The Role of Genetic Testing in Children Requiring Surgery for Ectopia Lentis
Abstract
:1. Introduction
2. Materials and Methods
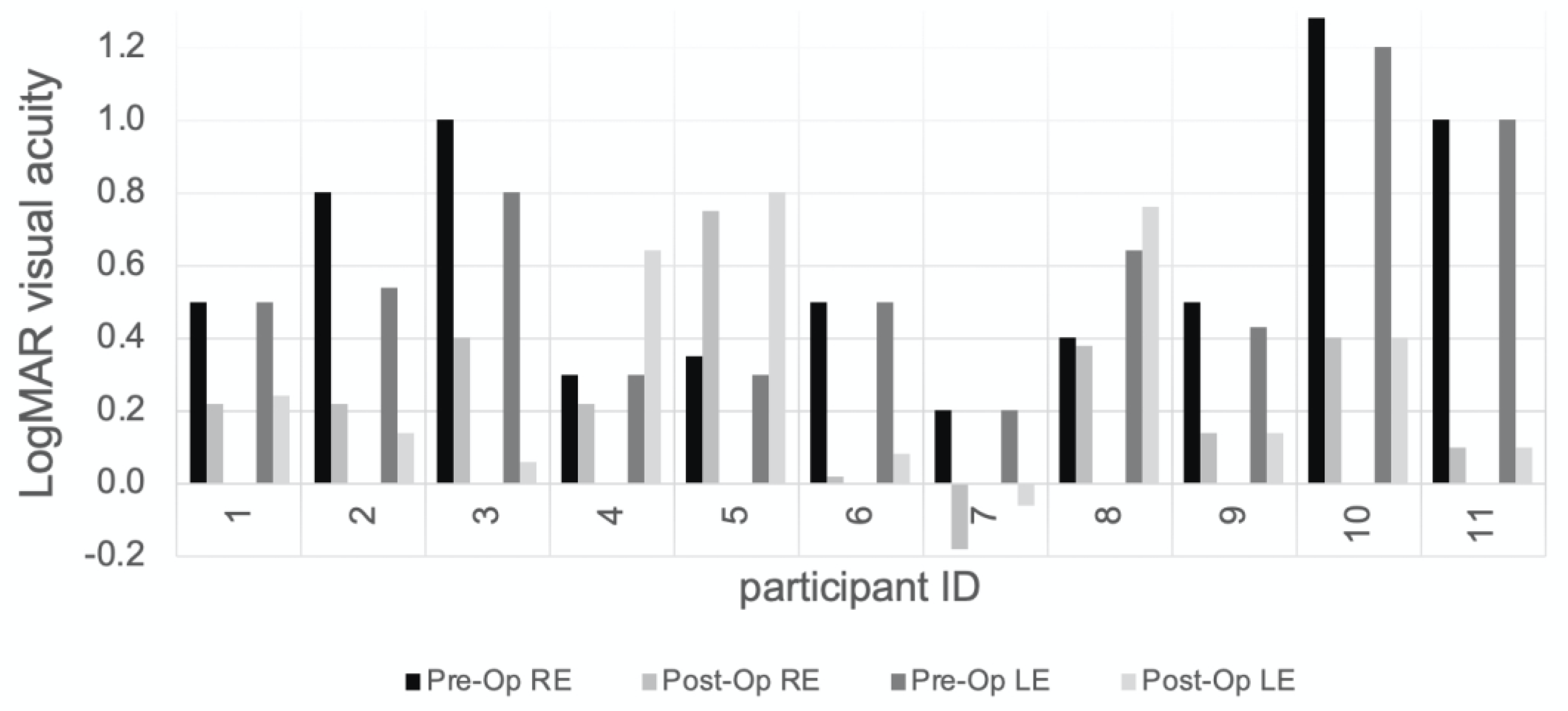
3. Results
4. Discussion
| Study (Cases Undergoing Surgery) | Age at Time of Surgery (Years) | Surgical Technique | IOL Implantation | Post-Operative Surgical Complications | Pre-op BCVA LogMAR | Final Post-op BCVA LogMAR |
|---|---|---|---|---|---|---|
| Current study (n = 22) | 5.0 (median) | Anterior approach (n = 18); Posterior approach (n = 4) § | Aphakia | Vitreous haemorrhage (n = 1); Glaucoma (n = 4) | 0.54 (median) | 0.22 (median) |
| Anteby et al. [8] (n = 38) | 6.4 (mean) | Anterior approach (n = 11); Posterior approach (n = 27) | Aphakia | Vitreous haemorrhage (n = 1); Retinal detachment (n = 1); Retinal tear n = 1 | 0.92 (mean) | 0.20 (mean) |
| Wu-Chen et al. [9] (n = 17) | 7.7 (mean) | Anterior approach (n = 1); Posterior approach (n = 16) | Aphakia (n = 17; scleral fixated lens 6 years later in one case) | Glaucoma (n = 1); Transient ocular hypertension (n = 1); Vitreous haemorrhage (n = 1); Partial PVD n = 2 | 0.7 (median) | 0.1 (median) |
| Konradsen et al. [10] (n = 37) | 4.3 (median) | Anterior approach (no anterior vitrectomy, posterior capsule intact) | Acrysof IOL (Alcon) in the capsular bag | Visual axis opacification (n = 31); IOL dislocation (n = 2); Suture-related discomfort (n = 2); Anterior synaechia (n = 1) | 0.59 (median) | 0.23 (median) |
| Català-Mora et al. [11] (n = 21) | 8.0 (mean) | Posterior approach | Artisan (iris-claw) IOL | Vitreous haemorrhage (n = 6); Anterior uveitis (n = 1); IOL dislocation (n = 1); Retinal detachment (n = 1); Macular oedema (n = 1) | 0.91 (mean) | 0.18 (mean) |
| Kopel et al. [12] (n = 22) | 6.5 (mean) | Posterior approach | Aphakia (n = 10); Iris-sutured 3-piece IOL (n = 12) | IOL dislocation (n = 4) | 0.81 (aphakic group); 0.83 (IOL group) (mean) | 0.41 (aphakic group) 0.24 (IOL group) (mean) |
| Cai et al. [13] (n = 101) | 6.1 (mean) | Anterior approach (posterior capsulectomy and anterior vitrectomy if <5 years) | IOL in the capsular bag | PCO (n = 55); Elevated IOP (n = 3); Vitreous haemorrhage (n = 2); Posterior synechiae (n = 3); IOL dislocation (n = 4) | 0.68 (mean) | 0.10 (mean) |
| Van Hoorde [19] (n = 2) | 17.5 (mean) | Anterior approach (posterior capsulorrhexis in one, anterior vitrectomy in the other) | Aphakia | Nil reported | 0.6 (mean) | 0.95 (mean) |
- require lensectomy in the first 3 years of life
- have unaffected parents (this is unsurprising, as Marfan syndrome is classically inherited as an autosomal dominant trait)
