Single-Nucleotide Polymorphisms as Biomarkers of Antipsychotic-Induced Akathisia: Systematic Review
Abstract
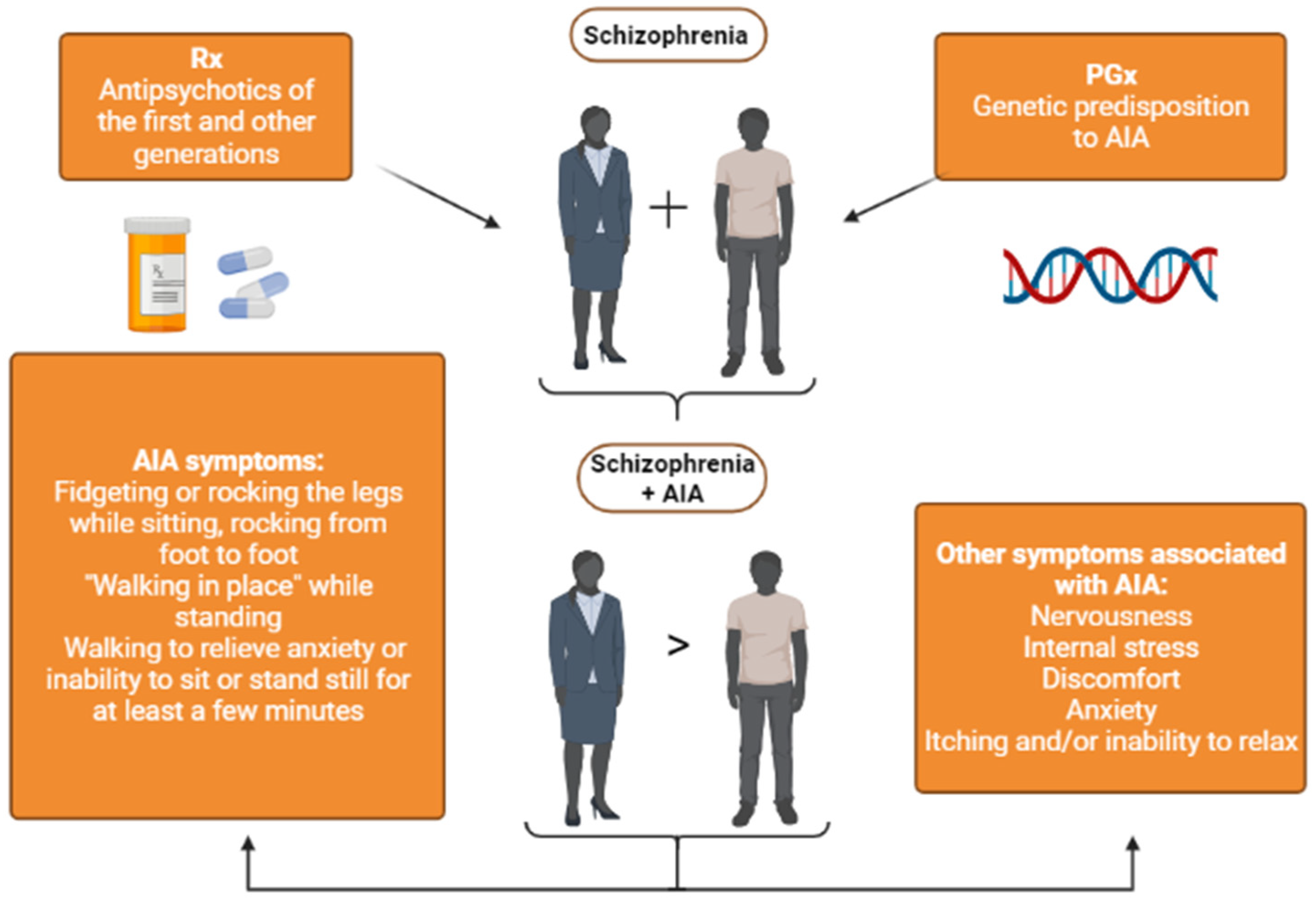
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Review Strategy
2.4. Data Synthesis
2.5. Data Not Included in the Review
3. Results
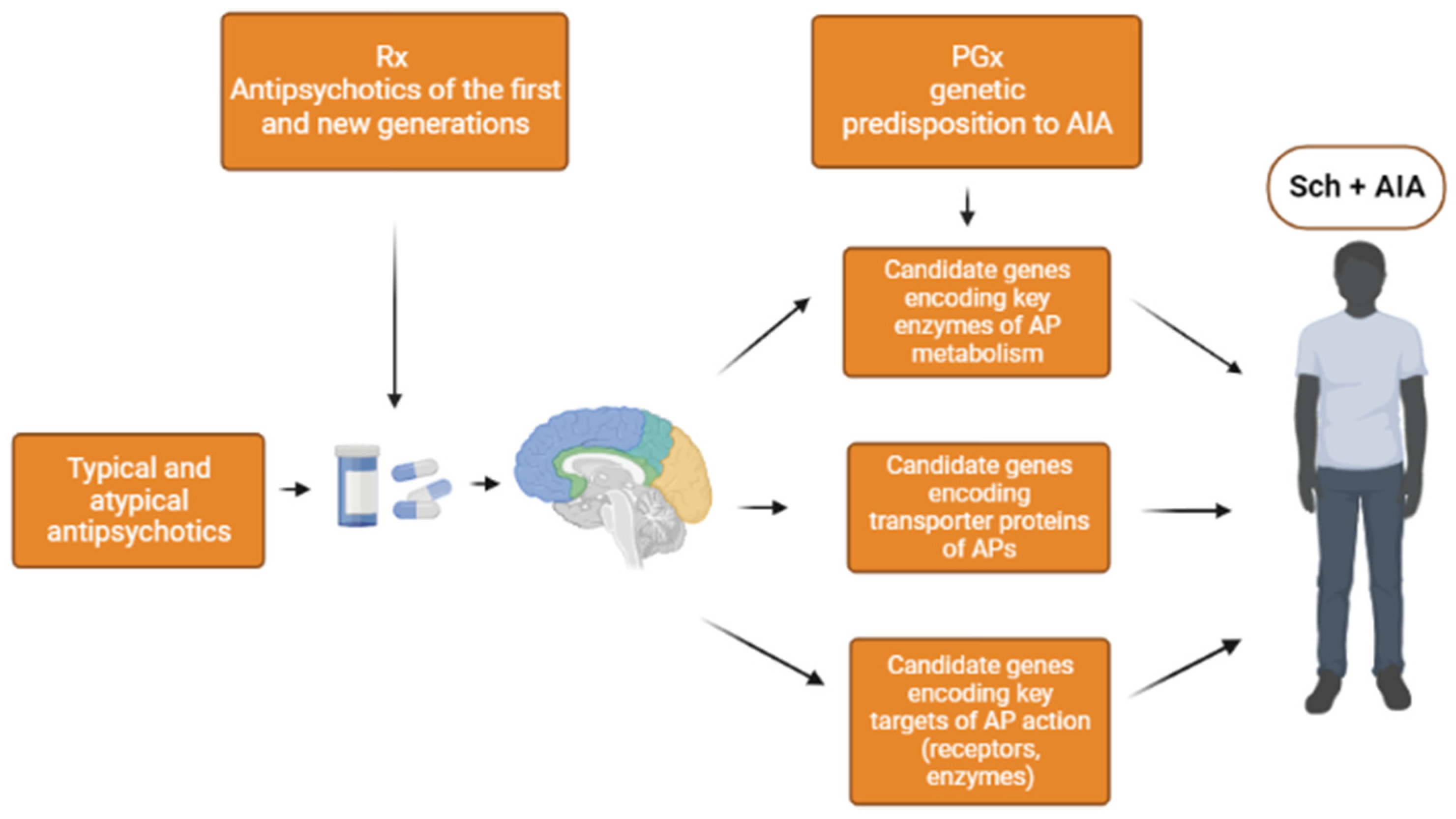
3.1. Genes Encoding Key Enzymes in Metabolism of Antipsychotics
3.2. Genes Encoding the Transport Proteins of Antipsychotics (via the Blood-Brain Barrier)
3.3. Genes Encoding Targets of Antipsychotics
3.3.1. Key Receptors for Antipsychotics Action
3.3.2. Key Enzymes of Antipsychotics Action
3.4. Evidence from a Systematic Review
| Gene (OMIM * Number) | SNP (Location) | Association with AIA | p-Value | Sample | Country | Reference |
|---|---|---|---|---|---|---|
| DRD2 (126450) | rs1800498 (NG_008841.1:g.59414C>T) (TaqI_D) | Major allele C is associated with the risk of AIA | 0.001 | 402 | The Netherlands | [76] |
| rs1800497 (NG_012976.1:g.17316G>A) (TaqIA) | Minor allele A is associated with the risk of AIA | 0.03 | 402 | The Netherlands | [76] | |
| 0.011 | 234 | Australia | [77] | |||
| HTR1B (182131) | rs13212041 (NC_000006.12:g.77461407C>T) | Homozygous genotype TT is associated with the risk of AIA | 0.004 | 229 | Croatia | [75] |
3.4.1. The DRD2 Gene
3.4.2. The HTR1B Gene
4. Discussion
4.1. Summary of Evidence
4.2. Comparison with the Existing Literature
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Primary Akathisia | Secondary Akathisia |
|---|---|
| Huntington’s disease | Drug-induced akathisia |
| Hereditary kinesiogenic dystonia | Sindengham`s disease |
| Hereditary non-kinesiogenic dystonia | Symptomatic Tourette’s syndrome |
| Familial Tourette syndrome |
| Drug | Frequency of Akathisia | Mechanism | Level of Evidence | References |
|---|---|---|---|---|
| ||||
| ||||
| Haloperidol | 24.8% | Blockade of dopaminergic D2 receptors in the limbic system and basal ganglia | A | [99] |
| Chlorpromazine | 15.9% | A | ||
| ||||
| Amisulpride | 11.3% | Blockade of serotonergic 5-HT2, dopaminergic D2, and adrenergic receptors in the limbic system and basal ganglia | A | [100] |
| Aripiprazole | 8.5% | A | [101] | |
| Ziprasidone | 8.3% | A | ||
| Clozapine | 7.9% | A | [102] | |
| Quetiapine | 5.2% | A | [101] | |
| Olanzapine | 8.7% | A | ||
| Paliperidone | 3.3% | A | ||
| Risperidone | 14.2% | A | [103] | |
| Sulpiride | 16.4% | A | [101] | |
| ||||
| ||||
| Fluoxetine | NA | Blockade of serotonergic 5-HT2C receptors | C | [104] |
| Sertraline | NA | Dopamine inhibition through its high-affinity sodium-dependent reuptake in presynaptic endings | C | |
| Paroxetine | NA | Blockade of dopaminergic D2 receptors | C | |
| Fluvoxamine | NA | Blockade of serotonin reuptake, increased action of serotonin on 5HT1A autoreceptors | C | |
| Escitalopram | NA | Blockade of dopaminergic D2 receptors | C | |
| ||||
| Venlafaxine | NA | Dopamine inhibition through its high-affinity sodium-dependent reuptake in presynaptic endings | C | [104] |
| Duloxetine | NA | C | ||
| ||||
| Mirtazapine | NA | Blockade of serotonergic 5-HT2- and 5-HT3-receptors as well as α2-adrenergic receptors | C | [105] |
| ||||
| Azithromycin | NA | Unknown | C | [106] |
| Clarithromycin | NA | C | [107] | |
| ||||
| Amlodipine | NA | Receptor hypersensitivity in the substantia nigra | C | [108] |
| Flunarizine | NA | Unknown | C | [109] |
| ||||
| Clonazepam | NA | Agonist of GABA receptors | C | [110] |
| Clorazepate | NA | C | ||
| Lorazepam | NA | C | ||
| ||||
| Betaxolol | NA | Unknown | A | [111] |
| ||||
| Propanolol | NA | Blockade of serotonergic 5-HT1A and 5-HT1B receptors | A | [112] |
| ||||
| Pregabalin | NA | Unknown | C | [113] |
| Lamotrigine | NA | Blockade of dopaminergic D2 receptors | C | [114] |
| ||||
| MDMA (Ecstasy) | NA | Blockade of dopaminergic and serotonergic 5-HT receptors, a decrease in the number of 5-HT receptors | C | [115] |
| Cocaine | NA | Dopamine reuptake inhibition | C | [116] |
| ||||
| Ciprofloxacin | NA | Unknown | C | [111] |
| ||||
| L-DOPA | NA | Increased activity of the direct dopaminergic striatal pathway | C | [117] |
| ||||
| Lithium | NA | Activation of dopaminergic neurotransmission | C | [118] |
| ||||
| Metoclopramide | 20–25% | Blockade of dopaminergic D2 receptors | B | [119] |
| Non-Modifiable Risk Factor | Modifiable Risk Factor |
|---|---|
| Second period of middle age (male: 36–60 years; female: 36–55 years) * | Reception APs:
|
| Female (for tardive AIA) | Brain injury |
| Caucasians | Malignant neoplasms |
| Genetic predisposition | Ferritin deficiency Low serum iron |
| Alcohol abuse | |
| Vitamin B6 deficiency | |
| Autoimmune NMDAR encephalitis |
| Gene (OMIM Number *) | Chromosome Location ** | Genomic Coordinate ** | Protein *** |
|---|---|---|---|
| CYP1A2 (124060) | 15q24.1 | chr15:74,748,845–74,756,607(GRCh38/hg38) | Cytochrome P450 Family 1 Subfamily A Member 2 |
| CYP2C9 (601130) | 10q23.33 | chr10:94,938,658–94,990,091(GRCh38/hg38) | Cytochrome P450 Family 2 Subfamily C Member 9 |
