Whole Transcriptome Analysis of Differentially Expressed Genes in Cultured Nile Tilapia (O. niloticus) Subjected to Chronic Stress Reveals Signaling Pathways Associated with Depressed Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experimental Fish
2.3. Experimental Design
Ammonia Stress Experiment
2.4. Stocking Density Stress Experiment
2.5. Sample Collection
2.6. Sample Processing
Physiological Parameters
2.7. Growth Performance
2.8. RNA Extraction
2.9. Reads Mapping to the Reference Genome
2.10. Quantification of Gene Expression Level
2.11. Real Time qPCR
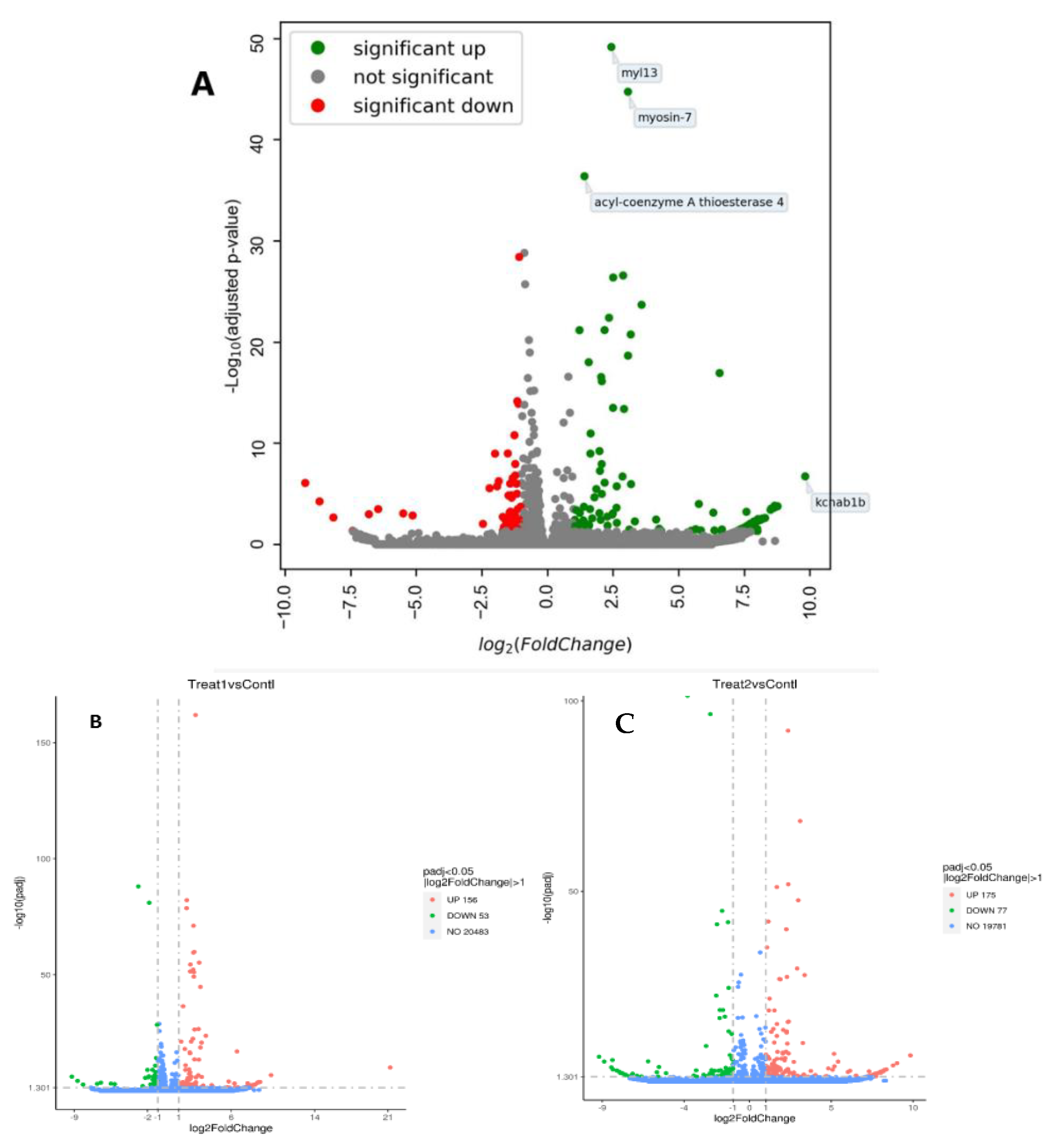
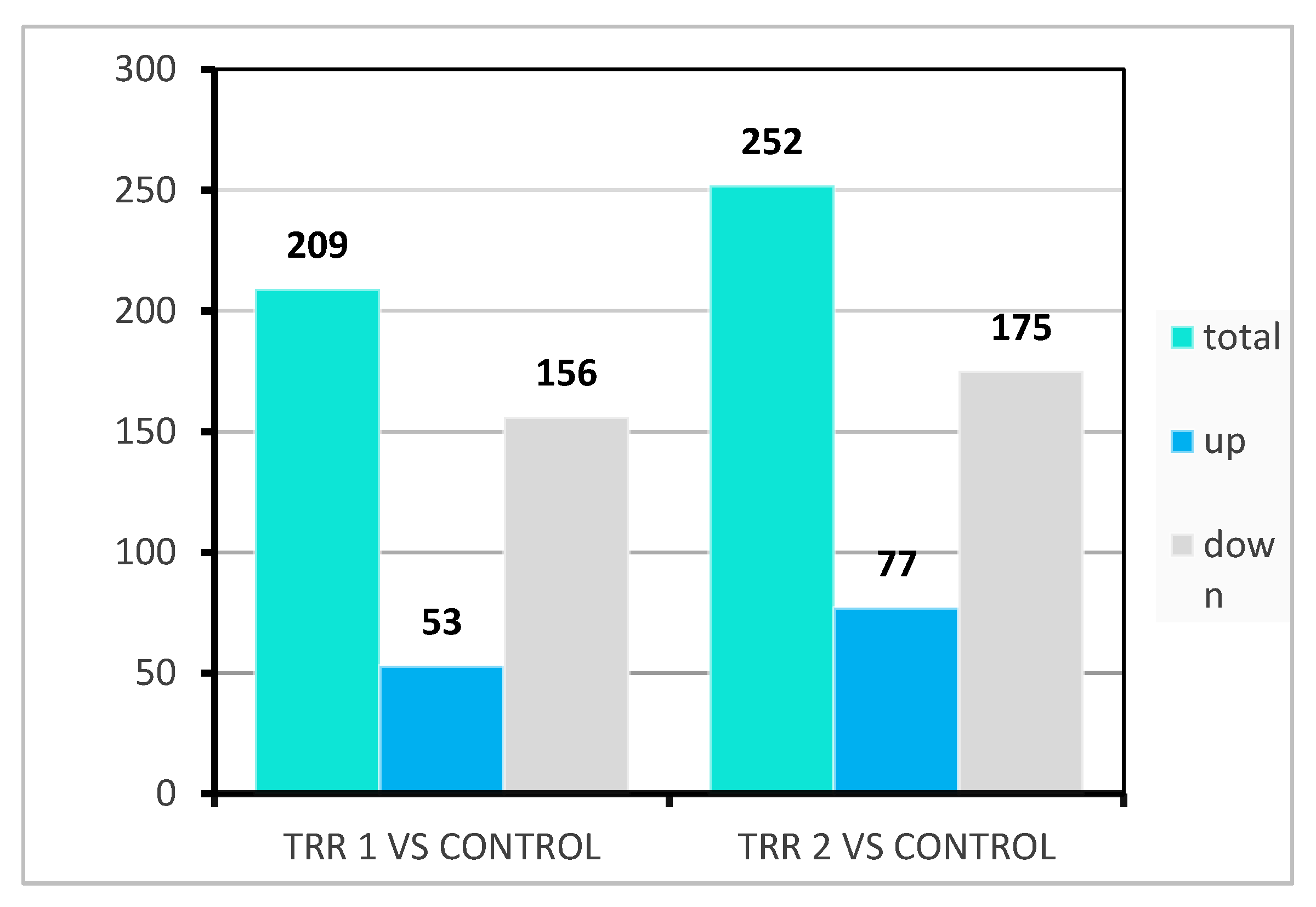
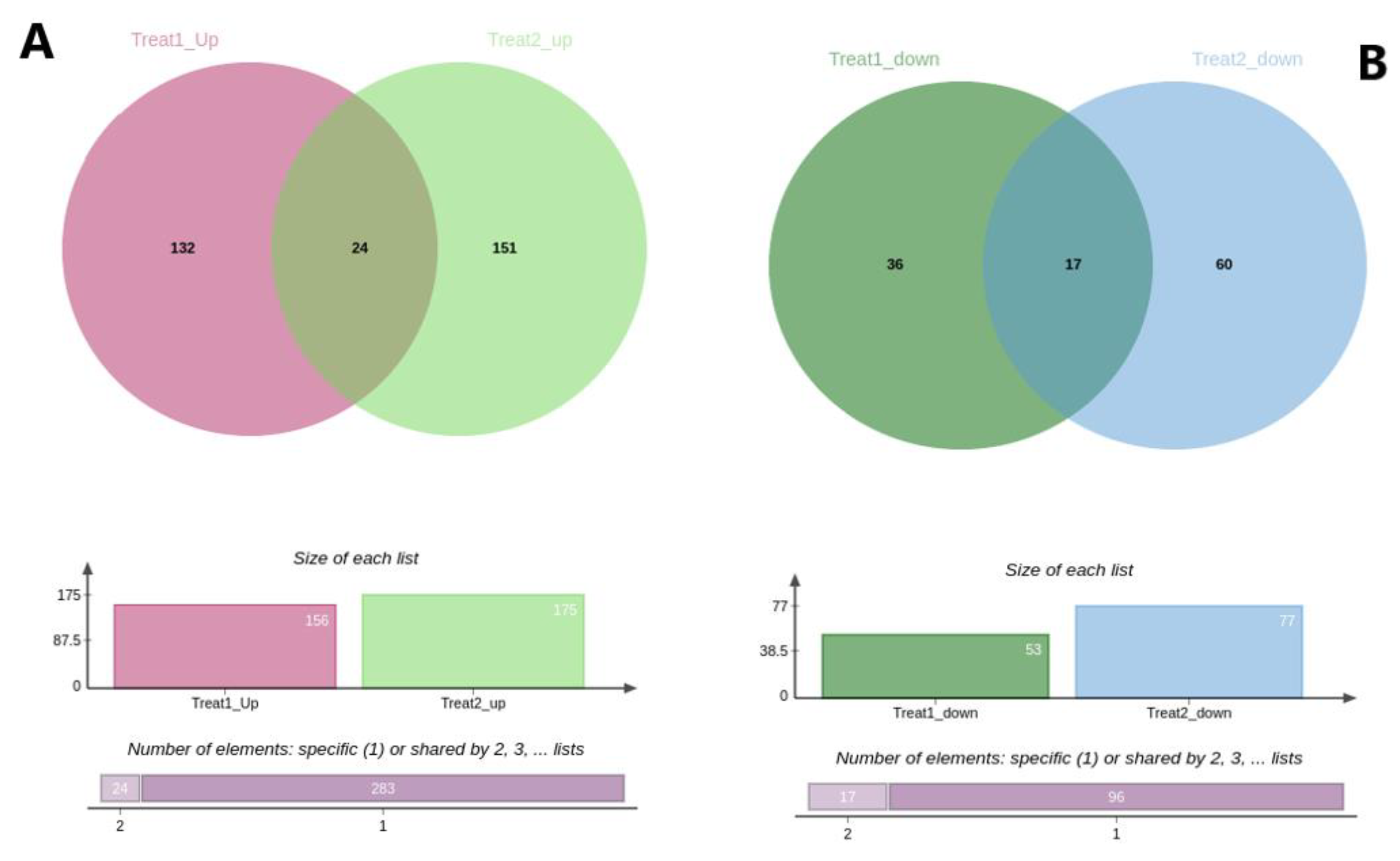
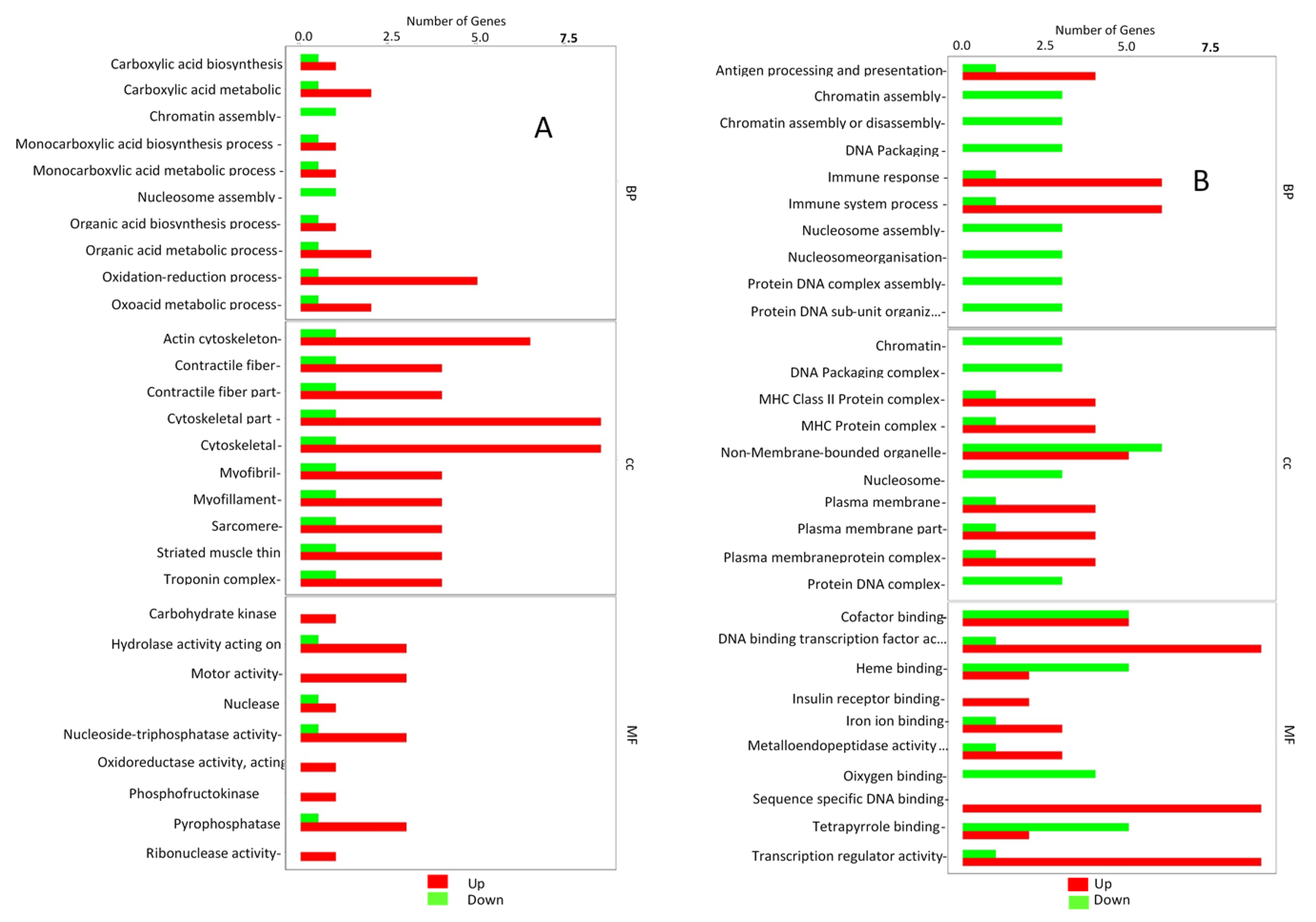
3. Results
3.1. Growth Performance
3.2. Cardiac Muscle Signaling Pathway
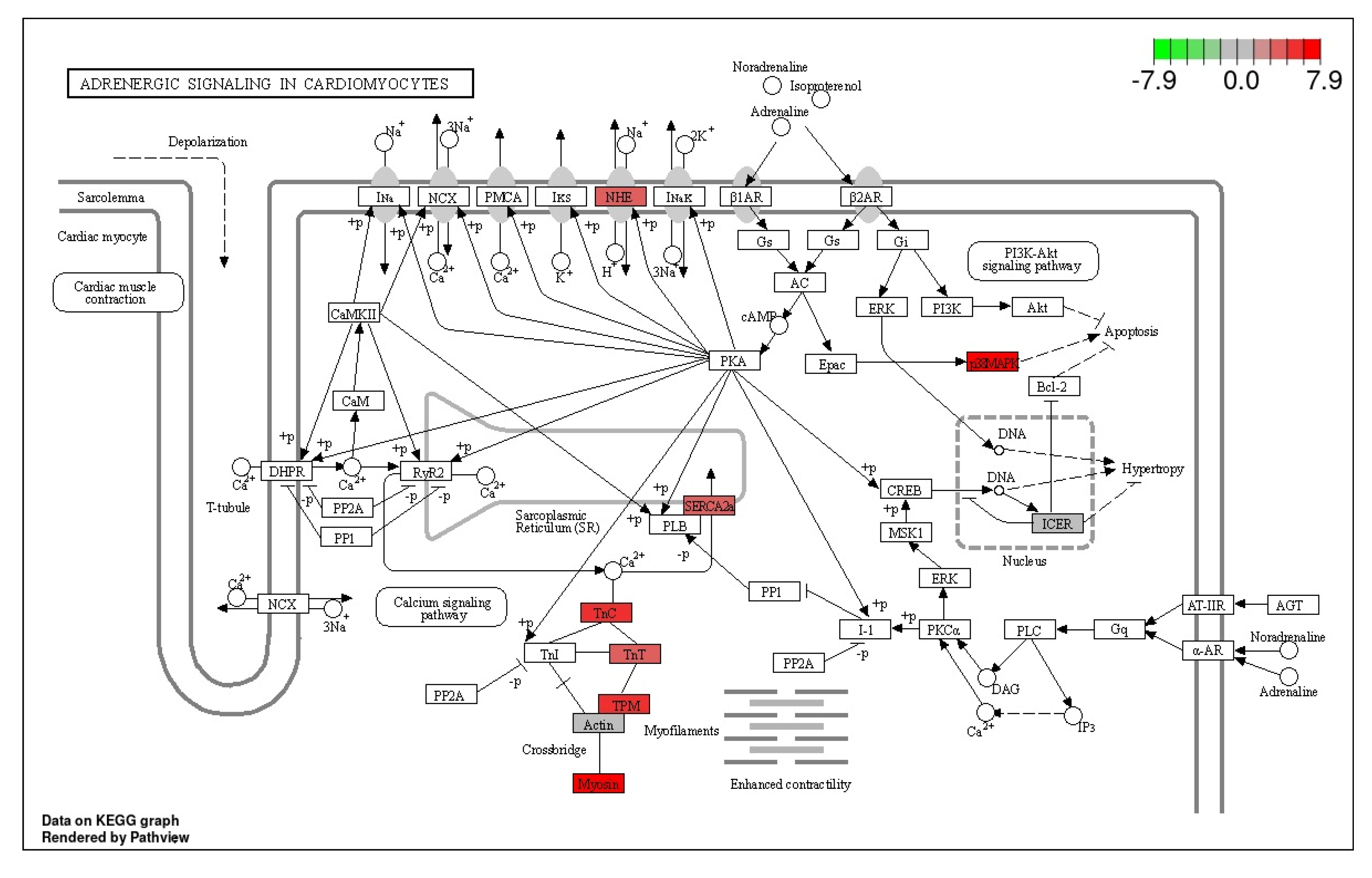
3.3. Adrenergic Signaling in Cardiomyocytes Pathway
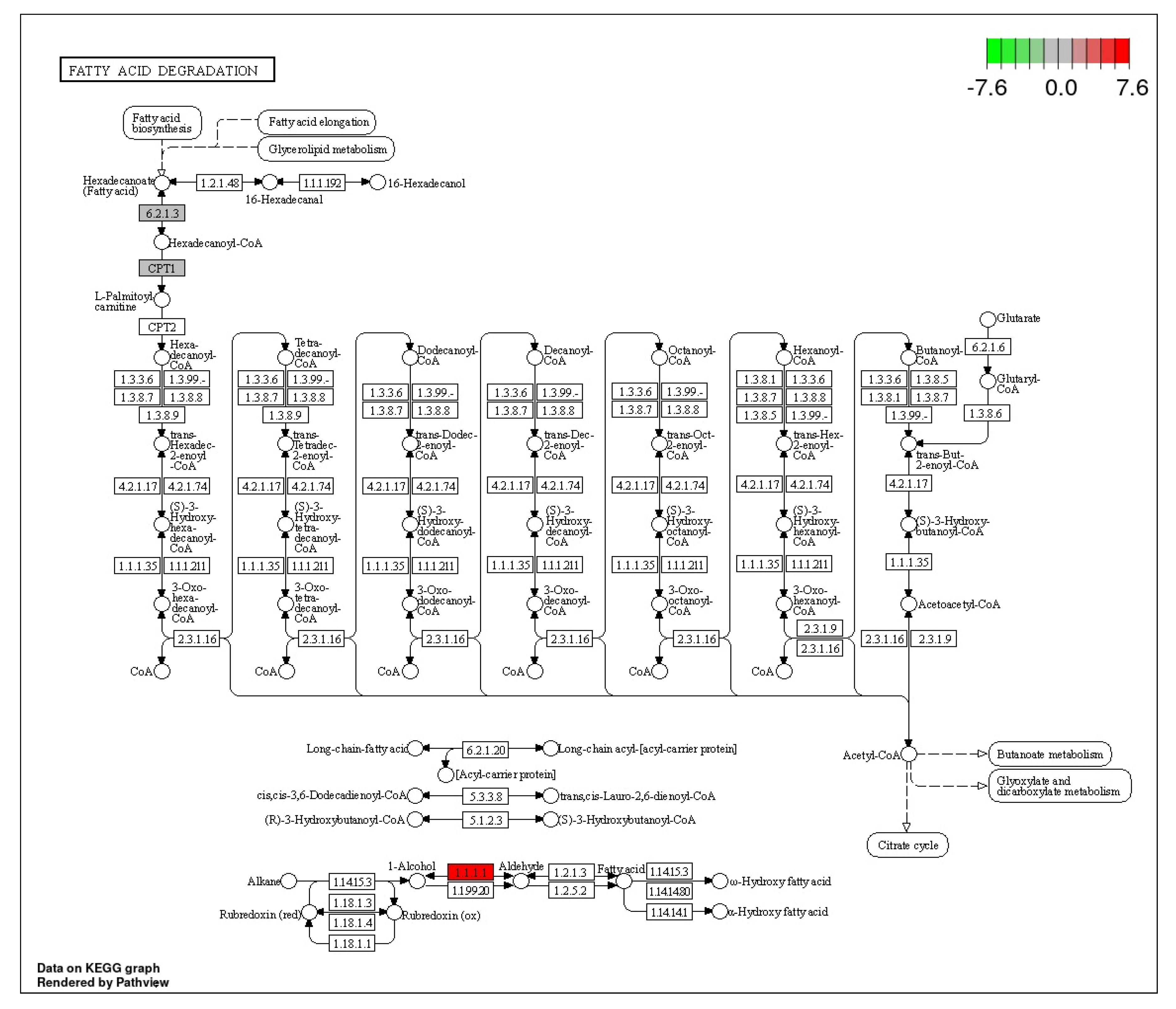
3.4. Fatty Acid Degradation
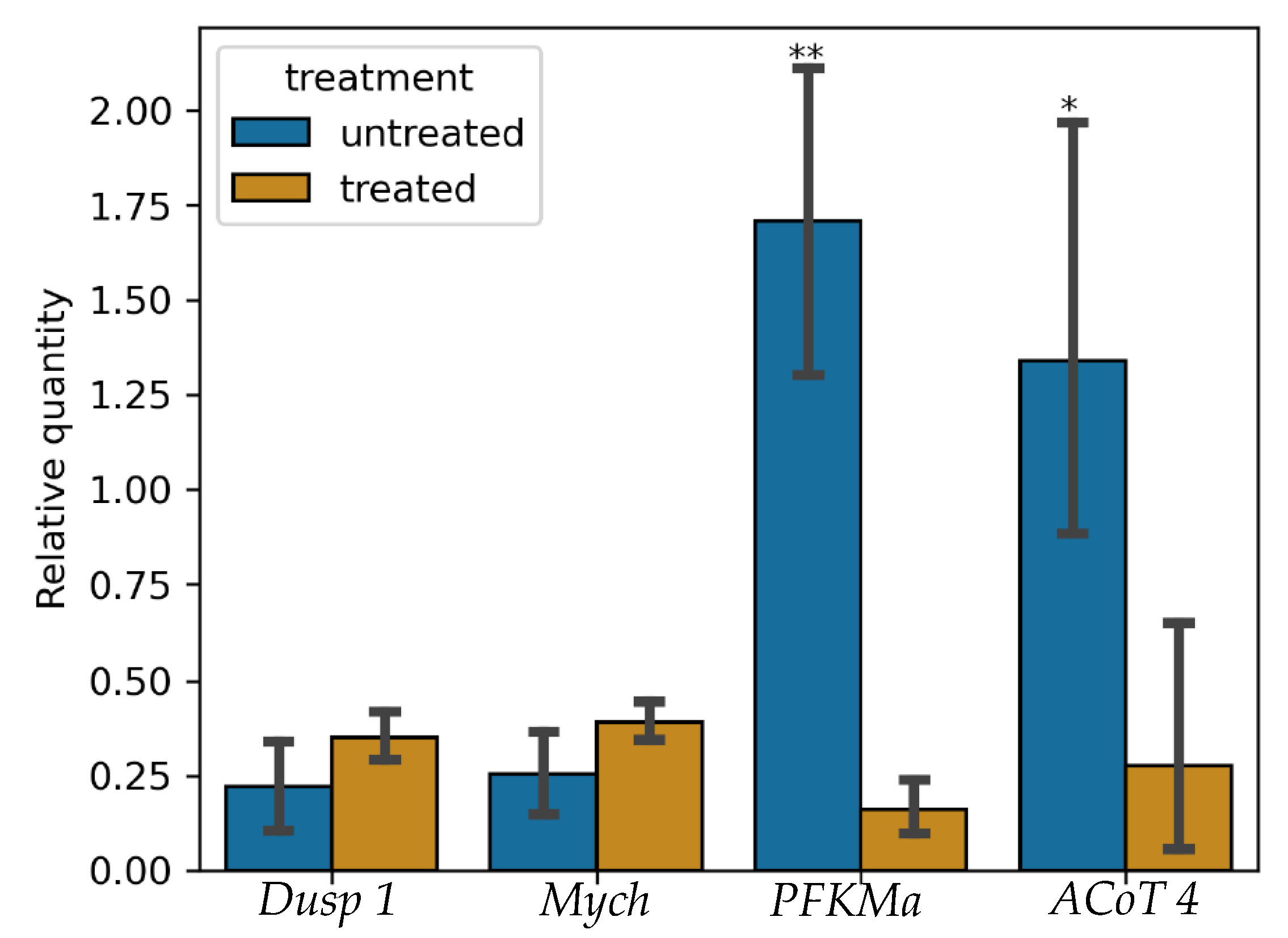
3.5. Validation of RNA-Seq Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lancker, K.; Bronnmann, J. Substitution Preferences for Fish in Senegal. Environ. Resour. Econ. 2022, 82, 1015–1045. [Google Scholar] [CrossRef]
- Parrao, C.G.; Shisler, S.; Moratti, M.; Yavuz, C.; Acharya, A.; Eyers, J.; Snilstveit, B. Aquaculture for improving productivity, income, nutrition and women’s empowerment in low- and middle-income countries: A systematic review and meta-analysis. Campbell Syst. Rev. 2021, 17, e1195. [Google Scholar] [CrossRef]
- Prabu, E.; Rajagopalsamy, C.; Ahilan, B.; Jeevagan, I.; Renuhadevi, M. Tilapia—An excellent candidate species for world aquaculture: A review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Bostock, J.; McAndrew, B.; Richards, R.; Jauncey, K.; Telfer, T.; Lorenzen, K.; Little, D.; Ross, L.; Handisyde, N.; Gatward, I.; et al. Aquaculture: Global status and trends. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2897–2912. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture-Sustainability in Action; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Kobayashi, M.; Msangi, S.; Batka, M.; Vannuccini, S.; Dey, M.; Anderson, J.L. Fish to 2030: The Role and Opportunity for Aquaculture. Aquac. Econ. Manag. 2015, 19, 282–300. [Google Scholar] [CrossRef] [Green Version]
- Ajani, F.; Olukunle, O.A.; Agbede, S.A. Hormonal and haematological responses of Clarias gariepinus (Burchell 1822) to ammonia toxicity. Afr. J. Biotechnol. 2008, 7, 3466–3471. [Google Scholar]
