1976, 5, 83–115. [Google Scholar]
- Stefaniak, K.; Ratajczak, U.; Kotowski, A.; Kozłowska, M.; Mackiewicz, P. Polish Pliocene and Quaternary deer and their biochronological implications. Quat. Int. 2020, 546, 64–83. [Google Scholar] [CrossRef]
- Kowalski, K. Historia i ewolucja lądowej fauny Polski (red.). Fol. Quat. 1989, 59–60, 1–276. [Google Scholar]
- Stefaniak, K. Neogene and Quaternary Cervidae from Poland. ISEA PAS: Kraków, Poland, 2015. [Google Scholar]
- Marciszak, A. Carnivores of the latest Pliocene from Węże 2, Poland. Acta Paleontol. Polonica, 2023; in review. [Google Scholar]
- Wiszniowska, T. Middle Pleistocene Carnivora (Mammalia) from Kozi Grzbiet in the Świętokrzyskie Mts., Poland. Acta Zool. Crac. 1989, 32, 589–630. [Google Scholar]
- Marciszak, A.; Sobczyk, A.; Kasprzak, M.; Gornig, W.; Ratajczak, U.; Wiśniewski, A.; Stefaniak, K. Taphonomic and paleoecological aspects of large mammals from Sudety Mts (Silesia, SW Poland), with particular interest to the carnivores. Quat. Int. 2020, 546, 42–63. [Google Scholar] [CrossRef]
- Kot, M.; Berto, C.; Krajcarz, M.T.; Moskal-del Hoyo, M.; Gryczewska, N.; Szymanek, M.; Marciszak, A.; Stefaniak, K.; Zarzecka-Szubińska, K.; Lipecki, G.; et al. Frontiers of the Lower Palaeolithic expansion in Europe: Tunel Wielki Cave (Poland). Sci. Rep. 2022, 12, 16355. [Google Scholar] [CrossRef] [PubMed]
- Sotnikova, M.V. 2001. Remains of Canidae from the Lower Pleistocene site of Untermassfeld. Monogr. Des. RGZM 2001, 40, 607–632. [Google Scholar]
- Marciszak, A.; Socha, P.; Nadachowski, A.; Stefaniak, K. Carnivores from Biśnik Cave. Quat. Hors-Ser. 2011, 4, 101–106. [Google Scholar]
- Wojtal, P. Zooarchaeological Studies of the Late Pleistocene Sites in Poland; ISEA PAS: Cracow, Poland, 2007. [Google Scholar]
- Nadachowski, A.; Żarski, M.; Urbanowski, M.; Wojtal, P.; Miękina, B.; Lipecki, G.; Ochman, K.; Krawczyk, M.; Jakubowski, G.; Tomek, T. Late Pleistocene Environment of the Częstochowa Upland (Poland) Reconstructed on the Basis of Faunistic Evidence from Archaeological Cave Sites; ISEA PAS: Kraków, Poland, 2009. [Google Scholar]
- Marciszak, A.; Schouwenburg, C.; Gornig, W.; Lipecki, G.; Mackiewicz, P. 2019. Morphometric comparison of Panthera spelaea (Goldfuss, 1810) from Poland with the lion remains from Eurasia over the last 700 ka. Quat. Sci. Rev. 2019, 223, 105950. [Google Scholar] [CrossRef]
- Marciszak, A. Carnivores from Naciekowa Cave (Połom Hill, Kaczawskie Mts., south-west Poland). In Proceedings of the 17th International Cave Bear Symposium, Einhornhöhle (Unicorn Cave); Harz, Germany, 15–18 September 2011, Döppes, D., Joger, U., Nielbock, R., Rosendahl, W., Eds.; Reiss-Engelhorn Museen: Osterode am Harz, Germany, 2011; p. 20. [Google Scholar]
- Made, V.D.J.; Stefaniak, K.; Marciszak, A. The Polish fossil record of the wolf Canis and the deer Alces, Capreolus, Megaloceros, Dama and Cervus in an evolutionary perspective. Quat. Int. 2014, 326–327, 406–430. [Google Scholar] [CrossRef]
- Marciszak, A.; Kotowski, A.; Przybylski, B.; Badura, J.; Wiśniewski, A.; Stefaniak, K. Large mammals from historical collections of open-air sites of Silesia (southern Poland) with special reference to carnivores and rhinoceros. Hist. Biol. 2019, 31, 696–730. [Google Scholar] [CrossRef]
- Lipecki, G.; Wojtal, P. Carnivores from the Open-Air Gravettian Site Kraków Spadzista. In A Gravettian Site in Southern Poland: Kraków Spadzista; Wilczyński, J., Wojtal, P., Haynes, G., Eds.; ISEA PAS: Kraków, Poland, 2015; pp. 117–157. [Google Scholar]
- Muzolf, B.; Stefaniak, K.; Tomek, T.; Wertz, K.; Socha, P.; Cyrek, K.; Mirosław-Grabowska, J.; Madeyska, T.; Nadachowski, A. Multiculturate sites on Mt. Birów in Podzamcze. Stud. Fac. Earth Scien. Univ. Sil. 2009, 56, 283–294. [Google Scholar]
- Herr, O. Diluviale und altalluviale Säugetierreste aus der Oberlausitz. Abh. Naturforsch. Ges. Görlitz 1924, 29, 92–101. [Google Scholar]
- Wiśniewski, A.; Stefaniak, K.; Wojtal, P.; Zych, J.; Nadachowski, A.; Musil, R.; Badura, J.; Przybylski, B. Archaeofauna or palaeontological record? Remarks on Pleistocene fauna from Silesia. Spraw. Archeol. 2009, 61, 1–62. [Google Scholar]
- Kropczyk, A.; Marciszak, A. Ssaki Stepu Mamuciego ze Śląskiego Stanowiska Skarszyn Koło Wrocławia. Monografia Pokonferencyjna XV; Kopernikańskie Seminarium Doktoranckie, UMK: Toruń, Poland, 2022; in press. [Google Scholar]
- Nadachowski, A.; Krajcarz, M.; Lemanik, A.; Lipecki, G.; Marciszak, A.; Miękina, B.; Stefaniak, K.; Tomek, T. Vertebrate Remains from Krucza Skała Rockshelter. In Late Magdalenian Campsite at Krucza Skała Rockshelter; Cyrek, K., Sudoł-Procyk, M., Czyżewski, Ł, Eds.; Wyd. Nauk. UMK: Toruń, Poland, 2022; pp. 138–160. [Google Scholar]
- Schild, R. Taphonomy and Chronology of the Settlement. In Wilczyce. A Late Magdalenian Winter Hunting Camp in Southern Poland; IAE PAS: Warszawa, Poland, 2014; pp. 87–104. [Google Scholar]
- Wyrost, P. Dawna fauna Polski w świetle badań kostnych materiałów archeologicznych (in Polish). Rocz. Ak. Rol. Poz. 1994, 259, 155–176. [Google Scholar]
- Piątkowska-Małecka, J.; Gubernat, J. Pies w neolicie na ziemiach polskich. Światowit B 2003, 5, 207–242. [Google Scholar]
- Dehnel, A. Nowy ssak dla fauny polskiej Nyctereutes procyonoides (Gray). Chrońmy Przyr. Ojcz. 1956, 12, 17–21. [Google Scholar]
- Dudziński, W.; Haber, A.; Matuszewski, G. Junat (Nyctereutes procyonoides) w Polsce. Chrońmy Przyr. Ojcz. 1965, 20, 21–30. [Google Scholar]
- Jabłońska, J. 1965. Uwagi o junacie, Nyctereutes procyonoides Gray. Przeg. Zool. 1965, 9, 299–300. [Google Scholar]