| CYP2C19 (124020) | 10q23.33 | chr10:94,762,681–94,855,547(GRCh38/hg38) | Cytochrome P450 Family 2 Subfamily C Member 19 |
| CYP2D6 (124030) | 22q13.2 | chr22:42,126,499–42,130,865(GRCh38/hg38) | Cytochrome P450 Family 2 Subfamily D Member 6 |
| CYP3A4 (124010) | 7q22.1 | chr7:99,756,960–99,784,248(GRCh38/hg38) | Cytochrome P450 Family 3 Subfamily A Member 4 |
| Antipsychotic | Metabolism in the Liver | Enzyme of Cytochrome P450 |
|---|---|---|
| ||
| Chlorpromazine | Hydroxylation N-dealkylation | CYP2D6 (the main path), CYP1A2, and CYP3A4 |
| Haloperidol | Glucuronization N-dealkylation | CYP2D6, CYP3A, and CYP1A2 |
| Perphenazine | Oxidation, N-dealkylation | CYP2D6, CYP1A2, and CYP3A4 |
| Thioridazine | Betaoxidation N-dealkylation | CYP2D6 and CYP1A2 |
| Flupentixol | Sulfonic acidification N-dealkylation Glucuronization | CYP2D6 |
| Tiapride | Oxidation (up to 15%) | Underexplored |
| Sulpiride | Not metabolized, excreted through the kidneys (about 95%) | Not involved in metabolism |
| ||
| Aripiprazole | Oxidation N-dealkylation | CYP2D6 and CYP3A |
| Amisulpiride | Oxidation (about 4%). | Underexplored |
| Asenapine | Glucuronization Oxidation Demethylation | CYP2D6 (the main path), CYP1A2, and CYP3A4 |
| Ziprasidone | Oxidation | CYP3A4 |
| Quetiapine | Oxidation | CYP3A4 and CYP2D6 |
| Clozapine | Oxidation | CYP1A2 |
| Lurasidone | Oxidation N-dealkylation | CYP3A4 |
| Olanzapine | Oxidation | CYP2D6 and CYP1A2 |
| Paliperidone | No hepatic metabolism. Excreted through the kidneys | Not involved in metabolism |
| Risperidone | Oxidation | CYP2D6 and CYP3A |
| Sertindol | Oxidation | CYP3A4 and CYP2D6 |
| Gene (OMIM * Number) |
Chromosome Location ** | Genomic Coordinate ** | Protein *** |
|---|---|---|---|
| ABCB1 (171050) | 7q21.12 | chr7:87,503,017–87,713,323(GRCh38/hg38) | Multidrug Resistance Protein 1 (MDR1) P-Glycoprotein 1 (P-gp) |
| ABCB2 (170260) | 6p21.32 | chr6:32,845,209–32,853,816(GRCh38/hg38) | Transporter 1 (TAP1) |
| ABCB3 (170261) | 6p21.32 | chr6:32,821,831–32,838,770(GRCh38/hg38) | Transporter 2 (TAP2) |
| ABCB4 (171060) | 7q21.12 | chr7:87,398,988–87,480,435(GRCh38/hg38) | Multidrug Resistance Protein 3 (MDR2) P-Glycoprotein 3 |
| ABCB11 (603201) | 2q31.1 | chr2:168,915,468–169,031,396(GRCh38/hg38) | Bile Salt Export Pump (BSEP) |
| ABCC1 (158343) | 16p13.11 | chr16:15,949,138–16,143,257(GRCh38/hg38) | Protein associated with multidrug resistance (MRP1) |
| ABCC2 (601107) | 10q24.2 | chr10:99,782,602–99,853,741(GRCh38/hg38) | Multidrug Resistance-Associated Protein 2 (MRP2) |
| ABCC3 (604323) | 17q21.33 | chr17:50,634,777–50,692,253(GRCh38/hg38) | Multidrug Resistance-Associated Protein 3 (MRP3) |
| ABCC4 (605250) | 13q32.1 | chr13:95,019,835–95,301,475(GRCh38/hg38) | Multidrug Resistance-Associated Protein 4 (MRP4) |
| ABCC5 (605251) | 3q27.1 | chr3:183,919,934–184,018,010(GRCh38/hg38) | Multidrug Resistance-Associated Protein 5 (MRP5) |
| ABCC6 (603234) | 16p13.11 | chr16:16,149,565–16,223,617(GRCh38/hg38) | Multidrug Resistance-Associated Protein 6 (MRP6) |
| ABCC1 (612509) | 6p21.1 | chr6:43,427,366–43,451,994(GRCh38/hg38) | Multidrug Resistance-Associated Protein 7 (MRP7) |
| ABCC11 (607040) | 16q12.1 | chr16:48,164,842–48,249,973(GRCh38/hg38) | Multidrug Resistance-Associated Protein 8 (MRP8) |
| ABCC12 (607041) | 16q12.1 | chr16:48,080,882–48,156,018(GRCh38/hg38) | Multidrug Resistance-Associated Protein 9 (MRP9) |
| ABCG2 (603756) | 4q22.1 | chr4:88,090,150–88,231,628(GRCh38/hg38) | Breast cancer resistance protein (BCRP) |
| P-Glycoprotein (P-gp) | Breast Cancer Resistance Protein (BCRP) | Multidrug Resistance-Associated Protein 1 (MRP1) |
|---|---|---|
| Amisulpride Aripiprazole Asenapine Chlorpromazine Chlorprothixene Clozapine Fluphenazine Flupentixol Olanzapine Paliperidone Periciazine Quetiapine Risperidone Sertindole Sulpiride Trifluoperazine Ziprasidone Zuclopenthixol | Aripiprazole Chlorpromazine Clozapine Haloperidol Olanzapine Paliperidone Quetiapine Risperidone Sulpiride | Clozapine |
| Gene (OMIM * Number) | Chromosome Location ** | Genomic Coordinate ** | Protein *** |
|---|---|---|---|
| Dopaminergic system | |||
| DRD2 (126450) | 11q23.2 | chr11:113,409,605–113,475,691(GRCh38/hg38) | Dopaminergic receptor type D2 |
| DRD3 (126451) | 3q13.31 | chr3:114,127,580–114,199,407(GRCh38/hg38) | Dopaminergic receptor type D3 |
| DRD4 (126452) | 11p15.5 | chr11:637,269–640,706(GRCh38/hg38) | Dopaminergic receptor type D4 |
| Serotoninergic system | |||
| HTR2A (182135) | 13q14.2 | chr13:46,831,546–46,898,082(GRCh38/hg38) | Serotonergic 5-hydroxytryptamine receptor type 2A |
| HTR2C (312861) | Xq23 | chrX:114,584,078–114,910,061(GRCh38/hg38) | Serotonergic 5-hydroxytryptamine receptor type 2C |
| Glutamatergic system | |||
| GRIN2A (138253) | 16p13.2 | chr16:9,753,404–10,182,928(GRCh38/hg38) | Glutamate ionotropic receptor type 2A |
| GRIN2B (138252) | 12p13.1 | chr12:13,437,942–13,982,134(GRCh38/hg38) | Glutamate ionotropic receptor type 2B |