- Odhiambo, E.; Angienda, P.O.; Okoth, P.; Onyango, D. Stocking Density Induced Stress on Plasma Cortisol and Whole Blood Glucose Concentration in Nile Tilapia Fish (Oreochromis niloticus) of Lake Victoria, Kenya. Int. J. Zool. 2020, 2020, 9395268. [Google Scholar] [CrossRef]
- Tsigos, C.; Kyrou, I.; Kassi, E.; Chrousos, G.P. Stress: Endocrine physiology and pathophysiology. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Hofland, J., Dungan, K., et al., Eds.; MDText.com, Inc.: Dartmouth, MA, USA, 2020. [Google Scholar] [PubMed]
- Gil Barcellos, L.J.; Volpato, G.L.; Barreto, R.E.; Coldebella, I.; Ferreira, D. Chemical communication of handling stress in fish. Physiol. Behav. 2011, 103, 372–375. [Google Scholar] [CrossRef] [Green Version]
- Dolomatov, S.I.; Shekk, P.V.; Zukow, W.; Kryukova, M.I. Features of nitrogen metabolism in fishes. Rev. Fish Biol. Fish. 2011, 21, 733–737. [Google Scholar] [CrossRef] [Green Version]
- Smutná, M.; Vorlová, L.; Svobodová, Z. Pathobiochemistry of Ammonia in the Internal Environment of Fish (Review). Acta Veter-Brno 2002, 71, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Paul, I.; Panigrahi, A.K.; Datta, S. Influence of Nitrogen Cycle Bacteria on Nitrogen Mineralisation, Water Quality and Productivity of Freshwater Fish Pond: A Review. Asian Fish. Sci. 2020, 33, 145–160. [Google Scholar] [CrossRef]
- Shin, K.W.; Kim, S.-H.; Kim, J.-H.; Hwang, S.D.; Kang, J.-C. Toxic effects of ammonia exposure on growth performance, hematological parameters, and plasma components in rockfish, Sebastes schlegelii, during thermal stress. Fish. Aquat. Sci. 2016, 19, 44. [Google Scholar] [CrossRef] [Green Version]
- Patra, R.W.; Chapman, J.C.; Lim, R.P.; Gehrke, P.C.; Sunderam, R.M. Interactions between water temperature and contaminant toxicity to freshwater fish. Environ. Toxicol. Chem. 2015, 34, 1809–1817. [Google Scholar] [CrossRef] [PubMed]
- Weihrauch, D.; Wilkie, M.P.; Walsh, P.J. Ammonia and urea transporters in gills of fish and aquatic crustaceans. J. Exp. Biol. 2009, 212, 1716–1730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randall, D.J.; Wright, P.A. The interaction between carbon dioxide and ammonia excretion and water pH in fish. Can. J. Zool. 1989, 67, 2936–2942. [Google Scholar] [CrossRef]
- Li, M.; Yu, N.; Qin, J.G.; Li, E.; Du, Z.; Chen, L. Effects of ammonia stress, dietary linseed oil and Edwardsiella ictaluri challenge on juvenile darkbarbel catfish Pelteobagrus vachelli. Fish Shellfish. Immunol. 2014, 38, 158–165. [Google Scholar] [CrossRef]
- Gall, G.A.; Bakar, Y. Stocking density and tank size in the design of breed improvement programs for body size of tilapia. Aquaculture 1999, 173, 197–205. [Google Scholar] [CrossRef]