- Pucek, Z.; Raczyński, J. Atlas Rozmieszczenia Ssaków w Polsce; PWN: Warszawa, Poland, 1983. [Google Scholar]
- Krzywiński, A.; Włodek, K. Jenot. Low. Pol. 1984, 4, 12–13. [Google Scholar]
- Sumiński, P.; Goszczyński, J.; Romanowski, J. Ssaki Drapieżne Europy; PWRiL: Warszawa, Poland, 1993. [Google Scholar]
- Kauhala, K.; Kowalczyk, R. Invasion of the raccoon dog Nyctereutes procyonoides in Europe: History of colonization, features behind its success, and threats to native fauna. Curr. Zool. 2011, 57, 584–598. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, R.; Kołodziej-Sobocińska, M.; Ruczyńska, I.; Wójcik, J.M. Range expansion of the golden jackal (Canis aureus) into Poland: First records. Mamm. Res. 2015, 60, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Reimer, P.J.; Austin, W.E.N.; Bard, E.; Bayliss, A.; Blackwell, P.G.; Bronk Ramsey, C.; Butzin, M.; Cheng, H.; Edwards, R.L.; Friedrich, M.; et al. The IntCal20 Northern Hemisphere radiocarbon age calibration curve (0–55 cal kBP). Radiocarbon 2020, 62, 725–757. [Google Scholar] [CrossRef]
- Nadachowski, A.; Harrison, D.L.; Szyndlar, Z.; Tomek, T.; Wolsan, M. Late Pleistocene vertebrate fauna from Obłazowa 2 (Carpathians, Poland): Palaeological reconstruction. Acta Zool. Crac. 1993, 36, 281–290. [Google Scholar]
- Lorenc, M. Radiocarbon ages of bones from Vistulian (Weichselian) cave deposits in Poland and their stratigraphy. Acta Geol. Pol. 2013, 63, 399–424. [Google Scholar] [CrossRef]
- Wilczyński, J.; Wojtal, P.; Sobczyk, K. Spatial organization of the Gravettian mammoth hunters site—Kraków Spadzista (southern Poland). J. Archaeol. Sci. 2012, 39, 3627–3642. [Google Scholar] [CrossRef]
- Wojtal, P.; Wilczyński, J. Zooarchaeological studies of large mammal remains from Kraków Spadzista site—trench C2 and trench E1 (2011-2012 excavations). In A Gravettian Site in Southern Poland: Kraków Spadzista; Wilczyński, J., Wojtal, P., Haynes, G., Eds.; ISEA PAS: Kraków, Poland, 2015; pp. 93–111. [Google Scholar]
- Tedford, R.H.; Qiu, Z. A new canid genus from the Pliocene of Yushe, Shanxi Province. Vert. PalAs. 1996, 34, 27–40. [Google Scholar]
- Wang, X.; Tedford, R.H. Dogs, Their Fossil Relatives and Evolutionary History; Columbia University Press: New York, NY, USA, 2008. [Google Scholar]
- Figueirido, B.; Martín-Serra, A.; Tseng, Z.J.; Janis, C.M. Habitat changes and changing predatory habits in North American fossil canids. Nat. Commun. 2015, 6, 7976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rook, L. “Canis” monticinensis sp. nov., a new Canidae (Carnivora, Mammalia) from the late Messinian of Italy. Boll. Soc. Paleontol. Ital. 1992, 31, 151–156. [Google Scholar]
- Rook, L. I cani dell’Eurasia dal Miocene Superiore al Pleistocene Medio. Ph.D. Dissertation, Firenze and Roma “La Sapienza” Universities, Bologna Italy, Modena, Italy, 1993. [Google Scholar]
- Rook, L. The wide ranging genus Eucyon Tedford & Qiu, 1996 (Mammalia, Carnivora, Canidae, Canini) in the Mio-Pliocene of the Old World. Geodiversitas 2009, 31, 723–741. [Google Scholar] [CrossRef]
- Pickford, M.; Soria, D.; Morales, J. Carnivores from the late Miocene and basal Pliocene of the Tugen Hills, Kenya. Rev. Soc. Geol. Esp. 2005, 18, 39–61. [Google Scholar]
- Howell, F.C.; Garcia, N. Carnivora (Mammalia) from Lemudong’o (late Miocene: Narok District, Kenya). Kirtlandia 2007, 46, 121–139. [Google Scholar]
- Lawrence, J.; Flynn, L.J.; Tedford, R.H.; Qiu, Z. Enrichment and stability in the Pliocene mammal faunas of North China. Paleobiology 1991, 17, 246–265. [Google Scholar] [CrossRef]
- Richard, H.; Tedford, R.H.; Flynn, L.J.; Qiu, Z.; Opdyke, N.D.; Downs, W.R. Yushe basin, China: Paleomagnetically calibrated mammalian biostratigraphic standard for the late Neogene of Eastern Asia. J. Vertebr. Paleontol. 1991, 11, 519–526. [Google Scholar] [CrossRef]
- Qiu, Z. Dispersals of Neogene carnivorans between Asia and North America. Bull. Am. Mus. Nat. Hist. 2003, 279, 18–31. [Google Scholar]
- Crusafont Pairó, M. El primer representante del género Canis en el Pontiense Eurasiatico (Canis cipio nova sp). Bol. R. Soc. Esp. Hist. Nat. Secc. Biol. 1950, 48, 43–56. [Google Scholar]
- Pons Moyá, J.; Crusafont Pairó, M. El Canis cipio Crusafont (1950), comparacion con los canidos del Plioceno y Pleistoceno europeo. Acta Geol. Hisp. 1978, 13, 133–136. [Google Scholar]
- Rook, L.; Ficcarelli, G.; Torre, D. Messinian carnivores from Italy. Boll. Soc. Paleontol. Ital. 1991, 30, 7–22. [Google Scholar]
- Torre, D.; Ficcarelli, G.; Masini, F.; Rook, L.; Sala, B. Mammal dispersal events in the Early Pleistocene of Western Europe. Cour. Forsch. Inst. Senckenberg 1992, 53, 51–58. [Google Scholar]
- Alcalá Martínez, L. Macromamíferos Neógenos de la Fosa de Alfambra-Teruel; Museo Nacional de Ciencias Naturales: Madrid, Spain, 1994. [Google Scholar]
- Wang, X.; Tedford, R.H. Evolutionary History of Canids. In The Behavioural Biology of Dogs; Jensen, P., Ed.; CABI: Wallingford, UK, 2007; pp. 3–20. [Google Scholar]
- Lydekker, R.B. Catalogue of the fossil Mammalia in the British Museum. Part I: Primates, Chiroptera, Insectivora, Carnivora; British Museum of the Natural History: London, UK, 1885. [Google Scholar]
- Martin, R. Trois nouvelles espèces de caninae (Canidae, Carnivora) des gisements Plio-Villafranchiens d’Europe. Doc. Lab. Géol. Fac. Sci. Lyon 1973, 57, 87–96. [Google Scholar]
- Odintzov, I.A. New species of Pliocene Carnivora, Vulpes odessana sp. nov. from the Karst Cave of Odessa. Paleontol. Sbornik 1967, 4, 130–137. [Google Scholar]