| Gene (OMIM * Number) | Chromosome Location ** | Genomic Coordinate ** | Protein *** |
|---|---|---|---|
| HSPG2 (142461) | 1p36.12 | chr1:21,822,244–21,937,310(GRCh38/hg38) | Perlecan |
| COMT (116790) | 22q11.21 | chr22:19,941,371–19,969,975(GRCh38/hg38) | Catechol-O-methyltransferase |
| NQO1 (125860) | 16q22.1 | chr16:69,706,996–69,726,668(GRCh38/hg38) | Quinone dehydrogenase nicotinamide adenine dinucleotide phosphate |
| RGS2 (600861) | 1q31.2 | chr1:192,809,039–192,812,275(GRCh38/hg38) | G protein signal transduction regulator 2 |
| GSTP1 (134660) | 11q13.2 | chr11:67,583,742–67,586,656(GRCh38/hg38) | Glutathione-S-transferase pi 1 |
| PPP1R1B (604399) | 17q12 | chr17:39,626,707–39,636,626(GRCh38/hg38) | Regulatory inhibitor of subunit 1B of protein phosphatase 1 |
| BDNF (113505) | 11p14.1 | chr11:27,654,893–27,722,058(GRCh38/hg38) | Brain-derived neurotrophic factor |
| MnSOD (147460) | 6q25.3 | chr6:159,669,069–159,762,281(GRCh38) | Manganese superoxide dismutase 2 |



References
- Mohr, P.; Volavka, J. Ladislav Haskovec and akathisia: 100th anniversary. Br. J. Psychiatry 2002, 181, 537. [Google Scholar] [CrossRef] [PubMed]
- Rummel-Kluge, C.; Komossa, K.; Schwarz, S.; Hunger, H.; Schmid, F.; Kissling, W.; Davis, J.M.; Leucht, S. Second-generation antipsychotic drugs and extrapyramidal side effects: A systematic review and meta-analysis of head-to-head comparisons. Schizophr. Bull. 2012, 38, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Brüne, M. Ladislav Haskovec and 100 years of akathisia. Am. J. Psychiatry 2002, 159, 727. [Google Scholar] [CrossRef]
- Kruse, W. Treatment of drug-induced extrapyramidal symptoms. (A comparative study of three antiparkinson agents). Dis. Nerv. Syst. 1960, 21, 79–81. [Google Scholar] [PubMed]
- Martino, D.; Karnik, V.; Osland, S.; Barnes, T.R.E.; Pringsheim, T.M. Movement disorders associated with antipsychotic medication in people with schizophrenia: An overview of Cochrane reviews and meta-analysis. Can. J. Psychiatry 2018, 63, 730–739. [Google Scholar] [CrossRef]
- Vaiman, E.E.; Shnayder, N.A.; Neznanov, N.G.; Nasyrova, R.F. Diagnostic methods of antipsychotic-induced extrapyramidal disorders. Sib. Med. Rev. 2019, 5, 5–13. (In Russian) [Google Scholar] [CrossRef]
- Vaiman, E.E.; Shnayder, N.A.; Novitsky, M.A.; Dobrodeeva, V.S.; Goncharova, P.S.; Bochanova, E.N.; Sapronova, M.R.; Popova, T.E.; Tappakhov, A.A.; Nasyrova, R.F. Candidate genes encoding dopamine receptors as predictors of the risk of antipsychotic-induced parkinsonism and tardive dyskinesia in schizophrenic patients. Biomedicines 2021, 9, 879. (In Russian) [Google Scholar] [CrossRef]
- Shin, H.W.; Chung, S.J. Drug-induced parkinsonism. J. Clin. Neurol. 2012, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.; Dencker, S.J.; Malm, U.; Dahl, M.L.; Svenson, J.O.; Halldin, C.; Naskashima, Y.; Farde, L. D(2)- and 5-HT(2) receptor occupancy in high-dose neuroleptic-treated patients. Int. J. Neuropsychopharmacol. 1998, 1, 95–101. [Google Scholar] [CrossRef]
- Sebastião, A.M.; Ribeiro, J.A. Adenosine receptors and the central nervous system. Handb. Exp. Pharm. 2009, 193, 471–534. [Google Scholar] [CrossRef]
- Jenner, P.; Mori, A.; Hauser, R.; Morelli, M.; Fredholm, B.B.; Chen, J.F. Adenosine, adenosine A 2A antagonists, and Parkinson’s disease. Park. Relat Disord 2009, 15, 406–413. [Google Scholar] [CrossRef]
- Hettinger, B.D.; Lee, A.; Linden, J.; Rosin, D.L. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J. Comp. Neurol. 2001, 431, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Varty, G.B.; Hodgson, R.A.; Pond, A.J.; Grzelak, M.E.; Parker, E.M.; Hunter, J.C. The effects of adenosine A2A receptor antagonists on haloperidol-induced movement disorders in primates. Psychopharmacology 2008, 200, 393–401. [Google Scholar] [CrossRef]
- Müller, C.E.; Ferré, S. Blocking striatal adenosine A2A receptors: A new strategy for basal ganglia disorders. Recent. Pat. CNS Drug Discov. 2007, 2, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.E.; Caballero, J.; Harrington, C.A. The Incidence of Akathisia in the Treatment of Schizophrenia with Aripiprazole, Asenapine and Lurasidone: A Meta-Analysis. Curr. Neuropharmacol. 2015, 13, 681–691. [Google Scholar] [CrossRef]
- Lencz, T.; Malhotra, A.K. Pharmacogenetics of antipsychotic-induced side effects. Dialogues Clin. Neurosci. 2009, 11, 405–415. [Google Scholar] [CrossRef] [PubMed]
- PRISMA. Available online: https://www.prisma-statement.org/ (accessed on 24 December 2022).
- Herken, M.E.; Erdal, K.; Esgi, O.; Virit, A.S.; Aynacioglu, A.S. The relationship between the response to risperidone treatment and 5-HT2A receptor gene (T102C and 1438G/A) polymorphism in schizophrenia. Bull Clin. Psychopharmacol. 2003, 13, 161–166. [Google Scholar] [CrossRef]
- Lu, J.; Yang, Y.; Lu, J.; Wang, Z.; He, Y.K.; Yan, Y.; Fu, K.; Jiang, W.; Xu, Y.; Wu, R.; et al. Effect of CYP2D6 polymorphisms on plasma concentration and therapeutic effect of risperidone. BMC Psychiatry 2021, 21, 70. [Google Scholar] [CrossRef]