- Fattah, A.F.A.; Ahmed, F.A.; Saleem, A.-S.Y.; Mohammed, H.H.; Youssef, M.I.; Said, E.N. Effect of the different stocking density on behavior, performance and welfare of the Nile tilapia (Oreochromis niloticus). Egypt. J. Aquat. Biol. Fish. 2020, 24, 539–560. [Google Scholar] [CrossRef]
- Barreto, D.R.; Rey, O.; Webster, T.U.; Castaldo, G.; Consuegra, S.; De Leaniz, C.G. Transcriptomic response to aquaculture intensification in Nile tilapia. Evol. Appl. 2019, 12, 1757–1771. [Google Scholar] [CrossRef] [Green Version]
- Champneys, T.; Castaldo, G.; Consuegra, S.; de Leaniz, C.G. Density-dependent changes in neophobia and stress-coping styles in the world’s oldest farmed fish. R. Soc. Open Sci. 2018, 5, 181473. [Google Scholar] [CrossRef] [Green Version]
- Samaras, A.; Santo, C.E.; Papandroulakis, N.; Mitrizakis, N.; Pavlidis, M.; Höglund, E.; Pelgrim, T.N.M.; Zethof, J.; Spanings, F.A.T.; Vindas, M.A.; et al. Allostatic Load and Stress Physiology in European Seabass (Dicentrarchus labrax L.) and Gilthead Seabream (Sparus aurata L.). Front. Endocrinol. 2018, 9, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreck, C.B.; Tort, L. The concept of stress in fish. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2016; Volume 35, pp. 1–34. [Google Scholar]
- Hoem, K.S.; Tveten, A. Current approaches in decoding the molecular mechanisms of long-term stress in adult farmed Atlantic salmon (Salmo salar). Rev. Aquac. 2019, 12, 1708–1720. [Google Scholar] [CrossRef] [Green Version]
- Wish, J.; Bulloch, P.; Oswald, L.; Halldorson, T.; Raine, J.C.; Jamshed, L.; Marvin, C.; Thomas, P.J.; Holloway, A.C.; Tomy, G.T. Kynurenine to tryptophan ratio as a biomarker of acute stress in fish. Chemosphere 2022, 288, 132522. [Google Scholar] [CrossRef] [PubMed]
- Gans, I.M.; Coffman, J.A. Glucocorticoid-Mediated Developmental Programming of Vertebrate Stress Responsivity. Front. Physiol. 2021, 12, 812195. [Google Scholar] [CrossRef]
- Farahani, M.M.; Hosseinian, S.A. Effects of dietary stinging nettle (Urtica dioica) on hormone stress and selected serum biochemical parameters of broilers subjected to chronic heat stress. Veter-Med. Sci. 2022, 8, 660–667. [Google Scholar] [CrossRef]
- Bonga, S.E.W. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Reid, S.G.; Bernier, N.J.; Perry, S.F. The adrenergic stress response in fish: Control of catecholamine storage and release. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1998, 120, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, J.; Thammaratsuntorn, J.; Wu, J.; Wei, J.; Wang, Y.; Xu, J.; Zhao, J. Comparative transcriptome analysis of Nile tilapia (Oreochromis niloticus) in response to alkalinity stress. Genet. Mol. Res. 2015, 14, 17916–17926. [Google Scholar] [CrossRef]