- Spassov, N.; Rook, L. Eucyon marinae sp. nov. (Mammalia, Carnivora), a new canid species from the Pliocene of Mongolia, with a review of forms referable to the genus. Riv. Ital. Paleontol. Stratig. 2006, 112, 123–133. [Google Scholar] [CrossRef]
- Bartolini Lucenti, S.; Rook, R. A review on the Late Villafranchian medium-sized canid Canis arnensis based on the evidence from Poggio Rosso (Tuscany, Italy). Quat. Sci. Rev. 2016, 151, 58–71. [Google Scholar] [CrossRef]
- Bartolini-Lucenti, S.; Spassov, N. Cave canem! The earliest Canis (Xenocyon) (Canidae, Mammalia) of Europe: Taxonomic affinities and paleoecology of the fossil wild dogs. Quat. Sci. Rev. 2022, 276, 107315. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, S. Additional note on the Cuon from Linyi, Shanxi Province, China. In Proceedings of the Tenth Annual Meeting of the Chinese Society of Vertebrate Paleontology; Dong, W., Ed.; China Ocean Press: Beijing, China, 2006; pp. 63–68. [Google Scholar]
- Wang, X.; Li, Q.; Qiu, Z.; Xie, G.; Wang, B.; Qiu, Z.; Tseng, Z.; Takeuchi, G.T.; Deng, T. Neogene Mammalian Biostratigraphy and Geochronology of the Tibetan Plateau. In Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology; Wang, X., Flynn, L.J., Fortelius, M., Eds.; Columbia University Press: New York, NY, USA, 2013; pp. 274–292. [Google Scholar]
- Wang, X.; Flynn, L.J.; Fortelius, M. Fossil Mammals of Asia: Neogene Biostratigraphy and Chronology; Columbia University Press: New York, NY, USA, 2013. [Google Scholar]
- Tao Deng, T.; Wu, F.; Wang, S.D.; Su, T.; Zhou, Z. Major turnover of biotas across the Oligocene/Miocene boundary on the Tibetan Plateau. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 567, 110241. [Google Scholar] [CrossRef]
- Wang, X.; Tseng, Z.; Li, Q.; Takeuchi, G.T.; Xie, G. From ‘third pole’ to north pole: A Himalayan origin for the arctic fox. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140893. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Xie, G. Earliest record of Sinicuon in Zanda Basin, southern Tibet and implications for hypercarnivores in cold environments. Quat. Int. 2015, 355, 3–10. [Google Scholar] [CrossRef]
- Hemmer, H. Die Feliden aus dem Epivillafranchium von Untermaßfeld bei Meiningen (Thüringen), Teil 3. Monogr. RGZM 2001, 40, 699–782. [Google Scholar]
- Hemmer, H.; Kahlke, R.-D. New results on felids from the Early Pleistocene site of Untermassfeld. Monogr. RGZM 2022, 40, 1465–1566. [Google Scholar]
- Turner, A. Remains of Pachycrocuta brevirostris (Carnivora, Hyaenidae) from the Lower Pleistocene site of Untermassfeld. Monogr. RGZM 2001, 2, 673–690. [Google Scholar]
- Turner, A. The evolution of the guild of large Carnivora of the British Isles during the Middle and Late Pleistocene. J. Quat. Sci. 2009, 24, 991–1005. [Google Scholar] [CrossRef]
- Turner, A.; Antón, M. The giant hyaena Pachycrocuta brevirostris (Mammalia, Carnivora, Hyaenidae). Geobios 1996, 29, 455–468. [Google Scholar] [CrossRef]
- Turner, A.; Antón, M.; Werdelin, L. Taxonomy and evolutionary patterns in the fossil Hyaenidae of Europe. Geobios 2008, 41, 677–687. [Google Scholar] [CrossRef]
- Marciszak, A.; Gornig, W.; Kropczyk, A.; Rössner, G.E. The Latest European Record of Chasmaporthetes lunensis lunensis (Del Campana, 1914) from Schernfeld (Bavaria, Germany) in Terms of the Changes in the European Carnivore Paleoguilds. In Stratigraphy and Timescales; Montenari, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 7, pp. 52–66. [Google Scholar]
- Eaton, R.L. Interference competition among carnivores: A model for the evolution of social behaviour. Carnivore 1979, 2, 9–16. [Google Scholar] [CrossRef]
- Meachen, J.A.; Samuels, J.X. Evolution in coyotes (Canis latrans) in response to the megafaunal extinctions. PNAS 2012, 109, 4191–4196. [Google Scholar] [CrossRef] [Green Version]
- Silvestro, D.; Antonelli, A.; Salamin, N.; Quental, T.B. The role of clade competition in the diversification of North American canids. PNAS 2015, 112, 8684–8689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antón, M.; Galobart, A.; Turner, A. Co-existence of scimitar-toothed cats, lions and hominins in the European Pleistocene. Implications of the post-cranial anatomy of Homotherium latidens (Owen) for comparative palaeoecology. Quat. Sci. Rev. 2005, 24, 1287–1301. [Google Scholar] [CrossRef]
- Palmqvist, P.; Martínez-Navarro, B.; Pérez-Claros, J.A.; Torregrosa, V.; Figueirido, B.; Jiménez-Arenas, J.M.; Patrocinio Espigares, M.; Ros-Montoya, S.; De Renzi, M. The giant hyena Pachycrocuta brevirostris: Modelling the bone-cracking behavior of an extinct carnivore. Quat. Int. 2011, 243, 61–79. [Google Scholar] [CrossRef]
- Iannucci, A.; Mecozzi, B.; Sardella, R.; Iurino, D.A. The extinction of the giant hyena Pachycrocuta brevirostris and a reappraisal of the Epivillafranchian and Galerian Hyaenidae in Europe: Faunal turnover during the Early-Middle Pleistocene Transition. Quat. Sci. Rev. 2021, 272, 107240. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Zhang, H.; Wagner, J.; Jiangzuo, Q.; Song, Y.; Liu, S.; Wang, Y.; Jin, C. The giant short-faced hyena Pachycrocuta brevirostris (Mammalia, Carnivora, Hyaenidae) from Northeast Asia: A reinterpretation of subspecies differentiation and intercontinental dispersal. Quat. Int. 2021, 577, 29–51. [Google Scholar] [CrossRef]
- Hemmer, H. Notes on the Ecological Role of European Cats (Mammalia: Felidae) of the Last Two Million Years. Misc. Homen. Emiliano Aquirre Paleontol. 2004, 2, 214–232. [Google Scholar]
- Marciszak, A.; Schouwenburg, C.; Darga, R. Decreasing size process in the cave (Pleistocene) lion Panthera spelaea (Goldfuss, 1810) evolution—A review. Quat. Int. 2014, 339–340, 245–257. [Google Scholar] [CrossRef]