- initially present to ophthalmology services (e.g., prior to clinical genetics or cardiology clinics).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chandra, A.; Charteris, D. Molecular pathogenesis and management strategies of ectopia lentis. Eye 2014, 28, 162–168. Available online: https://pubmed.ncbi.nlm.nih.gov/24406422 (accessed on 20 March 2023). [CrossRef] [PubMed] [Green Version]
- Matsuo, T. How far is observation allowed in patients with ectopia lentis? Springerplus 2015, 4, 461. Available online: https://pubmed.ncbi.nlm.nih.gov/26339562 (accessed on 20 March 2023). [CrossRef] [PubMed] [Green Version]
- Sadiq, M.A.; Vanderveen, D. Genetics of ectopia lentis. Semin. Ophthalmol. 2013, 28, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Overwater, E.; Floor, K.; van Beek, D.; de Boer, K.; van Dijk, T.; Hilhorst-Hofstee, Y.; Hoogeboom, A.; van Kaam, K.; van de Kamp, J.; Kempers, M.; et al. NGS panel analysis in 24 ectopia lentis patients; A clinically relevant test with a high diagnostic yield. Eur. J. Med. Genet. 2017, 60, 465–473. Available online: https://www.sciencedirect.com/science/article/pii/S1769721216302117 (accessed on 20 March 2023). [CrossRef] [PubMed]
- Lenassi, E.; Clayton-Smith, J.; Douzgou, S.; Ramsden, S.C.; Ingram, S.; Hall, G.; Hardcastle, C.L.; Fletcher, T.A.; Taylor, R.L.; Ellingford, J.M.; et al. Clinical utility of genetic testing in 201 preschool children with inherited eye disorders. Genet. Med. 2020, 22, 745–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, N.; Lloyd, I.C.; Ashworth, J.; Biswas, S.; Black, G.C.; Clayton-Smith, J. Traboulsi syndrome due to ASPH mutation: An under-recognised cause of ectopia lentis. Clin. Dysmorphol. 2019, 28, 184–189. Available online: https://journals.lww.com/clindysmorphol/Fulltext/2019/10000/Traboulsi_syndrome_due_to_ASPH_mutation__an.3.aspx (accessed on 20 March 2023). [CrossRef]
- Simon, M.A.; Origlieri, C.A.; Dinallo, A.M.; Forbes, B.J.; Wagner, R.S.; Guo, S. New management strategies for ectopia lentis. J. Pediatr. Ophthalmol. Strabismus 2015, 52, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Anteby, I.; Isaac, M.; BenEzra, D. Hereditary subluxated lenses: Visual performances and long-term follow-up after surgery. Ophthalmology 2003, 110, 1344–1348. [Google Scholar] [CrossRef]
- Wu-Chen, W.Y.; Letson, R.D.; Summers, C.G. Functional and structural outcomes following lensectomy for ectopia lentis. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2005, 9, 353–357. [Google Scholar] [CrossRef]