- Gunes, A.; Scordo, M.G.; Jaanson, P.; Dahl, M.L. Serotonin and dopamine receptor gene polymorphisms and the risk of extrapyramidal side effects in perphenazine-treated schizophrenic patients. Psychopharmacology 2007, 190, 479–484. [Google Scholar] [CrossRef]
- Gunes, A.; Dahl, M.L.; Spina, E.; Scordo, M.G. Further evidence for the association between 5-HT2C receptor gene, polymorphisms and extrapyramidal side effects in male schizophrenic patients. Eur. J. Clin. Pharm. 2008, 64, 477–482. [Google Scholar] [CrossRef]
- Güzey, C.; Scordo, M.G.; Spina, E.; Landsem, V.M.; Spigset, O. Antipsychotic-induced extrapyramidal symptoms in patients with schizophrenia: Associations with dopamine and serotonin receptor and transporter polymorphisms. Eur. J. Clin. Pharm. 2007, 63, 233–241. [Google Scholar] [CrossRef]
- Zivković, M.; Mihaljević-Peles, A.; Bozina, N.; Sagud, M.; Nikolac-Perkovic, M.; Vuksan-Cusa, B.; Muck-Seler, D. The association study of polymorphisms in DAT, DRD2, and COMT genes and acute extrapyramidal adverse effects in male schizophrenic patients treated with haloperidol. J. Clin. Psychopharmacol. 2013, 33, 593–599. [Google Scholar] [CrossRef]
- Lafuente, A.; Bernardo, M.; Mas, S.; Crescenti, A.; Aparici, M.; Gasso, P.; Deulofeu, R.; Mane, A.; Catalan, R.; Carne, X. Polymorphism of dopamine D2 receptor (TaqIA, TaqIB, and-141C Ins/Del) and dopamine degradation enzyme (COMT G158A, A-278G) genes and extrapyramidal symptoms in patients with schizophrenia and bipolar disorders. Psychiatry Res. 2008, 161, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Scordo, M.; Spina, E.; Romeo, P.; Dahl, M.L.; Bertilsson, L.; Johansson, I.; Sjöqvist, F. CYP2D6 genotype and antipsychotic-induced extrapyramidal side effects in schizophrenic patients. Eur. J. Clin. Pharm. 2000, 56, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Schillevoort, I.; de Boer, A.; van der Weide, J.; Steijns, L.S.; Roos, R.A.; Jansen, P.A.; Leufkens, H.G. Antipsychotic-induced extrapyramidal syndromes and cytochrome P450 2D6 genotype: A case–control study. Pharmacogenetics 2002, 12, 235–240. [Google Scholar] [CrossRef]
- Eum, S.; Lee, A.M.; Bishop, J.R. Pharmacogenetic tests for antipsychotic medications: Clinical implications and considerations. Dialogues Clin. Neurosci. 2016, 18, 323–337. [Google Scholar] [CrossRef]
- Sychev, D.A.; Burashnikova, I.S.; Kazakov, R.E. 1846G>A polymorphism of CYP2D6 gene and extrapyramidal side effects during antipsychotic therapy among Russians and Tatars: A pilot study. Drug Metab. Pers. 2016, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, O.A.; MacEwan, T.; Gulbrandsen, A.K.; McCreadie, R.G.; Steen, V.M. Non-functional CYP2D6 alleles and risk for neuroleptic-induced movement disorders in schizophrenic patients. Psychopharmacology 1997, 131, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, D.V.; Khoang, S.Z.; Makhmudova, B.V.; Buromskaya, N.I.; Shimanov, P.V.; Deitch, R.V.; Akmalova, K.A.; Shuev, G.N.; Dorina, I.V.; Nastovich, M.I.; et al. Pharmacogenetics of antipsychotics in adolescents with acute psychotic episode during first 14 days after admission: Effectiveness and safety evaluation. Drug Metab. Pers. 2020, 35, 20200102. [Google Scholar] [CrossRef] [PubMed]
- Bošković, M.; Vovk, T.; Saje, M.; Goričar, K.; Dolžan, V.; Kores Plesničar, B.; Grabnar, I. Association of SOD2, GPX1, CAT, and TNF genetic polymorphisms with oxidative stress, neurochemistry, psychopathology, and extrapyramidal symptoms in schizophrenia. Neurochem. Res. 2013, 38, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, D.V.; Khoang, S.Z.; Tazagulova, M.K.; Makhmudova, B.V.; Buromskaya, N.I.; Shimanov, P.V.; Deitch, R.V.; Dorina, I.V.; Nastovich, M.I.; Akmalova, K.A.; et al. The polymorphic variants DRD2 rs1800497 and ABCB1 3435C>T are associated with antipsychotic safety parameters in adolescents with an acute psychotic episode: The results of a pilot study. Neurol. Neuropsychiatry Psychosom. 2020, 12, 24–31. [Google Scholar] [CrossRef]
- Greenbaum, L.; Strous, R.D.; Kanyas, K.; Merbl, Y.; Horowitz, A.; Karni, O.; Katz, E.; Kotler, M.; Olender, T.; Deshpande, S.N.; et al. Association of the RGS2 gene with extrapyramidal symptoms induced by treatment with antipsychotic medication. Pharm. Genom. 2007, 17, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Zhilyaeva, T.V.; Akimova, E.V.; Sergeeva, A.V.; Blagonravova, A.S.; Mazo, G.E. Association of MTHFR 677C > T genetic polymorphism with extrapyramidal side effects of antipsychotic treatment. Gene Rep. 2020, 20, 100717. [Google Scholar] [CrossRef]
- Maruf, A.A.; Stein, K.; Arnold, P.D.; Aitchison, K.J.; Müller, D.J.; Bousman, C. CYP2D6 and antipsychotic treatment outcomes in children and youth: A systematic review. J. Child Adolesc. Psychopharmacol. 2021, 31, 33–45. [Google Scholar] [CrossRef]
- Zahari, Z.; Salleh, M.R.; The, L.K.; Ismail, R. Influence of CYP2D6 polymorphisms on symptomatology and side-effects of patients with schizophrenia in Malaysia. Malays. J. Med. Sci. 2009, 16, 12–20. [Google Scholar]