- Forsyth Major, C.I. Considerazioni sulla fauna dei mammiferi pliocenici e post pliocenici della Toscana e I. La Fauna dei Mammiferi del Val d’Arno superiore. Atti. Soc. Tosc. Sci. Nat. 1875, 1, 7–40. [Google Scholar]
- Forsyth Major, C.I. Considerazioni sulla fauna dei mammiferi pliocenici e post pliocenici della Toscana e II. La Fauna dei Mammiferi del Pliocene inferiore (Orizzonte di Casino). Atti. Soc. Tosc. Sci. Nat. 1875, 1, 223–245. [Google Scholar]
- Forsyth Major, C.I. Considerazioni sulla fauna dei mammiferi pliocenici e post pliocenici della Toscana e III. Cani fossili del Val d’Arno superiore e della Valle d’Era. Atti. Soc. Tosc. Sci. Nat. 1877, 3, 207–227. [Google Scholar]
- Del Campana, D. I cani Pliocenici di Toscana. Palaeontogr. Ital. 1913, 19, 189–254. [Google Scholar]
- Stehlík, A. Fosilní savci ze Stránské skály u Brna. Práce Moravské Přírodov. Společn. 1934, 9, 1–94. [Google Scholar]
- Kretzoi, M. Die Raubtiere von Gombaszög nebst einer übersicht der Gesamtfauna. Ann. Hist. Nat. Mus. Nat. Hung. 1938, 31, 88–157. [Google Scholar]
- Kretzoi, M. Weitere Beiträge zur Kenntnis der Fauna von Gombaszög. Ann. Hist. Nat. Mus. Nat. Hung. 1941, 34, 105–139. [Google Scholar]
- Kretzoi, M. Präokkupierte und durch ältere zu ersetzende Säugetiernamen. Földt. Közl. 1942, 71, 308–335. [Google Scholar]
- Torre, D. I cani villafranchiani della Toscana. Palaeontogr. Ital. 1967, 63, 113–138. [Google Scholar]
- Bonifay, M.-F. Carnivores quaternaires du sud-est de la France. Mém. Mus. Natl. Hist. Nat. Sér. C Sci. Terre C 1971, 21, 1–377. [Google Scholar]
- Musil, R. Die Caniden der Stránská Skála. In: Musil, R. (Ed.), Stránská Skála 1, 1910-1945. Anthropos 1972, 20, 77–106. [Google Scholar]
- Schütt, G. Revision der Cuon- und Xenocyon-Funde (Canidae, Mammalia) aus dem alpleistozänen Mosbacher sanden (Wiesbaden, Hessen). Mainz. Naturwissensch. Arch. 1973, 12, 49–77. [Google Scholar]
- Schütt, G. Die Carnivoren von Würzburg–Schalksberg: Mit einem Beitrag zur biostratigraphischen und zoogeographischen Stellung der altpleistozänen Wirbeltierfaunen von Mittelmain (Unterfranken). Neues Jahrb. Geol. Palaontol. Abh. 1974, 147, 61–90. [Google Scholar]
- Kurtén, B.; Poulianos, A.N. New stratigraphic and faunal material from Petralona Cave, with special reference to the Carnivora. Anthropos 1977, 4, 47–130. [Google Scholar]
- Kurtén, B.; Poulianos, A.N. Fossil Carnivora of Petralona Cave: Status of 1980. Anthropos 1981, 8, 9–56. [Google Scholar]
- Cordy, J.-M. Le paléokarst de la Belle-Roche (Sprimont, Liège), premier gisement paléontologique et archéologique du Pléistocène moyen ancien en Belgique. C. R. Acad. Sci. 1980, 291, 749–751. [Google Scholar]
- Cordy, J.-M. Biozonation du Quaternaire post-villafranchien continental d’Europe occidentale à partir des grands mammifères. Bull. Soc. Géol. Belgiq. 1982, 105, 303–314. [Google Scholar]
- Bishop, M.J. A preliminary report on the Middle Pleistocene mammal bearing deposits of Westbury-sub-Mendip, Somerset. Proc. Univ. Bristol Speleol. Soc. 1974, 13, 301–318. [Google Scholar]
- Bishop, M.J. The mammal fauna of the early Middle Pleistocene cavern infill site of Westbury-sub-Mendip, Somerset. Spec. Pap. Palaeontol. 1982, 28, 1–108. [Google Scholar]
- Jánossy, D. Pleistocene Vertebrae Faunas of Hungary; Akademiai Kiado: Budapest, Hungary, 1986. [Google Scholar]
- De Giuli, C.; Masini, F. Late Villafranchian faunas in Italy: The Casa Frata local fauna (Upper Valdarno, Tuscany). Palaeontogr. Ital. 1986, 74, 1–9. [Google Scholar]
- Sotnikova, M. Associacia melkij volk-ksenocion v pozdnem eoplejstocene-rannem plejstocene Zentral’noj Azii. Bull. Comm. Study Quat. Per. 1988, 57, 78–89. [Google Scholar]
- Rook, L. The Plio-Pleistocene Old World Canis (Xenocyon) ex gr. falconeri. Boll. Soc. Paleontol. Ital. 1994, 33, 71–82. [Google Scholar]
- Palmqvist, P.; Martínez-Navarro, B.; Arribas, A. Prey selection by terrestrial carnivores in a lower Pleistocene paleocommunity. Paleobiology 1996, 22, 514–534. [Google Scholar] [CrossRef]
- Palmqvist, P.; Arribas, A.; Martínez-Navarro, B. Ecomorphological study of large canids from the lower Pleistocene of southeastern Spain. Lethaia 1999, 32, 75–88. [Google Scholar] [CrossRef]
- Palmqvist, P.; Mendoza, M.; Arribas, A.; Gröcke, D.R. Estimating the body mass of Pleistocene canids: Discussion of some methodological problems and a new ‘taxon free’ approach. Lethaia 2002, 35, 358–360. [Google Scholar] [CrossRef]
- Palmqvist, P.; Gröcke, D.R.; Arribas, A.; Fariňa, R. Paleoecological reconstruction of a lower Pleistocene large mammals community using biogeochemical (d13C, d15N, d18O, Sr:Zn) and ecomorphological approaches. Paleobiology 2003, 29, 205–229. [Google Scholar] [CrossRef]
- Palmqvist, P.; Pérez-Claros, J.A.; Janis, C.M.; Gröcke, D.R. Tracing the ecophysiology of ungulates and predator-prey relationships in an early Pleistocene large mammal community. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 266, 95–111. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, P.; Pérez-Claros, J.A.; Gröcke, D.R.; Janis, C.M. Biogeochemical and ecomorphological inferences on prey selection and resource partitioning among mammalian carnivores in an early Pleistocene community. Palaios 2008, 23, 724–737. [Google Scholar] [CrossRef]
- Martinez-Navarro, B.; Rook, L. Gradual evolution in the hunting dog lineage. C. R. Palevol. 2003, 2, 695–702. [Google Scholar] [CrossRef]