- Konradsen, T.; Kugelberg, M.; Zetterström, C. Visual outcomes and complications in surgery for ectopia lentis in children. J. Cataract. Refract. Surg. 2007, 33, 819–824. Available online: https://journals.lww.com/jcrs/Fulltext/2007/05000/Visual_outcomes_and_complications_in_surgery_for.32.aspx (accessed on 20 March 2023). [CrossRef]
- Català-Mora, J.; Cuadras, D.; Díaz-Cascajosa, J.; Castany-Aregall, M.; Prat-Bartomeu, J.; García-Arumí, J. Anterior iris-claw intraocular lens implantation for the management of nontraumatic ectopia lentis: Long-term outcomes in a paediatric cohort. Acta Ophthalmol. 2017, 95, 170–174. [Google Scholar] [CrossRef]
- Kopel, A.C.; Carvounis, P.E.; Hamill, M.B.; Weikert, M.P.; Holz, E.R. Iris-sutured intraocular lenses for ectopia lentis in children. J. Cataract. Refract. Surg. 2008, 34, 596–600. [Google Scholar] [CrossRef]
- Cai, L.; Han, X.; Jiang, Y.; Qiu, X.; Qian, D.; Lu, Y.; Yang, J. Three-year outcomes of cionni-modified capsular tension ring implantation in children under 8 years old with ectopia lentis. Am. J. Ophthalmol. 2021, 224, 74–83. [Google Scholar] [CrossRef]
- Fuchs, J.; Rosenberg, T. Congenital ectopia lentis, a Danish national survey. Acta Ophthalmol. Scand. 1998, 76, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Chen, Z.-X.; Zhang, M.; Chen, J.-H.; Deng, M.; Zheng, J.-L.; Lan, L.-N.; Jiang, Y.-X. Combination of panel-based next-generation sequencing and clinical findings in congenital ectopia lentis diagnosed in chinese patients. Am. J. Ophthalmol. 2022, 237, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Maghsoudlou, P.; Khanam, T.; Banerjee, P.J.; Chandra, A. Are patients with ectopia lentis known to cardiology services? Eye 2019, 33, 516–517. Available online: https://pubmed.ncbi.nlm.nih.gov/30397254 (accessed on 20 March 2023). [CrossRef] [PubMed]
- Claerhout, H.; Witters, P.; Régal, L.; Jansen, K.; Van Hoestenberghe, M.-R.; Breckpot, J.; Vermeersch, P. Isolated sulfite oxidase deficiency. J. Inherit. Metab. Dis. 2018, 41, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Naughten, E.R.; Yap, S.; Mayne, P.D. Newborn screening for homocystinuria: Irish and world experience. Eur. J. Pediatr. 1998, 157, S84–S87. [Google Scholar] [CrossRef]
- Van Hoorde, T.; Nerinckx, F.; Kreps, E.; Roels, D.; Huyghe, P.; Van Heetvelde, M.; Verdin, H.; De Baere, E.; Balikova, I.; Leroy, B.P. Expanding the clinical spectrum and management of traboulsi syndrome: Report on two siblings homozygous for a novel pathogenic variant in ASPH. Ophthalmic. Genet. 2021, 42, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, K.J.; Clayton-Smith, J.; Harris, R. Evolving phenotype of marfan’s syndrome. Arch. Dis. Child 1997, 76, 41 LP–46 LP. Available online: http://adc.bmj.com/content/76/1/41.abstract (accessed on 20 March 2023). [CrossRef] [Green Version]