- Bakker, P.R.; Bakker, E.; Amin, N.; van Duijn, C.M.; van Os, J.; van Harten, P.N. Candidate gene-based association study of antipsychotic-induced movement disorders in long-stay psychiatric patients: A prospective study. PLoS ONE 2012, 7, e36561. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.R.; Al Hadithy, A.F.; Amin, N.; van Duijn, C.M.; van Os, J.; van Harten, P.N. Antipsychotic-induced movement disorders in long-stay psychiatric patients and 45 tag SNPs in 7 candidate genes: A prospective study. PLoS ONE 2012, 7, e50970. [Google Scholar] [CrossRef]
- Dolzan, V.; Serretti, A.; Mandelli, L.; Koprivsek, J.; Kastelic, M.; Plesnicar, B.K. Acute antipsychotic efficacy and side effects in schizophrenia: Association with serotonin transporter promoter genotypes. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1562–1566. [Google Scholar] [CrossRef]
- Ruda, D.; Jensen, K.G.; Decara, M.S.; Klauber, D.G.; Fagerlund, B.; Møllegaard, J.R.; Linnet, K.; Werge, T.; Correll, C.U.; Fink-Jensen, A.; et al. CYP2D6 Genotyping and antipsychotic-associated extrapyramidal adverse effects in a randomized trial of aripiprazole versus quetiapine extended release in children and adolescents, aged 12-17 years, with first episode psychosis. J. Clin. Psychopharmacol. 2021, 41, 667–672. [Google Scholar] [CrossRef]
- Plesnicar, B.K.; Zalar, B.; Breskvar, K.; Dolzan, V. The influence of the CYP2D6 polymorphism on psychopathological and extrapyramidal symptoms in the patients on long-term antipsychotic treatment. J. Psychopharmacol. 2006, 20, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Pouget, J.G.; Shams, T.A.; Tiwari, A.K.; Müller, D.J. Pharmacogenetics and outcome with antipsychotic drugs. Dialogues Clin. Neurosci. 2014, 16, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Gassó, P.; Mas, S.; Oliveira, C.; Bioque, M.; Parellada, E.; Bernardo, M.; Trias, G.; Comeche, J.; Lafuente, A. Searching for functional SNPs or rare variants in exonic regions of DRD3 in risperidone-treated patients. Eur. Neuropsychopharmacol. 2011, 21, 294–299. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, E.; Kang, D.H.; Choi, J.S.; Yu, K.S.; Jang, I.J.; Shin, S.G. APLUS study group. Taq1A polymorphism in the dopamine D2 receptor gene as a predictor of clinical response to aripiprazole. Eur. Neuropsychopharmacol. 2008, 18, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Khot, A.M.; Anuradha, H.V.; Prakash, V.S.; Shivamurathy, M.C. Antianginal efficacy and tolerability of ranolazine as an add-on drug to concomitant medications primarily metoprolol in chronic stable angina patients: A prospective, open-label study. J. Pharmacol. Pharmacother. 2017, 8, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Gorgun, O.V.; Goloenko, I.M.; Obiedkov, V.G.; Levdansky, O.D.; Shimkevich, A.M.; Shatarnova, T.M.; Ruban, A.E. Risk Relationship occurrence of acute drug-induced extrapyramidal disorders in antipsychotic therapy of paranoid schizophrenia. Mil. Med. 2019, 1, 32–40. [Google Scholar]
- Zastrozhin, M.S.; Sychev, D.A.; Grishina, E.A.; Savchenko, L.M.; Bryun, E.A. Pharmacodynamic gene polymorphism and adverse drug reactionsthen applying antipsychotic drugs. World J. Pers. Med. 2017, 1, 5–12. (In Russian) [Google Scholar] [CrossRef]
- Kaiser, R.; Tremblay, P.B.; Klufmöller, F.; Roots, I.; Brockmöller, J. Relationship between adverse effects of antipsychotic treatment and dopamine D(2) receptor polymorphisms in patients with schizophrenia. Mol. Psychiatry 2002, 7, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Shnayder, N.A.; Abdyrakhmanova, A.K.; Nasyrova, R.F. Oxidation of antipsychotics. Encyclopedia 2022, 2, 974–989. [Google Scholar] [CrossRef]
- Shen, W.W. The metabolism of atypical antipsychotic drugs: An Update. Ann. Clin. Psychiatry 1999, 11, 145–158. [Google Scholar] [CrossRef]
- Beedham, C. The role of non-P450 enzymes in drug oxidation. Pharm. World Sci. 1997, 19, 255–263. [Google Scholar] [CrossRef]
- Manikandan, P.; Nagini, S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets 2018, 19, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Nasyrova, R.F.; Kravtsov, V.V.; Dobrodeeva, V.S.; Shnayder, N.A.; Neznanov, N.G. Pharmacogenetics of antipsychotics. In Clinical Psychopharmacogenetics; Nasyrova, R.F., Neznanov, N.G., Eds.; DEAN Publishing House: Saint Petersburg, Russia, 2019; pp. 93–174. (In Russian) [Google Scholar]
- Danielson, P.B. The cytochrome P450 superfamily: Biochemistry, evolution and drug metabolism in humans. Curr. Drug Metab. 2002, 3, 561–597. [Google Scholar] [CrossRef] [PubMed]
- Naumovska, Z.; Nestorovska, A.K.; Filipce, A.; Sterjev, Z.; Brezovska, K.; Dimovski, A.; Suturkova, L.J. Pharmacogenetics and antipsychotic treatment response. Pril (Makedon Akad Nauk Umet Odd Med. Nauki) 2015, 36, 53–67. [Google Scholar] [PubMed]
- Arranz, M.J.; Gallego, C.; Salazar, J.; Arias, B. Pharmacogenetic studies of drug response in schizophrenia. Expert Rev. Precis. Med. Drug Dev. 2016, 1, 79–91. [Google Scholar] [CrossRef]