- Bertè, D.F. L’evoluzione del Genere Canis (Carnivora, Canidae, Caninae) in Italia Dal Wolf-Event a Oggi: Implicazioni Biocronologiche, Paleoecologiche e Paleoambientali. Ph.D. Dissertation, Università di Roma, Sapienza, Italy, 2014; p. 390. [Google Scholar]
- Petrucci, M.; Cipullo, A.; Martínez-Navarro, B.; Rook, L.; Sardella, R. The Late Villafranchian (Early Pleistocene) carnivores (Carnivora, Mammalia) from Pirro Nord (Italy). Palaeontogr. Abt. A 2013, 298, 113–145. [Google Scholar] [CrossRef] [Green Version]
- Madurell-Malapeira, J.; Alba, D.M.; Moyà-Solà, S. Carnivora from the late Early Pleistocene of Cal Guardiola (Terrassa, Vallès-Pendès Basin, Catalonia, Spain). J. Paleontol. 2009, 83, 969–974. [Google Scholar] [CrossRef]
- Madurell-Malapeira, J.; Minwer-Barakat, R.; Alba, D.M.; Garcés, M.; Gómez, M.; Aurell-Garrido, J.; Ros-Montoya, S.; Moyà-Solà, S.; Berástegui, X. The Vallparadís section (Terrassa, Iberian Peninsula) and the latest Villafranchian faunas of Europe. Quat. Sci. Rev. 2010, 29, 2972–2982. [Google Scholar] [CrossRef]
- Madurell-Malapeira, J.; Rook, L.; Martínez-Navarro, B.M.; Alba, D.M.; Aurell-Garrido, J.; Moyà-Solà, S. The latest European painted dog. J. Vertebr. Paleontol. 2013, 33, 1244–1249. [Google Scholar] [CrossRef]
- Madurell-Malapeira, J.; Ros-Montoya, S.; Espigares, M.P.; Alba, D.M.; Aurell-Garrido, J. Villafrachian large mammals from the Iberian Peninsula: Paleobiogeography, paleoecology and dispersal events. J. Iber. Geol. 2014, 40, 167–178. [Google Scholar] [CrossRef] [Green Version]
- García, N.; Virgós, E. Evolution of community composition in several carnivore palaeoguilds from the European Pleistocene: The role of interspecific competition. Lethaia 2007, 40, 33–44. [Google Scholar] [CrossRef]
- Merkle, J.A.; Stahler, D.R.; Smith, D.W. Interference competition between gray wolves and coyotes in Yellowstone National Park. Can. J. Zool. 2009, 87, 56–63. [Google Scholar] [CrossRef]
- Venkataraman, A.B.; Arumugan, R.; Sukumar, R. The foraging ecology of dhole (Cuon alpinus) in Mudumalai Sanctuary, southern India. J. Zool. 1995, 237, 543–561. [Google Scholar] [CrossRef]
- Ghezzo, E.; Rook, L. Cuon alpinus (Pallas, 1811) (Mammalia, Carnivora) from Equi (Late Pleistocene, Massa-Carrara, Italy): Anatomical analysis and palaeoethological contextualisation. Rend. Lincei Sci. Fis. Nat. 2014, 25, 491–504. [Google Scholar] [CrossRef]
- Hayward, M.W.; Lyngdoh, S.; Habib, B. Diet and prey preferences of dholes (Cuon alpinus): Dietary competition within Asia’s apex predator guild. J. Zool. 2014, 294, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Volmer, R.; Hertler, C.; van der Geer, A. Niche overlap and competition potential among tigers (Panthera tigris), saber-toothed cats (Homotherium ultimum, Hemimachairodus zwierzyckii) and Merriam’s dog (Megacyon merriami) in the Pleistocene of Java. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 441, 901–911. [Google Scholar] [CrossRef]
- Volmer, R.; van der Geer, A.A.E.; Cabera, P.A.; Prasetyo, W.U.; Kurniawan, I. When did Cuon reach Java?—Reinvestigation of canid fossils from Homo erectus faunas. Geobios 2019, 55, 89–102. [Google Scholar] [CrossRef]
- Kamler, J.F.; Davies-Mostert, H.T.; Hunter, L.; Macdonald, D.W. Predation on black-backed jackals (Canis mesomelas) by African wild dogs (Lycaon pictus). Afr. J. Ecol. 2007, 45, 667–668. [Google Scholar] [CrossRef]
- Thenius, E. Die Caniden (Mammalia) aus dem Altquartär von Hundsheim (Niederösterreich) nebst Bemerkungen zur stammesgeschichte der Gattung Cuon. Neues Jahrb. Fur Geol. Palaontol. Abh. 1954, 99, 230–286. [Google Scholar]
- Tobien, H. Cuon Hodg. und Gulo Frisch (Carnivora, Mammalia) aus den altpleistozän Sanden von Mosbach bei Wiesbaden. Acta Zool. Cracov. 1957, 18, 433–451. [Google Scholar]
- Adam, K.D. Mittelpleistozäne Caniden aus den Heppenloch bei Gutenberg (Würtemberg). Stuttg. Beitr. Naturkd. A 1959, 27, 1–46. [Google Scholar]
- Adam, K.D. Die Bedeutung der pleistozänen Säugetier-Faunen Mitteleuropas für die Geschichte des Eiszeitalters. Stuttg. Beitr. Naturkd. A 1961, 78, 1–34. [Google Scholar]
- Moullé, P.-É.; Echassoux, A. Étude du Xenocyon lycaonoides de la grotte du Vallonnet (Roquebrune-Cap-Martin, Alpes-Maritimes). Bull. Mus. Anthropol. Préhist. Monaco 2005, 45, 13–22. [Google Scholar]
- Moullé, P.-É.; Echassoux, A.; Lacombat, F. Taxonomie du grand canidé de la grotte du Vallonnet (Roquebrune-Cap-Martin, Alpes-Maritimes, France). L’Anthropologie 2006, 110, 832–836. [Google Scholar] [CrossRef]
- Tedford, R.H.; Wang, X.; Taylor, B.E. Phylogenetic systematics of the North American fossil Caninae (Carnivora, Canidae). Bull. Am. Mus. Nat. Hist. 2009, 325, 1–218. [Google Scholar] [CrossRef]
- Baryshnikov, G.F. Pleistocene Canidae (Mammalia, Carnivora) from the Palaeolithic Kudaro caves in the Caucasus. Russ. J. Theriol. 2012, 11, 77–120. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sinding, M.-H.S.; Ramos-Madrigal, J.; Niemann, J.; Samaniego Castruita, J.A.; Vieira, F.G.; Carøe, C.; Montero, M.d.M.; Kuderna, L.; Serres, A.; et al. Interspecific gene flow shaped the evolution of the genus Canis. Curr. Biol. 2018, 28, 3441–3449. [Google Scholar] [CrossRef] [Green Version]
- Jánossy, D. Vertebrate Fauna of Site II. In Vertesszölös. Site, Man and Culture; Kretzoi, M., Dobosi, V.T., Eds.; Akademiai Kiado: Budapest, Hungary, 1990; pp. 187–219. [Google Scholar]
- Pienaar, U.d.V. Predator-prey relationships amongst the larger mammals of the Kruger National Park. Koedoe 1969, 12, 108–176. [Google Scholar] [CrossRef] [Green Version]
- Kruuk, H. The spotted Hyena: A Study of Predation and Social Behavior; University of California Press: Chicago, IL, USA, 1972. [Google Scholar]