- Xu, Z.; Gan, L.; Li, T.; Xu, C.; Chen, K.; Wang, X.; Qin, J.G.; Chen, L.; Li, E. Transcriptome Profiling and Molecular Pathway Analysis of Genes in Association with Salinity Adaptation in Nile Tilapia Oreochromis niloticus. PLoS ONE 2015, 10, e0136506. [Google Scholar] [CrossRef] [Green Version]
- Li, H.L.; Gu, X.H.; Li, B.J.; Chen, X.; Lin, H.R.; Xia, J.H. Characterization and functional analysis of hypoxia-inducible factor HIF1α and its inhibitor HIF1αn in tilapia. PLoS ONE 2017, 12, e0173478. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Bai, B.; Huang, S.; Liu, P.; Wan, Z.Y.; Ye, B.; Wu, J.; Yue, G.H. QTL Mapping for Resistance to Iridovirus in Asian Seabass Using Genotyping-by-Sequencing. Mar. Biotechnol. 2017, 19, 517–527. [Google Scholar] [CrossRef]
- Yue, X.; Huan, P.; Hu, Y.; Liu, B. Integrated transcriptomic and proteomic analyses reveal potential mechanisms linking thermal stress and depressed disease resistance in the turbot Scophthalmus maximus. Sci. Rep. 2018, 8, 1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galt, N.J.; Froehlich, J.M.; McCormick, S.D.; Biga, P.R. A comparative evaluation of crowding stress on muscle HSP90 and myostatin expression in salmonids. Aquaculture 2018, 483, 141–148. [Google Scholar] [CrossRef]
- Hu, S.; Ni, W.; Sai, W.; Zi, H.; Qiao, J.; Wang, P.; Sheng, J.; Chen, C. Knockdown of Myostatin Expression by RNAi Enhances Muscle Growth in Transgenic Sheep. PLoS ONE 2013, 8, e58521. [Google Scholar] [CrossRef]
- Sternstein, I.; Reissmann, M.; Maj, D.; Bieniek, J.; Brockmann, G.A. A new single nucleotide polymorphism in the rabbit (Oryctolagus cuniculus) myostatin (MSTN) gene is associated with carcass composition traits. Anim. Genet. 2014, 45, 596–599. [Google Scholar] [CrossRef]
- Ings, J.S.; Servos, M.R.; Vijayan, M.M. Exposure to municipal wastewater effluent impacts stress performance in rainbow trout. Aquat. Toxicol. 2011, 103, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Telli, G.S.; Ranzani-Paiva, M.J.T.; Dias, D.D.C.; Sussel, F.R.; Ishikawa, C.M.; Tachibana, L. Dietary administration of Bacillus subtilis on hematology and non-specific immunity of Nile tilapia Oreochromis niloticus raised at different stocking densities. Fish Shellfish. Immunol. 2014, 39, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Herkenhoff, M.E.; Ribeiro, A.O.; Costa, J.M.; Oliveira, A.C.; Dias, M.A.D.; Neto, R.V.R.; Hilsdorf, A.W.S.; Pinhal, D. Expression profiles of growth-related genes in two Nile tilapia strains and their crossbred provide insights into introgressive breeding effects. Anim. Genet. 2020, 51, 611–616. [Google Scholar] [CrossRef]