- Stuart, A.G.; Williams, A. Marfan’s syndrome and the heart. Arch. Dis. Child 2007, 92, 351–356. Available online: https://pubmed.ncbi.nlm.nih.gov/17376944 (accessed on 20 March 2023). [CrossRef] [PubMed]
- Bösenberg, M.T.; Bösenberg, A.T. Anaesthesia for marfan’s syndrome. South. Afr. J. Anaesth. Analg. 2007, 13, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Gong, N.; Cao, Q.; Zhou, Y.; Cai, Y.; Jin, G.; Young, C.A.; Yang, J.; Wang, Y.; Zheng, D. What hinders congenital ectopia lentis patients’ follow-up visits? A qualitative study. BMJ Open 2020, 10, e030434. Available online: http://bmjopen.bmj.com/content/10/3/e030434.abstract (accessed on 20 March 2023). [CrossRef] [PubMed] [Green Version]
- Stark, V.C.; Hensen, F.; Kutsche, K.; Kortüm, F.; Olfe, J.; Wiegand, P.; Von Kodolitsch, Y.; Kozlik-Feldmann, R.; Müller, G.C.; Mir, T.S. Genotype-phenotype correlation in children: The impact of FBN1 variants on pediatric Marfan care. Genes 2020, 11, 799. [Google Scholar] [CrossRef]
- Li, J.; Lu, C.; Wu, W.; Liu, Y.; Wang, R.; Si, N.; Meng, X.; Zhang, S.; Zhang, X. Application of next-generation sequencing to screen for pathogenic mutations in 123 unrelated Chinese patients with marfan syndrome or a related disease. Sci. China Life Sci. 2019, 62, 1630–1637. [Google Scholar] [CrossRef]

| Case ID | Sex | Broad Ancestral Group | Presenting Symptom | Initial Presentation to Ophthalmology? | History of Consanguinity | Family History of Ectopia Lentis or Marfan Syndrome | Age at Presentation (Years) | Age at Time of First Lens Procedure (Years) | Age at Genetic Testing (Years) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | White European | Iris flickering | Yes | No | No | <1 | 2 | 1 |
| 2 | Female | South Asian | Reduced vision | Yes | Yes | Yes | 3 | 5 | 5 |
| 3 | Male | White European | Iris flickering | Yes | No | No | 1 | 3 | 5 |
| 4 | Female | South Asian | Iris dislocation noted following minor trauma | Yes | Yes | No | 1 | 1 | 1 |
| 5 | Female | White European | Iris flickering | Yes | No | No | 4 | 5 | 4 |
| 6 | Male | White European | Reduced vision | Yes | No | No | 3 | 3 | 3 |
| 7 | Female | White European | High myopia | Yes | No | Yes | 5 | 8 | 8 |
| 8 | Male | South Asian | Screening due to family history of Marfan syndrome | No | No | Yes | 2 | 8 | 8 |
| 9 | Female | South Asian | High arch palate at postnatal screening | No | No | Yes | 3 | 5 | 3 |