- Schaller, G.B. The Serengeti Lion: A Study of Predator-Prey Relations; University of Chicago Press: Chicago, IL, USA, 1972. [Google Scholar]
- Creel, S.; Creel, N.M. Limitation of African wild dogs by competition with larger carnivores. Conserv. Biol. 1996, 10, 526–538. [Google Scholar] [CrossRef]
- Creel, S.; Creel, N.M. Six ecological factors that may limit African wild dogs, Lycaon pictus. Anim. Conserv. 1998, 1, 1–9. [Google Scholar] [CrossRef]
- Creel, S.; Creel, N.M. The African Wild Dog: Behaviour, Ecology, and Conservation; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- McNutt, J.; Boggs, L.P. Running wild: Dispelling the Myths of the African Wild Dog; D.C. Smithsonian Books: Washington, DC, USA, 1997. [Google Scholar]
- Mecozzi, B.; Iurino, D.A.; Profico, A.; Rosa, C.; Sardella, R. The wolf from the Middle Pleistocene site of Ostiense (Rome): The last occurrence of Canis mosbachensis (Canidae, Mammalia) in Italy. Hist Biol. 2021, 33, 2031–2042. [Google Scholar] [CrossRef]
- Woodroffe, R.; Ginsberg, J.R. Past and Future Causes of Wild Dogs Population Decline. In Status Survey and Conservation Plan: The African Wild Dog; Woodroffe, R., Ginsberg, J., MacDonald, D., Eds.; IUCN/SSC Canid Specialist Group: Gland, CA, USA, 1997; pp. 58–73. [Google Scholar]
- Woodroffe, R.; Ginsberg, J.R. Conserving the African wild dog Lycaon pictus. I. Diagnosing and treating causes of decline. Oryx 1999, 33, 132–142. [Google Scholar] [CrossRef] [Green Version]
- Boudadi-Maligne, M. Les Canis Pléistocénes du Sud de la France: Approche Biosystématique, Évolutive et Biochronologique. Ph.D Thesis, Université Bordeaux-Talence, Bordeaux, France, 2010. [Google Scholar]
- Flower, L.O.H. Canid Evolution and Palaeoecology in the Pleistocene of Western Europe, with Particular Reference to the Wolf Canis lupus L. 1758; University of London: London, UK, 2014. [Google Scholar]
- Flower, L.O.H. New body mass estimates of British Pleistocene wolves: Palaeoenvironmental implications and competitive interactions. Quat. Sci. Rev. 2016, 149, 230–247. [Google Scholar] [CrossRef]
- Berto, C.; Nadachowski, A.; Pereswiet–Soltan, A.; Lemanik, A.; Kot, M. The Middle Pleistocene small mammals from the lower layers of Tunel Wielki Cave (Kraków-Częstochowa Upland): An Early Toringian assemblage in Poland. Quat. Int. 2021, 577, 52–70. [Google Scholar] [CrossRef]
- Ehlers, J.; Gibbard, L. The extent and chronology of Cenozoic global glaciation. Quat. Int. 2007, 164–165, 6–20. [Google Scholar] [CrossRef]
- Flower, L.O.H.; Schreve, D.C. An investigation of palaeodietary variability in European Pleistocene canids. Quat. Sci. Rev. 2014, 96, 188–203. [Google Scholar] [CrossRef]
- Parfitt, S.A. Mammalia. In Boxgrove. A Middle Pleistocene hominid site at Eartham Quarry, Boxgrove, West Sussex; Roberts, M.B., Ed.; University College London: London, UK, 1999; pp. 197–290. [Google Scholar]
- Iurino, D.A.; Mecozzi, B.; Iannucci, A.; Moscarella, A.; Strani, F.; Bona, M.; Gaeta, M.; Sardella, R. A Middle Pleistocene wolf from central Italy provides insights on the first occurrence of Canis lupus in Europe. Sci. Rep. 2022, 12, 2882. [Google Scholar] [CrossRef]
- Portis, A. Di una formazione stagnale presso la Basalica di Ostiense di Roma e degli avanzi di fossili vertebrati in essa rinvenuti. Boll. Soc. Geol. Ital. 1900, 19, 179–240. [Google Scholar]
- Portis, A. Avanzi di canidi fossili dai terreni sedimento-tufacei di Roma. Boll. Soc. Geol. Ital. 1909, 28, 203–247. [Google Scholar]
- Adam, K.D. Vom Heppenloch zur Sibyllenhöhle. Ein Bericht über alte Funde eiszeitlicher Säugetiere auf der Kirchheimer Alb. Jahresh. Karst-Höhlenk. 1963, 4, 271–285. [Google Scholar]
- Adam, K.D. Die mittelpleistozäne Säugetier–Fauna aus dem Heppenloch bei Gutenberg (Württemberg). Stutt. Beitr. Naturkd. B 1975, 3, 1–247. [Google Scholar]
- Musil, R. Eine karstspalte mit mittelpleistozänen Funden im Kalksteinbruch Žernavá. Čas. Morav. Mus. Vědy Přírodní 1969, 54, 85–96. [Google Scholar]
- Abbazzi, L.; Fanfani, F.; Ferretti, M.P.; Rook, L.; Cattani, L.; Masini, F.; Mallegni, F.; Negrino, F.; Tozzi, C. New human remains of archaic Homo sapiens and lower palaeolithic industries from Visogliano (Duino Aurisina, Trieste, Italy). J. Archaeol. Sci. 2000, 27, 1173–1186. [Google Scholar] [CrossRef]
- Pilot, M.; Moura, A.E.; Okhlopkov, I.M.; Mamaev, N.V.; Alagaili, A.N.; Mohammed, O.B.; Yavruyan, E.G.; Manaseryan, N.H.; Hayrapetyan, V.; Kopaliani, N.; et al. Global phylogeographic and admixture patterns in grey wolves and genetic legacy of an ancient Siberian lineage. Sci. Rep. 2019, 9, 17328. [Google Scholar] [CrossRef] [Green Version]
- Morrell, V. Ch2.1-From wolf to dog. Our furry friends: The science of pets. Sci. Am. 2015, 313, 60–67. [Google Scholar] [CrossRef]
- Freedman, A.H.; Schweizer, R.M.; Ortega-Del Vecchyo, D.; Han, E.; Davis, B.W.; Gronau, I.; Silva, P.M.; Galaverni, M.; Fan, Z.; Marx, P.; et al. Demographically-based evaluation of genomic regions under selection in domestic dogs. PLoS Genet. 2016, 12, e1005851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssens, L.; Giemsch, L.; Schmitz, R.; Street, M.; Van Dongen, S.; Crombé, P. A new look at an old dog: Bonn-Oberkassel reconsidered. J. Archaeol. Sci. 2018, 92, 126–138. [Google Scholar] [CrossRef] [Green Version]
- Bergström, A.; Stanton, D.W.G.; Taron, U.H.; Frantz, L.; Sinding, M.-H.S.; Ersmark, E.; Pfrengle, S.; Cassatt-Johnstone, M.; Lebrasseur, O.; Girdland-Flink, L.; et al. Grey wolf genomic history reveals a dual ancestry of dogs. Nature 2022, 607, 313–320. [Google Scholar] [CrossRef]