- Li, W.; Hu, Z.-F.; Chen, B.; Ni, G.-X. Response of C2C12 Myoblasts to Hypoxia: The Relative Roles of Glucose and Oxygen in Adaptive Cellular Metabolism. BioMed Res. Int. 2013, 2013, 326346. [Google Scholar] [CrossRef] [Green Version]
- Langley, B.; Thomas, M.; Bishop, A.; Sharma, M.; Gilmour, S.; Kambadur, R. Myostatin Inhibits Myoblast Differentiation by Down-regulating MyoD Expression. J. Biol. Chem. 2002, 277, 49831–49840. [Google Scholar] [CrossRef] [Green Version]
- Khalil, K.; Elayat, M.; Khalifa, E.; Daghash, S.; Elaswad, A.; Miller, M.; Abdelrahman, H.; Ye, Z.; Odin, R.; Drescher, D.; et al. Generation of Myostatin Gene-Edited Channel Catfish (Ictalurus punctatus) via Zygote Injection of CRISPR/Cas9 System. Sci. Rep. 2017, 7, 7301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuentes, E.N.; Pino, K.; Navarro, C.; Delgado, I.; Valdés, J.A.; Molina, A. Transient inactivation of myostatin induces muscle hypertrophy and overcompensatory growth in zebrafish via inactivation of the SMAD signaling pathway. J. Biotechnol. 2013, 168, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.-M.; Khosravi, S.; Kothari, D.; Lee, W.-D.; Lim, J.-M.; Lee, B.-J.; Kim, K.-W.; Lim, S.-G.; Lee, S.-M.; Kim, S.-K. Effects of dietary multi-strain probiotics supplementation in a low fishmeal diet on growth performance, nutrient utilization, proximate composition, immune parameters, and gut microbiota of juvenile olive flounder (Paralichthys olivaceus). Fish Shellfish. Immunol. 2019, 93, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.D.C.; Favero, G.C.; Beier, S.L.; Ferreira, N.S.; Palheta, G.D.A.; De Melo, N.F.A.C.; Luz, R.K. Physiological and metabolic responses in juvenile Colossoma macropomum exposed to hypoxia. Fish Physiol. Biochem. 2020, 46, 2157–2167. [Google Scholar] [CrossRef]
- Cho, S.H. Effect of Intermittent Feeding on Growth, Feed Utilization and Body Composition of Subadult Olive Flounder Paralichthys olivaceus in Suboptimal Temperature. Asian-Australas. J. Anim. Sci. 2011, 24, 394–399. [Google Scholar] [CrossRef]
- Emerson, K.; Russo, R.C.; Lund, R.E.; Thurston, R.V. Aqueous Ammonia Equilibrium Calculations: Effect of pH and Temperature. J. Fish. Res. Board Can. 1975, 32, 2379–2383. [Google Scholar] [CrossRef]
- Ighwela, K.A.; Ahmed, A.B.; Abol-Munafi, A.B. Condition Factor as an Indicator of Growth and Feeding Intensity of Nile Tilapia Fingerlings (Oreochromis niloticus) Feed on Different Levels of Maltose. Am.-Eurasian J. Agric. Environ. Sci. 2011, 11, 559–563. [Google Scholar]
- Brandine, G.D.S.; Smith, A.D. Falco: High-speed FastQC emulation for quality control of sequencing data. F1000Research 2021, 8, 1874. [Google Scholar] [CrossRef]