- Kirchheiner, J.; Nickchen, K.; Bauer, M.; Wong, M.L.; Licinio, J.; Roots, I.; Brockmoller, J. Pharmacogenetics of antidepressants and antipsychotics: The contribution of allelic variations to the phenotype of drug response. Mol. Psychiatry 2004, 9, 442–473. [Google Scholar] [CrossRef]
- Vaiman, E.E.; Shnayder, N.A.; Neznanov, N.G.; Nasyrova, R.F. Candidate genes involved in the development of anti-psychotic-induced tardive dyskinesia in patients with schizophrenia. Neuromuscul. Dis. 2020, 10, 10–26. [Google Scholar] [CrossRef]
- Gorbachev, V.I.; Bragina, N.V. Blood-brain barrier from the point of view of anesthesiologist. Review. Part 1. Ann. Crit. Care 2020, 3, 35–45. [Google Scholar] [CrossRef]
- Barar, J.; Rafi, M.A.; Pourseif, M.M.; Omidi, Y. Blood-brain barrier transport machineries and targeted therapy of brain diseases. Bioimpacts 2016, 6, 225–248. [Google Scholar] [CrossRef]
- Mahringer, A.; Ott, M.; Reimold, I.; Reichel, V.; Fricker, G. The ABC of the blood-brain barrier—regulation of drug efflux pumps. Curr. Pharm. Des. 2011, 17, 2762–2770. [Google Scholar] [CrossRef]
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.M.; Decleves, X.; Menet, M.C. ABC Transporters at the blood–brain interfaces, their study models, and drug delivery implications in gliomas. Pharmaceutics 2020, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Girardin, F. Membrane transporter proteins: A challenge for CNS drug development. Dialogues Clin. Neurosci. 2006, 8, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Miller, D.S.; Pasinelli, P.; Trotti, D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res. 2015, 1628, 298–316. [Google Scholar] [CrossRef]
- Alemayehu, D.; Melisie, G.; Taye, K.; Tadesse, E. The role of ABC efflux transporter in treatment of pharmaco-resistant schizophrenia: A review article. Clin. Pharm. Biopharm 2019, 8, 1–8. [Google Scholar] [CrossRef]
- Chen, L.; Manautou, J.E.; Rasmussen, T.P.; Zhong, X. Development of precision medicine approaches based on inter-individual variability of BCRP/ABCG2. Acta Pharm. Sin. 2019, 9, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Sarkadi, B.; Homolya, L.; Hegedűs, T. The ABCG2/BCRP transporter and its variants—From structure to pathology. FEBS Lett. 2020, 594, 4012–4034. [Google Scholar] [CrossRef] [PubMed]
- Bruckmueller, H.; Cascorbi, I. ABCB1, ABCG2, ABCC1, ABCC2, and ABCC3 drug transporter polymorphisms and their impact on drug bioavailability: What is our current understanding. Expert Opin. Drug Metab. Toxicol. 2021, 17, 369–396. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S. Regulation of ABC transporters blood-brain barrier: The good, the bad, and the ugly. Adv. Cancer Res. 2015, 125, 43–70. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Blood-brain barrier active efflux transporters: ATP-binding cassette gene family. NeuroRx 2005, 2, 86–98. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, X.; Xiong, Y.; Xing, Q.; He, L.; Qin, S. Pharmacogenomics can improve antipsychotic treatment in schizophrenia. Front. Med. 2013, 7, 180–190. [Google Scholar] [CrossRef]
- Daood, M.; Tsai, C.; Ahdab-Barmada, M.; Watchko, J.F. ABC transporter (P-gp/ABCB1, MRP1/ABCC1, BCRP/ABCG2) expression in the developing human CNS. Neuropediatrics 2008, 39, 211–218. [Google Scholar] [CrossRef]
- Nasyrova, R.F.; Ivanov, M.V.; Neznanov, N.G. Introduction to Psychopharmacogenetics: Monography; DEAN Publishing House: St Petersburg, Russia, 2015; p. 27. ISBN 978-5-7452-0020-5. [Google Scholar]
- Musco, S.; McAllister, V.; Caudle, I. Dopamine-receptor blocking agent-associated akathisia: A summary of current understanding and proposal for a rational approach to treatment. Adv. Psychopharmacol. 2020, 10, 2045125320937575. [Google Scholar] [CrossRef]
- Grubor, M.; Zivkovic, M.; Sagud, M.; Nikolac Perkovic, M.; Mihaljevic-Peles, A.; Pivac, N.; Muck-Seler, D.; Svob Strac, D. HTR1A, HTR1B, HTR2A, HTR2C and HTR6 gene polymorphisms and extrapyramidal side effects in haloperidol-treated patients with schizophrenia. Int. J. Mol. Sci. 2020, 21, 2345. [Google Scholar] [CrossRef]
- Koning, J.P.; Vehof, J.; Burger, H.; Wilffert, B.; Al Hadithy, A.; Alizadeh, B.; van Harten, P.N.; Snieder, H. Genetic Risk and Outcome in Psychosis (GROUP) investigators. Association of two DRD2 gene polymorphisms with acute and tardive antipsychotic-induced movement disorders in young Caucasian patients. Psychopharmacology 2012, 219, 727–736. [Google Scholar] [CrossRef]
- Lawford, B.R.; Barnes, M.; Swagell, C.D.; Connor, J.P.; Burton, S.C.; Heslop, K.; Voisey, J.; Morris, C.P.; Nyst, P.; Noble, E.P.; et al. DRD2/ANKK1 Taq1A (rs 1800497 C>T) genotypes are associated with susceptibility to second generation antipsychotic-induced akathisia. J. Psychopharmacol. 2013, 27, 343–348. [Google Scholar] [CrossRef] [PubMed]
- OMIM: Online Mendelian Inheritance in Man. Available online: https://www.omim.org/ (accessed on 24 December 2022).