- Bryce, C.M.; Davis, M.S.; Gompper, M.E.; Hurt, A.; Koster, J.M.; Larson, G.; Ostrander, E.A.; Udell, M.A.R.; Urfer, S.; Wirsing, A.J.; et al. Biology’s best friend: Bridging disciplinary gaps to advance canine science. Integr. Comp. Biol. 2021, 61, 76–92. [Google Scholar] [CrossRef]
- Perri, A.R.; Feuerborn, T.R.; Frantz, L.A.F.; Larson, G.; Malhi, R.S.; Meltzer, D.J.; Witt, K.E. Dog domestication and the dual dispersal of people and dogs into the Americas. PNAS 2021, 118, e2010083118. [Google Scholar] [CrossRef] [PubMed]
- Germonpré, M.; Lázničková-Galetová, M.; Sablin, M.V.; Bocherens, H. Self-Domestication or Human Control? The Upper Palaeolithic Domestication of the Wolf. In Hybrid Communities. Biosocial Approaches to Domestication and Other Transspecies Relationships; Stepanoff, C., Vigne, J.-D., Eds.; Routledge: London, UK, 2018; pp. 39–64. [Google Scholar]
- Wilczyński, J.; Haynes, G.; Sobczyk, Ł.; Svoboda, J.; Roblíčková, M.; Wojtal, P. Friend or foe? Large canid remains from Pavlovian sites and their archaeozoological context. J. Anthropol. Archaeol. 2020, 59, 101197. [Google Scholar] [CrossRef]
- Marciszak, A.; Kasprzak, M.; Matyaszczyk, L. Canids and ursids from Południowa Cave (Sudety Mts, Silesia, SW Poland): Biostratigraphy and palaeoecology. Palaeontol. Electron. 2023; in review. [Google Scholar]
- Bartolini Lucenti, S.; Rook, L.; Morales, J. Nyctereutes (Mammalia, Carnivora, Canidae) from Layna and the Eurasian raccoon-dogs: An updated revision. Riv. It. Paleontol. Strat. 2018, 124, 597–616. [Google Scholar] [CrossRef]
- Domingo, L.; Koch, P.L.; Fernández, M.H.; Fox, D.L.; Domingo, M.S.; Alberdi, M.T. Late Neogene and early Quaternary paleoenvironmental and paleoclimatic conditions in southwestern Europe: Isotopic analyses on mammalian taxa. PLoS ONE 2013, 8, e63739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clauzon, G.; Le Strat, P.; Duvail, C.; Do Couto, D.; Suc, J.P.; Molliex, S.; Bache, F.; Besson, D.; Lindsay, E.H.; Opdyke, N.D.; et al. The Roussillon basin (S. France): A case-study to distinguish local and regional events between 6 and 3 Ma. Mar. Pet. Geol. 2015, 66, 18–40. [Google Scholar] [CrossRef] [Green Version]
- Bartolini Lucenti, S. Nyctereutes megamastoides (Canidae, Mammalia) from the early and middle Villafranchian (late Pliocene and early Pleistocene) of the Lower Valdarno (Firenze and Pisa, Tuscany, Italy). Riv. It. Paleontol. Strat. 2017, 123, 211–218. [Google Scholar] [CrossRef]
- Bartolini Lucenti, S. Nyctereutes Temminck, 1838 (Mammalia, Canidae): A revision of the genus across the Old World during Plio-Pleistocene times. Fossilia 2018, 2018, 7–10. [Google Scholar] [CrossRef] [Green Version]
- Tamvakis, A.; Savvidou, A.; Spassov, N.; Youlatos, D.; Merceron, G.; Kostopoulos, D.S. New insights on Early Pleistocene Nyctereutes from the Balkans based on material from Dafnero-3 (Greece) and Varshets (Bulgaria). Palaeoworld 2022. [Google Scholar] [CrossRef]
- Teilhard de Chardin, P.; Pivetau, J. Les mammiferes fossils de Nihewan (Chine). Ann. Paleontol. 1930, 19, 1–134. [Google Scholar]
- Viret, J. Le loess à bancs durcis de Saint-Vallier (Drôme) et sa faune de mammifères villafranchiens. Nouv. Arch. Mus. Hist. Nat. Lyon 1954, 4, 3–67. [Google Scholar]
- Kurtén, B.; Crusafont Pairó, M. Villafranchian carnivores (Mammalia) from La Puebla de Valverde (Teruel, Spain). Commentat. Biol. 1977, 85, 1–39. [Google Scholar]
- Geraads, D. Carnivores du Pliocene terminal de Ahl al Oughlam (Casablanca, Maroc). Géobios 1997, 30, 127–164. [Google Scholar] [CrossRef]
- Koufos, G.D. The canids Eucyon and Nyctereutes from the Ruscinian of Macedonia, Greece. Paleont. Evol. 1997, 30–31, 39–48. [Google Scholar]
- Ginsburg, L. Le gisement de vertébrés pliocènes de Çalta, Ankara, Turquie. 5. Carnivores. Geodiversitas 1998, 20, 379–396. [Google Scholar]
- Bartolini Lucenti, S.; Madurell-Malapeira, J. Unravelling the fossil record of foxes: An updated review on the Plio-Pleistocene Vulpes ssp. from Europe. Quat. Sci. Rev. 2020, 236, 106296. [Google Scholar] [CrossRef]
- Kormos, T. Die Füchse des ungarischen Oberpliozän. Fol. Zool. Hydrobiol. 1932, 4, 167–188. [Google Scholar]
- Crégut, E. La faune de mammifères du Pléistocène moyen de la Caune de l’Arago à Tautavel (Pyrénées-Orientales). Trav. Laborat. Paléontol. Hum. Préh. Marseille 1979, 3, 1–381. [Google Scholar]
- García, N.; Arsuaga, J.L.; Torres, T.D. The carnivore remains from the Sima de los Huesos Middle Pleistocene site (Sierra de Atapuerca, Spain). J. Hum. Evol. 1997, 33, 155–174. [Google Scholar] [CrossRef] [Green Version]
- Moigne, A.-M.; Palombo, M.R.; Belda, V.; Heriech-Briki, D.; Kacimi, S.; Lacombat, F.; de Lumley, M.-A.; Moutoussamy, J.; Rivals, F.; Quilèsa, J.; et al. Les faunes de grands mammifères de la Caune de l’Arago (Tautavel) dans le cadre biochronologique des faunes du Pléistocène moyen italien. L’Anthropologie 2006, 110, 788–831. [Google Scholar] [CrossRef]
- Rabeder, G. Die Carnivore (Mammalia) aus dem Altpleistozän von Deutsch-Altenburg 2. Mit Beiträgen zur Systematik einiger Musteliden und Caniden. Beit. Paläontol. 1976, 1, 5–119. [Google Scholar]
- Spassov, N. Villafranchian succession of mammalian megafaunas from Bulgaria and the biozonation of southeast Europe. Geol. Balc. 1997, 27, 83–94. [Google Scholar]
- Spassov, N. Biochronology and zoogeographical affinities of the Villafranchian faunas of Bulgaria and south Europe. Hist. Nat. Bulg. 2000, 12, 89–128. [Google Scholar]
- Spassov, N. The Plio-Pleistocene vertebrate fauna in South-Eastern Europe and the megafaunal migratory waves from the east to Europe. Rev. Paléobiol. 2003, 22, 197–229. [Google Scholar]