- Piñero, J.; Saüch, J.; Sanz, F.; Furlong, L.I. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 2021, 19, 2960–2967. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. ClusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. Feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wekesa, C.; Asudi, G.O.; Okoth, P.; Reichelt, M.; Muoma, J.O.; Furch, A.C.U.; Oelmüller, R. Rhizobia Contribute to Salinity Tolerance in Common Beans (Phaseolus vulgaris L.). Cells 2022, 11, 3628. [Google Scholar] [CrossRef]
- Untergasser, A.; Nijveen, H.; Rao, X.; Bisseling, T.; Geurts, R.; Leunissen, J.A.M. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 2007, 35, W71–W74. [Google Scholar] [CrossRef] [Green Version]
- Ekström, A.; Sundell, E.; Morgenroth, D.; Sandblom, E. Adrenergic tone benefits cardiac performance and warming tolerance in two teleost fishes that lack a coronary circulation. J. Comp. Physiol. B 2021, 191, 701–709. [Google Scholar] [CrossRef]
- Valentim, M.A.; Brahmbhatt, A.N.; Tupling, A.R. Skeletal and cardiac muscle calcium transport regulation in health and disease. Biosci. Rep. 2022, 42, BSR20211997. [Google Scholar] [CrossRef]
- Carneiro, V.C.L.; Delicio, H.C.; Barreto, R.E. Effects of stress-associated odor on ventilation rate and feeding performance in Nile tilapia. J. Appl. Anim. Welf. Sci. 2022, 1–11. [Google Scholar] [CrossRef]
- Xu, H.; Van Remmen, H. The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: A potential target for intervention in aging and skeletal muscle pathologies. Skelet. Muscle 2021, 11, 25. [Google Scholar] [CrossRef]
- Bkaily, G.; Jacques, D. Na+–H+ exchanger and proton channel in heart failure associated with Becker and Duchenne muscular dystrophies. Can. J. Physiol. Pharmacol. 2017, 95, 1213–1223. [Google Scholar] [CrossRef] [Green Version]
- Bathe-Peters, M.; Gmach, P.; Boltz, H.-H.; Einsiedel, J.; Gotthardt, M.; Hübner, H.; Gmeiner, P.; Lohse, M.J.; Annibale, P. Visualization of β-adrenergic receptor dynamics and differential localization in cardiomyocytes. Proc. Natl. Acad. Sci. USA 2021, 118, e2101119118. [Google Scholar] [CrossRef]
- Svendsen, E.; Føre, M.; Økland, F.; Gräns, A.; Hedger, R.; Alfredsen, J.; Uglem, I.; Rosten, C.; Frank, K.; Erikson, U.; et al. Heart rate and swimming activity as stress indicators for Atlantic salmon (Salmo salar). Aquaculture 2021, 531, 735804. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Ripa, R.; George, T.; Sattar, Y. Physiology, Cardiac Muscle. [Updated 2 Jun 2022]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572070/ (accessed on 11 February 2023).