| 10 | Female | White European | Screening due to family history of Marfan syndrome | No | No | Yes | 3 | 7 | 4 |
| 11 | Female | South Asian | Reduced vision | Yes | No | No | 3 | 3 | 3 |
| Case ID | Pre-op BCVA LogMAR | Pre-op Refraction (SE) | Axial Length (mm) | Post-op Refraction (SE) | BCVA at Last Visit (LogMAR) | Surgical Approach | Grade of Operating Surgeon | Lens Implant (Correction) | Glaucoma Present (Treatment) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.80 R; 0.80 L; 0.50 BEO | +12.00 RE; +12.00 LE | 24.01 RE; 22.86 LE | +10.50 RE; +10.00 LE | 0.22 RE; 0.24 LE | Anterior approach | Consultant | Aphakia (contact lenses) | No |
| 2 | 0.80 R; 0.54 L | −9.00 RE; −4.00 LE | 24.01 RE; 22.86 LE | +9.50 RE; +12.00 LE | 0.22 RE; 0.14 LE | Anterior approach | Consultant | Aphakia (glasses) | No |
| 3 | 1.00 R; 0.80 L | −9.75 RE; −7.50 LE | 20.95 RE; 21.13 LE | +14.50 RE; +15.00 LE | 0.40 RE; 0.06 LE | Anterior approach | Consultant | Aphakia | No |
| 4 | 0.30 BEO | unknown RE; +19.00 LE | 19.19 RE; 19.25 LE | +21.75 RE; +19.50 LE | 0.22 RE; 0.64 LE | Anterior approach | Consultant | Aphakia (glasses) | No |
| 5 | 0.35 R; 0.30 L | −2.75 RE; −3.25 LE | 22.27 RE; 22.39 LE | +13.375 RE; +13.00 LE | 0.75 RE; 0.80 LE | Anterior approach | Consultant | Aphakia (glasses) | Yes (bilateral filtration surgery) |
| 6 | 0.50 BEO | −4.25 RE; −4.25 LE | unknown | +13.25 RE; +15.00 LE | 0.00 RE; 0.08 LE | Anterior approach | Fellow | Aphakia (glasses) | No |
| 7 | 0.20 R; 0.20 L | −20.00 RE; −18.25 LE | 21.22 RE; 21.91 LE | +11.75 RE; +12.875 LE | −0.18 RE; −0.06 LE | Anterior approach | Consultant | Aphakia (glasses) | No |
| 8 | 0.40 R; 0.64 L | +5.375 RE; +6.50 LE | unknown | +4.875 RE; +5.75 LE | 0.38 RE; 0.76 LE | Posterior approach | Fellow | Aphakia (glasses) | No |
| 9 | 0.50 R; 0.43 L | −9.00 RE; −13.00 LE | 22.76 RE; 22.77 LE | +12.75 RE; +13.00 LE | 0.14 RE; 0.14 LE | Anterior approach | Consultant | Aphakia (glasses) | No |
| 10 | 1.28 RE; 1.20 LE | −6,75 RE; −6.75 LE | 23.89 RE; 24.06 LE | +12.125 RE; +13.00 LE | 0.40 RE; 0.40 LE | Anterior approach | Consultant | Aphakia (glasses) | No |
| 11 | 1.00 RE; 1.00 LE | −20.75 RE; +11.50 LE | 22.40 RE; 22.21 LE | +9.25 RE; +9.75 LE | 0.10 RE; 0.10 LE | Posterior approach | Consultant | Aphakia (glasses) | Yes (drops) |
| Case ID | Gene | Variant and Zygosity | Other Ocular Abnormalities | Extraocular Features |
|---|---|---|---|---|
| 1 | ADAMTSL4 | c.767_786del20 (p.Gln256ProfsTer8) homozygous | Ectopia pupillae (RE and LE) | None |
| 2 | No causal variant(s) detected | Not applicable | High myopia (RE) | Patent ductus arteriosus (corrected with cardiac catheterisation at age 3 years) |