- Odintzov, I.A. Vulpes praecorsac Kormos from Pliocene deposits of Odessa. Paleont. Zb. 1965, 2, 57–64. [Google Scholar]
- Rook, L.; Bartolini Lucenti, S.; Bukhsianidze, M.; Lordkipanidze, D. The Kvabebi Canidae record revisited (late Pliocene, Sighnaghi, eastern Georgia). J. Paleontol. 2017, 91, 1258–1271. [Google Scholar] [CrossRef] [Green Version]
- Madurell-Malapeira, J.; Bartolini Lucenti, S.; Alba, D.M. Middle Pleistocene fox from the Vallparadís Section (Vallès-Penedès Basin, NE Iberian Peninsula) and the earliest records of the extant red fox. Riv. It. Paleontol. Strat. 2021, 127, 179–187. [Google Scholar]
- Geraads, D.; Drapeau, M.S.; Bobe, R.; Fleagle, J.G. Vulpes mathisoni, sp. nov., a new fox from the Pliocene Mursi Formation of southern Ethiopia and its contribution to the origin of African foxes. J. Vertebr. Paleontol. 2015, 35, e943765. [Google Scholar] [CrossRef]
- Szuma, E. Geography of dental polymorphism in the red fox Vulpes vulpes and its evolutionary implications. Biol. J. Linn. Soc. 2007, 90, 61–84. [Google Scholar] [CrossRef] [Green Version]
- Szuma, E. Geography of sexual dimorphism in the tooth size of the red fox Vulpes vulpes (Mammalia, Carnivora). J. Zool. Syst. Evol. Res. 2008, 46, 73–81. [Google Scholar] [CrossRef]
- Szuma, E. Evolutionary and climatic factors affecting tooth size in the red fox Vulpes vulpes in the Holarctic. Mamm. Res. 2008, 53, 289–332. [Google Scholar] [CrossRef]
- Szuma, E. Geographic variation of tooth and skull sizes in the arctic fox Vulpes (Alopex) lagopus. Ann. Zool. Fenn. 2008, 45, 185–199. [Google Scholar] [CrossRef]
- Szuma, E. Ecological and evolutionary determinants of dental polymorphism in the arctic fox Vulpes (Alopex) lagopus. Ann. Zool. Fenn. 2011, 48, 191–214. [Google Scholar] [CrossRef]
- Sher, A.V. Olyorian land mammal age of northeastern Siberia. Palaeontogr. Ital. 1986, 74, 97–112. [Google Scholar]
- Lister, A.M. The impact of Quaternary Ice Ages on mammalian evolution. Phil Trans. Royal Soc. B Biol. Sci. 2004, 359, 221–241. [Google Scholar] [CrossRef] [Green Version]
- Krakhmalnaya, T.V. Carnivore remains from Late Pleistocene and Holocene deposits in the Ukraine. Archäol. Eurasien 1999, 6, 223–235. [Google Scholar]


| Site | Age | E. odessanus | N. donnezani | L. falconeri | L. lycaonoides | Cuon alpinus priscus | Cuon alpinus fossilis | Cuon alpinus europaeus | Canis etruscus | Canis mosbachensis | Canis lupus lunellensis | Canis lupus spelaeus | Canis lupus lupus/ssp. | Vulpes alopecoides | Vulpes praeglacialis | Vulpes vulpes | Vulpes lagopus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Węże 1 | 3.8–3.4 myr | ||||||||||||||||
| Węże 2 | 2.8–2.6 myr | ||||||||||||||||
| Rębielice Królewskie 1A | 2.4–2.2 myr | ||||||||||||||||
| Zamkowa Dolna Cave A | 2.4–2.2 myr | ||||||||||||||||
| Kielniki 3B | 2.2–1.8 myr | ||||||||||||||||
| Kadzielnia | 2.2–1.8 myr | ||||||||||||||||
| Kamyk | 1.9–1.8 myr | ||||||||||||||||
| Żabia Cave | 1.7–1.5 myr | ||||||||||||||||
| Kielniki 3A | 1.5–1.3 myr | ||||||||||||||||
| Zalesiaki 1A | 1.1–0.9 myr | ||||||||||||||||
| Kielniki 1 | 1.1–0.8 myr | ||||||||||||||||
| Zamkowa Dolna Cave C | 1.0–0.8 myr | ||||||||||||||||
| Przymiłowice | MIS 22-17 | ||||||||||||||||
| Kozi Grzbiet | MIS 19-17 | ||||||||||||||||
| Sitkówka | MIS 19-17 | ||||||||||||||||
| Południowa Cave | MIS 17-15 | ||||||||||||||||
| Rębielice Królewskie 2 | MIS 15-13 | ||||||||||||||||
| Tunel Wielki Cave | MIS 14-12 | ||||||||||||||||
| Draby 3 | MIS 11 | ||||||||||||||||
| Biśnik Cave, l. 19ad-19 | MIS 9 | ||||||||||||||||
| Deszczowa Cave, l. 1-5 | MIS 9-8 | ||||||||||||||||
| Wschodnia Cave | MIS 8-7 | ||||||||||||||||
| Biśnik Cave, l. 18-13 | MIS 7-5e | ||||||||||||||||
| Wierzchowska Górna Cave | MIS 7-1 | ||||||||||||||||
| Nietoperzowa Cave | MIS 6-1 | ||||||||||||||||
| Komarowa Cave | MIS 6-1 | ||||||||||||||||
| Stajnia Cave | MIS 6-1 | ||||||||||||||||
| Dziadowa Skała Cave | MIS 5e-1 | ||||||||||||||||
| Mamutowa Cave | MIS 5-1 | ||||||||||||||||
| Koziarnia Cave | MIS 5-1 | ||||||||||||||||
| Niedźwiedzia Cave | MIS 5d-1 | ||||||||||||||||
| Raj Cave | MIS 5-1 | ||||||||||||||||
| Biśnik Cave, l. 12-1 | MIS 5d-1 | ||||||||||||||||
| Radochowska Cave | MIS 5d-1 | ||||||||||||||||
| Skarszyn | MIS 3 | ||||||||||||||||
| Kraków-Spadzista | MIS 3 | ||||||||||||||||
| Late Pleistocene 1 | MIS 5d-1 | ||||||||||||||||
| Holocene 2 | MIS 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marciszak, A.; Kropczyk, A.; Gornig, W.; Kot, M.; Nadachowski, A.; Lipecki, G. History of Polish Canidae (Carnivora, Mammalia) and Their Biochronological Implications on the Eurasian Background. Genes 2023, 14, 539. https://doi.org/10.3390/genes14030539
Marciszak A, Kropczyk A, Gornig W, Kot M, Nadachowski A, Lipecki G. History of Polish Canidae (Carnivora, Mammalia) and Their Biochronological Implications on the Eurasian Background. Genes. 2023; 14(3):539. https://doi.org/10.3390/genes14030539
Chicago/Turabian StyleMarciszak, Adrian, Aleksandra Kropczyk, Wiktoria Gornig, Małgorzata Kot, Adam Nadachowski, and Grzegorz Lipecki. 2023. "History of Polish Canidae (Carnivora, Mammalia) and Their Biochronological Implications on the Eurasian Background" Genes 14, no. 3: 539. https://doi.org/10.3390/genes14030539
APA StyleMarciszak, A., Kropczyk, A., Gornig, W., Kot, M., Nadachowski, A., & Lipecki, G. (2023). History of Polish Canidae (Carnivora, Mammalia) and Their Biochronological Implications on the Eurasian Background. Genes, 14(3), 539. https://doi.org/10.3390/genes14030539