- Kitagawa, H.; Sugo, N.; Morimatsu, M.; Arai, Y.; Yanagida, T.; Yamamoto, N. Activity-Dependent Dynamics of the Transcription Factor of cAMP-Response Element Binding Protein in Cortical Neurons Revealed by Single-Molecule Imaging. J. Neurosci. 2016, 37, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Horinouchi, T.; Koike, K. New insights into β-adrenoceptors in smooth muscle: Distribution of receptor subtypes and molecular mechanisms triggering muscle relaxation. Clin. Exp. Pharmacol. Physiol. 2005, 32, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Lee, J.-A.; Nam, Y.K. Molecular characterization of three muscle alpha actin genes in mud loach (Misgurnus mizolepis; Cypriniformes). Fish. Aquat. Sci. 2017, 20, 27. [Google Scholar] [CrossRef] [Green Version]
- Landeira, B.S.; Santana, T.T.D.S.; Araújo, J.A.D.M.; Tabet, E.I.; Tannous, B.A.; Schroeder, T.; Costa, M.R. Activity-Independent Effects of CREB on Neuronal Survival and Differentiation during Mouse Cerebral Cortex Development. Cereb. Cortex 2016, 28, 538–548. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.; Hu, T.; An, Y.; Huang, J.; Xu, Y. Purification and enzymatic characterization of alcohol dehydrogenase from Arabidopsis thaliana. Protein Expr. Purif. 2013, 90, 74–77. [Google Scholar] [CrossRef]
- Liu, L.; Long, X.; Deng, D.; Cheng, Y.; Wu, X. Molecular characterization and tissue distribution of carnitine palmitoyltransferases in Chinese mitten crab Eriocheir sinensis and the effect of dietary fish oil replacement on their expression in the hepatopancreas. PLoS ONE 2018, 13, e0201324. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Katerelos, M.; Gleich, K.; Galic, S.; Kemp, B.E.; Mount, P.F.; Power, D.A. Phosphorylation of Acetyl-CoA Carboxylase by AMPK Reduces Renal Fibrosis and Is Essential for the Anti-Fibrotic Effect of Metformin. J. Am. Soc. Nephrol. 2018, 29, 2326–2336. [Google Scholar] [CrossRef] [Green Version]
- Rotte, A.; Pasham, V.; Eichenmüller, M.; Bhandaru, M.; Föller, M.; Lang, F. Upregulation of Na+/H+ exchanger by the AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2010, 398, 677–682. [Google Scholar] [CrossRef]
- Vizin, T.; Kos, J. Gamma-enolase: A well-known tumour marker, with a less-known role in cancer. Radiol. Oncol. 2015, 49, 217–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chau, A.C.; Smith, A.E.; Hordacre, B.; Kumar, S.; Cheung, E.Y.; Mak, H.K. A scoping review of resting-state brain functional alterations in Type 2 diabetes. Front. Neuroendocr. 2022, 65, 100970. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Bi, W.; Yang, G.; Zhao, J.; Wang, J.; Li, X.; Zhou, X. Hyperglycemia Induced by Chronic Restraint Stress in Mice Is Associated With Nucleus Tractus Solitarius Injury and Not Just the Direct Effect of Glucocorticoids. Front. Neurosci. 2018, 12, 983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulei, I.R.; Mbuthia, P.G.; Waruiru, R.M.; Nyaga, P.N.; Mutoloki, S.; Evensen, Ø. Management Practices, Farmers’ Knowledge of Diseased Fish, and Their Occurrence in Fish Farms in Nyeri County, Kenya. Veter-Med. Int. 2021, 2021, 8896604. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yuan, Z.; Liu, M.; Stock, C. (Patho-)Physiology of Na+/H+ Exchangers (NHEs) in the Digestive System. Front. Physiol. 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, C.-H.; Woo, J.S.; Perez, C.F.; Lee, E.H. A focus on extracellular Ca2+ entry into skeletal muscle. Exp. Mol. Med. 2017, 49, e378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.Y.; Takeda, M.; Briggs, L.E.; Graham, M.L.; Lu, J.T.; Horikoshi, N.; Weinberg, E.O.; Aoki, H.; Sato, N.; Chien, K.R.; et al. Identification of Cardiac-Specific Myosin Light Chain Kinase. Circ. Res. 2008, 102, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Masri, B.; Knibiehler, B.; Audigier, Y. Apelin signalling: A promising pathway from cloning to pharmacology. Cell. Signal. 2005, 17, 415–426. [Google Scholar] [CrossRef]
- Fearnley, C.J.; Roderick, H.L.; Bootman, M.D. Calcium Signaling in Cardiac Myocytes. Cold Spring Harb. Perspect. Biol. 2011, 3, a004242. [Google Scholar] [CrossRef] [Green Version]
- Chapman, N.A.; Dupré, D.J.; Rainey, J.K. The apelin receptor: Physiology, pathology, cell signalling, and ligand modulation of a peptide-activated class A GPCR. Biochem. Cell Biol. 2014, 92, 431–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkoff, H.; Wyatt, J.L. Apelin in goldfish (Carassius auratus): Cloning, distribution and role in appetite regulation. Peptides 2009, 30, 1434–1440. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.; Hu, G.; Zhang, X.; Ruan, Z.; Zhao, X.; Guo, C.; Tang, Z.; Li, X.; You, X.; et al. Comparative Transcriptomic Study of Muscle Provides New Insights into the Growth Superiority of a Novel Grouper Hybrid. PLoS ONE 2016, 11, e0168802. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.B.; Kim, Y.S.; Oh, M.-Y.; Jeong, I.-H.; Seong, K.-B.; Jin, H.-J. Improving rainbow trout (Oncorhynchus mykiss) growth by treatment with a fish (Paralichthys olivaceus) myostatin prodomain expressed in soluble forms in E. coli. Aquaculture 2010, 302, 270–278. [Google Scholar] [CrossRef]