- Michalczyk, A.; Pełka-Wysiecka, J.; Kucharska-Mazur, J.; Wroński, M.; Misiak, B.; Samochowiec, J. Association between DRD2 and ANKK1 polymorphisms with the deficit syndrome in schizophrenia. Ann. Gen. Psychiatry 2020, 19, 39. [Google Scholar] [CrossRef]
- Luykx, J.J.; Broersen, J.L.; de Leeuw, M. The DRD2 rs1076560 polymorphism and schizophrenia-related intermediate phenotypes: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2017, 74, 214–224. [Google Scholar] [CrossRef] [PubMed]
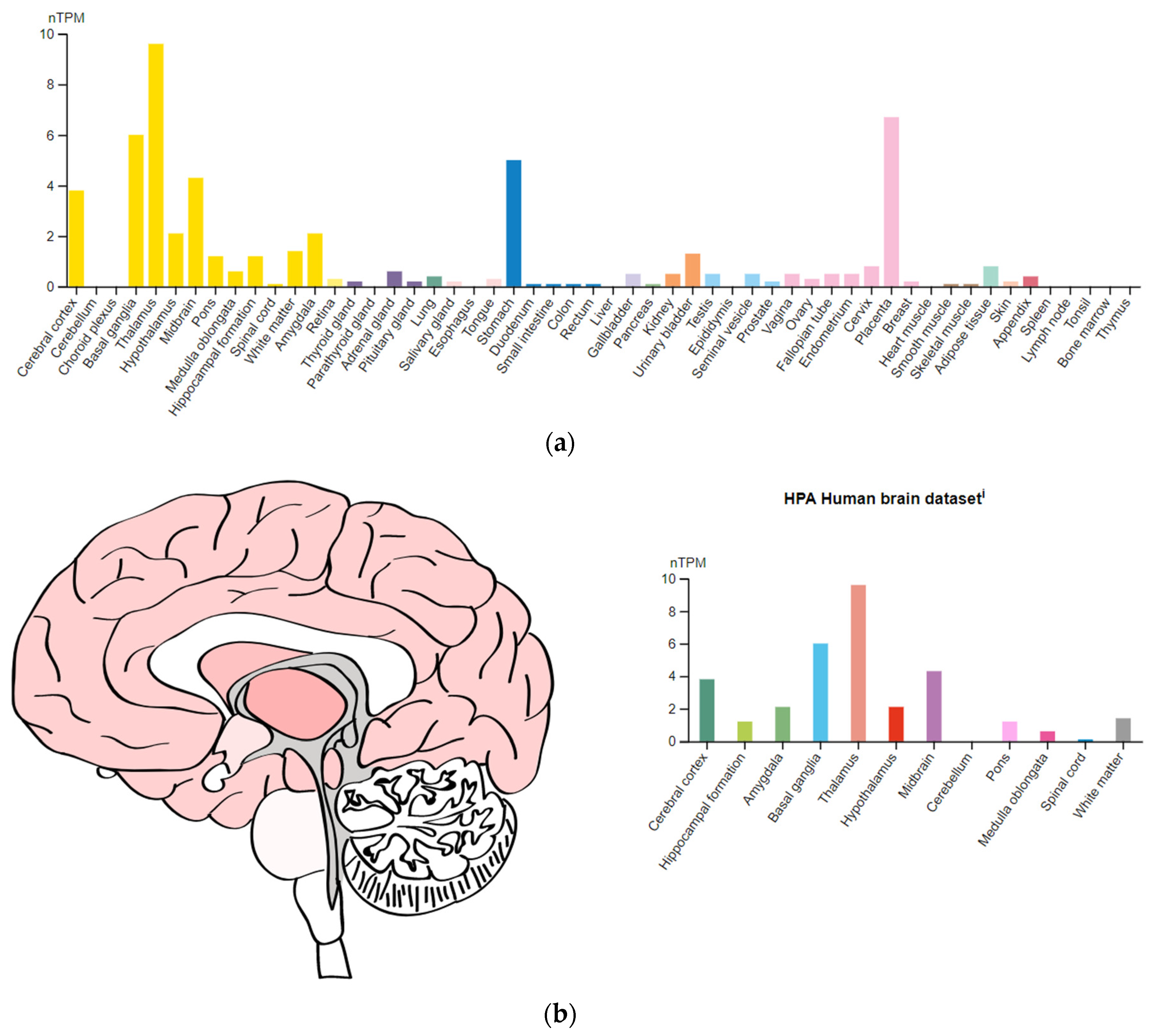
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000149295-DRD2 (accessed on 24 December 2022).
- Vaiman, E.E.; Novitsky, M.A.; Nasyrova, R.F. Pharmacogenetics of chlorpromazine and its role in the development of antipsychotic-induced parkinsonism. Pers. Psychiatry Neurol. 2021, 1, 11–17. [Google Scholar] [CrossRef]
- Martel, J.C.; Gatti McArthur, S. Dopamine receptor subtypes, physiology and pharmacology: New ligands and concepts in schizophrenia. Front. Pharm. 2020, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Wasmuth, J.; McPherson, J.D.; Wagner, C.; Grandy, D.; Civelli, O.; Potkin, S.; Litt, M. Cosegregation of an 11q22.3-9p22 translocation with affective disorder: Proximity of the dopamine D2 receptor gene relative to the translocation breakpoint. Am. J. Hum. Genet 1989, 45, A220. [Google Scholar]
- Oliveri, R.L.; Annesi, G.; Zappia, M.; Civitelli, D.; Montesanti, R.; Branca, D.; Nicoletti, G.; Spadafora, P.; Pasqua, A.A.; Cittadella, R.; et al. Dopamine D2 receptor gene polymorphism and the risk of levodopa-induced dyskinesias in PD. Neurology 1999, 53, 1425–1430. [Google Scholar] [CrossRef]
- The Human Gene database. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=DRD2 (accessed on 24 December 2022).
- Xia, X.; Ding, M.; Xuan, J.F.; Xing, J.X.; Yao, J.; Wu, X.; Wang, B.J. Functional polymorphisms and transcriptional analysis in the 5’ region of the human serotonin receptor 1B gene (HTR1B) and their associations with psychiatric disorders. BMC Psychiatry 2020, 20, 499. [Google Scholar] [CrossRef]
- Pozhidaev, I.V.; Paderina, D.Z.; Fedorenko, O.Y.; Kornetova, E.G.; Semke, A.V.; Loonen, A.J.M.; Bokhan, N.A.; Wilffert, B.; Ivanova, S.A. 5-Hydroxytryptamine Receptors and Tardive Dyskinesia in Schizophrenia. Front. Mol. Neurosci. 2020, 13, 63. [Google Scholar] [CrossRef] [PubMed]
- Al Hadithy, A.F.; Wilffert, B.; Stewart, R.E.; Looman, N.M.; Bruggeman, R.; Brouwers, J.R.; Matroos, G.E.; van Os, J.; Hoek, H.W.; van Harten, P.N. Pharmacogenetics of parkinsonism, rigidity, rest tremor, and bradykinesia in African-Caribbean inpatients: Differences in association with dopamine and serotonin receptors. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147B, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Drugbank online. Available online: https://go.drugbank.com (accessed on 24 December 2022).
- Bork, J.A.; Rogers, T.; Wedlund, P.J.; de Leon, J. A pilot study on risperidone metabolism: The role of cytochromes P450 2D6 and 3A. J. Clin. Psychiatry 1999, 60, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Brockmöller, J.; Kirchheiner, J.; Schmider, J.; Walter, S.; Sachse, C.; Müller-Oerlinghausen, B.; Roots, I. The impact of the CYP2D6 polymorphism on haloperidol pharmacokinetics and on the outcome of haloperidol treatment. Clin. Pharm. 2002, 72, 438–452. [Google Scholar] [CrossRef]
- de Leon, J.; Susce, M.T.; Pan, R.M.; Fairchild, M.; Koch, W.H.; Wedlund, P.J. The CYP2D6 poor metabolizer phenotype may be associated with risperidone adverse drug reactions and discontinuation. J. Clin. Psychiatry 2005, 66, 15–27. [Google Scholar] [CrossRef]
- Eichhammer, P.; Albus, M.; Borrmann-Hassenbach, M.; Schoeler, A.; Putzhammer, A.; Frick, U.; Klein, H.E.; Rohrmeier, T. Association of dopamine D3-receptor gene variants with neuroleptic induced akathisia in schizophrenic patients: A generalization of Steen’s study on DRD3 and tardive dyskinesia. Am. J. Med. Genet. 2000, 96, 187–191. [Google Scholar] [CrossRef]
- Higa, M.; Ohnuma, T.; Maeshima, H.; Hatano, T.; Hanzawa, R.; Shibata, N.; Sakai, Y.; Suzuki, T.; Arai, H. Association analysis between functional polymorphism of the rs4606 SNP in the RGS2 gene and antipsychotic-induced Parkinsonism in Japanese patients with schizophrenia: Results from the Juntendo University Schizophrenia Projects (JUSP). Neurosci. Lett. 2010, 469, 55–59. [Google Scholar] [CrossRef]
- Turčin, A.; Dolžan, V.; Porcelli, S.; Serretti, A.; Plesničar, B.K. Adenosine hypothesis of antipsychotic drugs revisited: Pharmacogenomics variation in nonacute schizophrenia. OMICS 2016, 20, 283–289. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/ (accessed on 24 December 2022).