| 3 | ADAMTSL4 | c.767_786del20 (p.Gln256ProfsTer38) heterozygous; c.2236C > T (p.Arg746Cys) heterozygous | High myopia (RE and LE) | None |
| 4 | LTBP2 | c.3427delC (p.Gln1143ArgfsTer35) homozygous | Increased corneal diameter, high intraocular pressure (RE and LE) | None |
| 5 | FBN1 | c.6354C > T (p.Ile2118Ile) heterozygous | None | Pectus carinatum; flat feet, long toes and fingers, high-arched palate; normal cardiology assessment |
| 6 | FBN1 | c.356G > A (p.Cys119Tyr) heterozygous | None | Flat feet, long toes and fingers; normal cardiology assessment |
| 7 | ASPH | c.1965C > A (p.Tyr565Ter) heterozygous; c.2127-2delA heterozygous | Spherophakia, high myopia, high intraocular pressure (RE and LE) | Skeletal and facial features of Marfan syndrome; normal cardiology assessment |
| 8 | FBN1 | exons 46 to 48 deletion heterozygous; exons 56 to 58 duplication heterozygous | None | None |
| 9 | FBN1 | c.7204 + 1G > A heterozygous | High myopia | Skeletal and facial features of Marfan syndrome; normal cardiology assessment |
| 10 | FBN1 | c.5789-9_5794del15insA heterozygous | High myopia | Skeletal and facial features of Marfan syndrome; aortic aneurysm, fenestrated atrial septal defect |
| 11 | LTBP2 | c.507C > G (p.Cys169Trp) homozygous | Increased corneal diameter (RE) | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musleh, M.; Bull, A.; Linton, E.; Liu, J.; Waller, S.; Hardcastle, C.; Clayton-Smith, J.; Sharma, V.; Black, G.C.; Biswas, S.; et al. The Role of Genetic Testing in Children Requiring Surgery for Ectopia Lentis. Genes 2023, 14, 791. https://doi.org/10.3390/genes14040791
Musleh M, Bull A, Linton E, Liu J, Waller S, Hardcastle C, Clayton-Smith J, Sharma V, Black GC, Biswas S, et al. The Role of Genetic Testing in Children Requiring Surgery for Ectopia Lentis. Genes. 2023; 14(4):791. https://doi.org/10.3390/genes14040791
Chicago/Turabian StyleMusleh, Mohammud, Adam Bull, Emma Linton, Jingshu Liu, Sarah Waller, Claire Hardcastle, Jill Clayton-Smith, Vinod Sharma, Graeme C. Black, Susmito Biswas, and et al. 2023. "The Role of Genetic Testing in Children Requiring Surgery for Ectopia Lentis" Genes 14, no. 4: 791. https://doi.org/10.3390/genes14040791
APA StyleMusleh, M., Bull, A., Linton, E., Liu, J., Waller, S., Hardcastle, C., Clayton-Smith, J., Sharma, V., Black, G. C., Biswas, S., Ashworth, J. L., & Sergouniotis, P. I. (2023). The Role of Genetic Testing in Children Requiring Surgery for Ectopia Lentis. Genes, 14(4), 791. https://doi.org/10.3390/genes14040791