- Yarahmadi, P.; Miandare, H.K.; Fayaz, S.; Caipang, C.M.A. Increased stocking density causes changes in expression of selected stress- and immune-related genes, humoral innate immune parameters and stress responses of rainbow trout (Oncorhynchus mykiss). Fish Shellfish. Immunol. 2016, 48, 43–53. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, W.-N.; He, W.-Y.; Zheng, Y.; Wang, L.; Xin, Y.; Liu, Y.; Wang, A.-L. Expression of HSP60 and HSP70 in white shrimp, Litopenaeus vannamei in response to bacterial challenge. J. Invertebr. Pathol. 2010, 103, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Xue, D.; Li, Z.; Dong, S.; Jiang, F.; Hu, Z.; Zheng, Y. Evaluation of dietary Haematococcus pluvialis in pilot-scale farming of Litopenaeus vannamei: Effects on production performance, physiological health, bacterial communities, and eukaryotic plankton. Aquaculture 2023, 563. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Yu, Y.; Sun, M.; Xiang, J.; Li, F. Effects of ammonia stress on the hemocytes of the Pacific white shrimp Litopenaeus vannamei. Chemosphere 2019, 239, 124759. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, R.; Zhao, D.; Wang, L.; Sun, M.; Wang, M.; Song, L. Ammonia exposure induces oxidative stress, endoplasmic reticulum stress and apoptosis in hepatopancreas of pacific white shrimp (Litopenaeus vannamei). Fish Shellfish. Immunol. 2016, 54, 523–528. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Yang, F.-F.; Ling, R.-Z.; Liao, S.-A.; Miao, Y.-T.; Ye, C.-X.; Wang, A.-L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef]
- Fardell, L.L.; Bedoya-Pérez, M.A.; Dickman, C.R.; Crowther, M.S.; Pavey, C.R.; Narayan, E.J. Are physiological and behavioural responses to stressors displayed concordantly by wild urban rodents? Sci. Nat. 2021, 108, 5. [Google Scholar] [CrossRef] [PubMed]
- Shourbela, R.M.; Khatab, S.A.; Hassan, M.M.; Van Doan, H.; Dawood, M.A.O. The Effect of Stocking Density and Carbon Sources on the Oxidative Status, and Nonspecific Immunity of Nile tilapia (Oreochromis niloticus) Reared under Biofloc Conditions. Animals 2021, 11, 184. [Google Scholar] [CrossRef]




| Gene Name | Primer Sequence | Annealing Temp. | Amplicon Size | GC Content% |
|---|---|---|---|---|
| dusp1_F | ACTTGAACACATCGTCCCCAAC | 61.1 °C | 181 bp | 50.0 |
| dusp1_R | TGTGCCGTCCTTTTTCACTTGG | 61.8 °C | 50.0 | |
| mych_F | AACTGTTTCATGCGCTTGCG | 60.7 °C | 190 bp | 50.0 |
| mych_R | TTTCCTGGTTTGCAGTCTGTGG | 61.3 °C | 50.0 | |
| pfkma_F | TGCAATTAAGGCCAAGCACCAC | 62.2 °C | 196 bp | 50.0 |
| pfkma_R | GCATGGTGTTGTAAAGGCTCACC | 62.5 °C | 52.2 | |
| AcoT4_F | TTGAGGCAGGTGGTGAAGACAAG | 62.9 °C | 185 bp | 52.2 |
| AcoT4_R | TGTGCAACATAACAGGCAGCTG | 62.0 °C | 50.0 | |
| GAPDH_F | TGGCATTGCACTCAACGACAAC | 62.5 °C | 182 bp | 50.0 |
| GAPDH_R | GTGCAGCAAACAAGCTTTGGTC | 61.6 °C | 50.0 |
| Ammonia | Length–Weight Relationship | |||||
|---|---|---|---|---|---|---|
| Concentration | Length | Weight | a | b | Kn | SGR (%) |
| Control (0 Mg/L) | 13.4 ± 1.85 | 56.32 ± 22.28 | 0.01397 | 3.12202 | 1.17 | 1.81341 |
| 0.4 Mg/L | 13.2 ± 1.67 | 52.56 ± 20.92 | 0.01415 | 3.12788 | 1.13 | 1.78352 |
| 0.8 Mg/L | 13.1 ± 1.33 | 51.18 ± 17.88 | 0.01418 | 3.1277 | 1.11 | 1.64517 |
| 1.2 Mg/L | 13.0 ± 1.80 | 46.37 ± 17.86 | 0.01437 | 3.11417 | 1.08 | 1.56446 |
| 1.6 Mg/L | 12.8 ± 1.39 | 40.93 ± 12.30 | 0.01484 | 3.08808 | 1.02 | 1.5114 |
| 2.0 Mg/L | 12.7 ± 1.34 | 38.70 ± 10.00 | 0.0154 | 3.07442 | 0.99 | 1.3249 |
| 2.4 Mg/L | 12.5 ± 1.14 | 35.09 ± 8.87 | 0.01586 | 3.06278 | 0.93 | 1.22908 |
| Density | Length–Weight Relationship | |||||
|---|---|---|---|---|---|---|
| Fish/Tank | Length | Weight | a | B | Kn | SGR (%) |
| Control (20 Fish/Tank) | 14.1 ± 1.95 | 52.05 ± 21.34 | 0.012961 | 3.0635 | 1.17 | 1.567213 |
| 25 Fish/Tank | 14.0 ± 1.86 | 48.54 ± 20.46 | 0.013067 | 3.0524 | 1.14 | 1.540296 |
| 40 Fish/Tank | 13.8 ± 1.66 | 46.44 ± 17.64 | 0.013103 | 3.0601 | 1.11 | 1.329046 |
| 55 Fish/Tank | 13.7 ± 1.65 | 41.62 ± 10.88 | 0.013156 | 3.0578 | 1.07 | 1.065474 |
| 70 Fish/Tank | 13.4 ± 1.19 | 37.10 ± 8.46 | 0.013323 | 3.0490 | 1.01 | 0.905001 |
| 85 Fish/Tank | 13.1 ± 1.17 | 34.34 ± 8.15 | 0.013327 | 3.0711 | 0.95 | 0.893896 |
| 100 Fish/Tank | 13.1 ± 1.21 | 32.20 ± 7.43 | 0.013445 | 3.0627 | 0.91 | 0.812368 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mwaura, J.G.; Wekesa, C.; Ogutu, P.A.; Okoth, P. Whole Transcriptome Analysis of Differentially Expressed Genes in Cultured Nile Tilapia (O. niloticus) Subjected to Chronic Stress Reveals Signaling Pathways Associated with Depressed Growth. Genes 2023, 14, 795. https://doi.org/10.3390/genes14040795
Mwaura JG, Wekesa C, Ogutu PA, Okoth P. Whole Transcriptome Analysis of Differentially Expressed Genes in Cultured Nile Tilapia (O. niloticus) Subjected to Chronic Stress Reveals Signaling Pathways Associated with Depressed Growth. Genes. 2023; 14(4):795. https://doi.org/10.3390/genes14040795
Chicago/Turabian StyleMwaura, John Gitau, Clabe Wekesa, Philip A. Ogutu, and Patrick Okoth. 2023. "Whole Transcriptome Analysis of Differentially Expressed Genes in Cultured Nile Tilapia (O. niloticus) Subjected to Chronic Stress Reveals Signaling Pathways Associated with Depressed Growth" Genes 14, no. 4: 795. https://doi.org/10.3390/genes14040795
APA StyleMwaura, J. G., Wekesa, C., Ogutu, P. A., & Okoth, P. (2023). Whole Transcriptome Analysis of Differentially Expressed Genes in Cultured Nile Tilapia (O. niloticus) Subjected to Chronic Stress Reveals Signaling Pathways Associated with Depressed Growth. Genes, 14(4), 795. https://doi.org/10.3390/genes14040795