- Ensembl Genomes. Available online: https://www.ensembl.org/index.html (accessed on 24 December 2022).
- Berna, F.; Misdrahi, D.; Boyer, L.; Aouizerate, B.; Brunel, L.; Capdevielle, D.; Chereau, I.; Danion, J.M.; Dorey, J.M.; Dubertret, C.; et al. FACE-SZ (FondaMental Academic Centers of Expertise for Schizophrenia) group. Akathisia: Prevalence and risk factors in a community-dwelling sample of patients with schizophrenia. Results from the FACE-SZ dataset. Schizophr. Res. 2015, 169, 255–261. [Google Scholar] [CrossRef]
- Korkmaz, S. Delayed-onset akathisia due to amisulpride. Indian J. Pharm. 2011, 43, 460–462. [Google Scholar] [CrossRef]
- Chow, C.L.; Kadouh, N.K.; Bostwick, J.R.; Vanden Berg, A.M. Akathisia and Newer Second-Generation Antipsychotic Drugs: A Review of Current Evidence. Pharmacotherapy 2020, 40, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Sahoo, S. Clozapine induced akathisia: A case report and review of the evidence. Indian J. Pharmacol. 2015, 47, 234–235. [Google Scholar] [CrossRef]
- Mishra, B.R.; Ranjan, R.; Mohapatra, D.; Nath, S. Risperidone-Induced Tardive Akathisia: A Rare Antipsychotic Side Effect with Management Issues. Ann. Indian Acad. Neurol. 2019, 22, 499–501. [Google Scholar] [CrossRef]
- Hawthorne, J.M.; Caley, C.F. Extrapyramidal Reactions Associated with Serotonergic Antidepressants. Ann. Pharmacother. 2015, 49, 1136–1152. [Google Scholar] [CrossRef]
- Girishchandra, B.G.; Johnson, L.; Cresp, R.M.; Orr, K.G. Mirtazapine-induced akathisia. Med. J. Aust. 2002, 176, 242. [Google Scholar] [CrossRef] [PubMed]
- Riesselman, A.; El-Mallakh, R.S. Akathisia With Azithromycin. Ann. Pharmacother. 2015, 49, 609. [Google Scholar] [CrossRef]
- Gbinigie, I.I.; Lasserson, D. Clarithromycin-induced akathisia: A class effect of macrolides. BMJ Case Rep. 2016, 2016, bcr2016217421. [Google Scholar] [CrossRef]
- Kuzuhara, S.; Kohara, N.; Ohkawa, Y.; Fuse, S.; Yamanouchi, H. Parkinsonism, depression and akathisia induced by flunarizine, a calcium entry blockade--report of 31 cases. RinshoShinkeigaku 1989, 29, 681–686. (In Japanese) [Google Scholar]
- Dressler, D. Tardive dystonic syndrome induced by the calcium-channel blocker amlodipine. J. Neural Transm. 2014, 121, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.B.; Wroblewski, B.A. Paradoxical akathisia caused by clonazepam, clorazepate and lorazepam in patients with traumatic encephalopathy and seizure disorders: A subtype of benzodiazepine-induced disinhibition? Behav. Neurol. 1993, 6, 221–223. [Google Scholar] [CrossRef]
- Owusu Aboagye, G.; Ankrah, D. Drug-drug-induced akathisia: Two case reports. Case Rep. Psychiatry 2020, 2020, 9649483. [Google Scholar] [CrossRef] [PubMed]
- Dumon, J.P.; Catteau, J.; Lanvin, F.; Dupuis, B.A. Randomized, double-blind, crossover, placebo-controlled comparison of propranolol and betaxolol in the treatment of neuroleptic-induced akathisia. Am. J. Psychiatry 1992, 149, 647–650. [Google Scholar] [CrossRef]
- Dag, E.; Gokce, B.; Buturak, S.V.; Tiryaki, D.; Erdemoglu, A.K. Pregabalin-induced akathisia. Ann. Pharmacother. 2013, 47, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Ponde, M.P.; Freire, A.C. Increased anxiety, akathisia, and suicidal thoughts in patients with mood disorder on aripiprazole and lamotrigine. Case Rep. Psychiatry 2015, 2015, 419746. [Google Scholar] [CrossRef]
- Duma, S.R.; Fung, V.S.C. Drug-induced movement disorders. Aust. Prescr. 2019, 42, 56–61. [Google Scholar] [CrossRef]
- Maat, A.; Fouwels, A.; de Haan, L. Cocaine is a major risk factor for antipsychotic induced akathisia, parkinsonism and dyskinesia. Psychopharmacol. Bull. 2008, 41, 5–10. [Google Scholar] [PubMed]
- Lang, A.E.; Johnson, K. Akathisia in idiopathic Parkinson’s disease. Neurology 1987, 37, 477. [Google Scholar] [CrossRef]
- Poyurovsky, M.; Kreinin, A.; Modai, I.; Weizman, A. Lithium-induced akathisia responds to low-dose mianserin: Case report. Int. Clin. Psychopharmacol. 1995, 10, 261–263. [Google Scholar] [CrossRef]
- Miller, L.G.; Jankovic, J. Metoclopramide-induced movement disorders. Clinical findings with a review of the literature. Arch. Intern. Med. 1989, 149, 2486–2492. [Google Scholar] [CrossRef]
- Straus, S.; Glasziou, P.; Richardson, W.S.; Haynes, R.B. Evidence-Based Medicine How to Practice and Teach EBM, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018; 336p. [Google Scholar]
- World Health Organization. Available online: https://www.who.int/ (accessed on 24 December 2022).
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000135312-HTR1B (accessed on 24 December 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasyrova, R.F.; Vaiman, E.E.; Repkina, V.V.; Khasanova, A.K.; Asadullin, A.R.; Shipulin, G.A.; Altynbekov, K.S.; Al-Zamil, M.; Petrova, M.M.; Shnayder, N.A. Single-Nucleotide Polymorphisms as Biomarkers of Antipsychotic-Induced Akathisia: Systematic Review. Genes 2023, 14, 616. https://doi.org/10.3390/genes14030616
Nasyrova RF, Vaiman EE, Repkina VV, Khasanova AK, Asadullin AR, Shipulin GA, Altynbekov KS, Al-Zamil M, Petrova MM, Shnayder NA. Single-Nucleotide Polymorphisms as Biomarkers of Antipsychotic-Induced Akathisia: Systematic Review. Genes. 2023; 14(3):616. https://doi.org/10.3390/genes14030616
Chicago/Turabian StyleNasyrova, Regina F., Elena E. Vaiman, Vera V. Repkina, Aiperi K. Khasanova, Azat R. Asadullin, German A. Shipulin, Kuanysh S. Altynbekov, Mustafa Al-Zamil, Marina M. Petrova, and Natalia A. Shnayder. 2023. "Single-Nucleotide Polymorphisms as Biomarkers of Antipsychotic-Induced Akathisia: Systematic Review" Genes 14, no. 3: 616. https://doi.org/10.3390/genes14030616
APA StyleNasyrova, R. F., Vaiman, E. E., Repkina, V. V., Khasanova, A. K., Asadullin, A. R., Shipulin, G. A., Altynbekov, K. S., Al-Zamil, M., Petrova, M. M., & Shnayder, N. A. (2023). Single-Nucleotide Polymorphisms as Biomarkers of Antipsychotic-Induced Akathisia: Systematic Review. Genes, 14(3), 616. https://doi.org/10.3390/genes14030616









