Cytoskeletal Keratins Are Overexpressed in a Zebrafish Model of Idiopathic Scoliosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry and Crossing
2.2. Genotyping
2.3. RNA Extraction and Sequencing (Novogene)
2.4. Bioinformatic Filtering
2.5. Keratin Staining and Histology
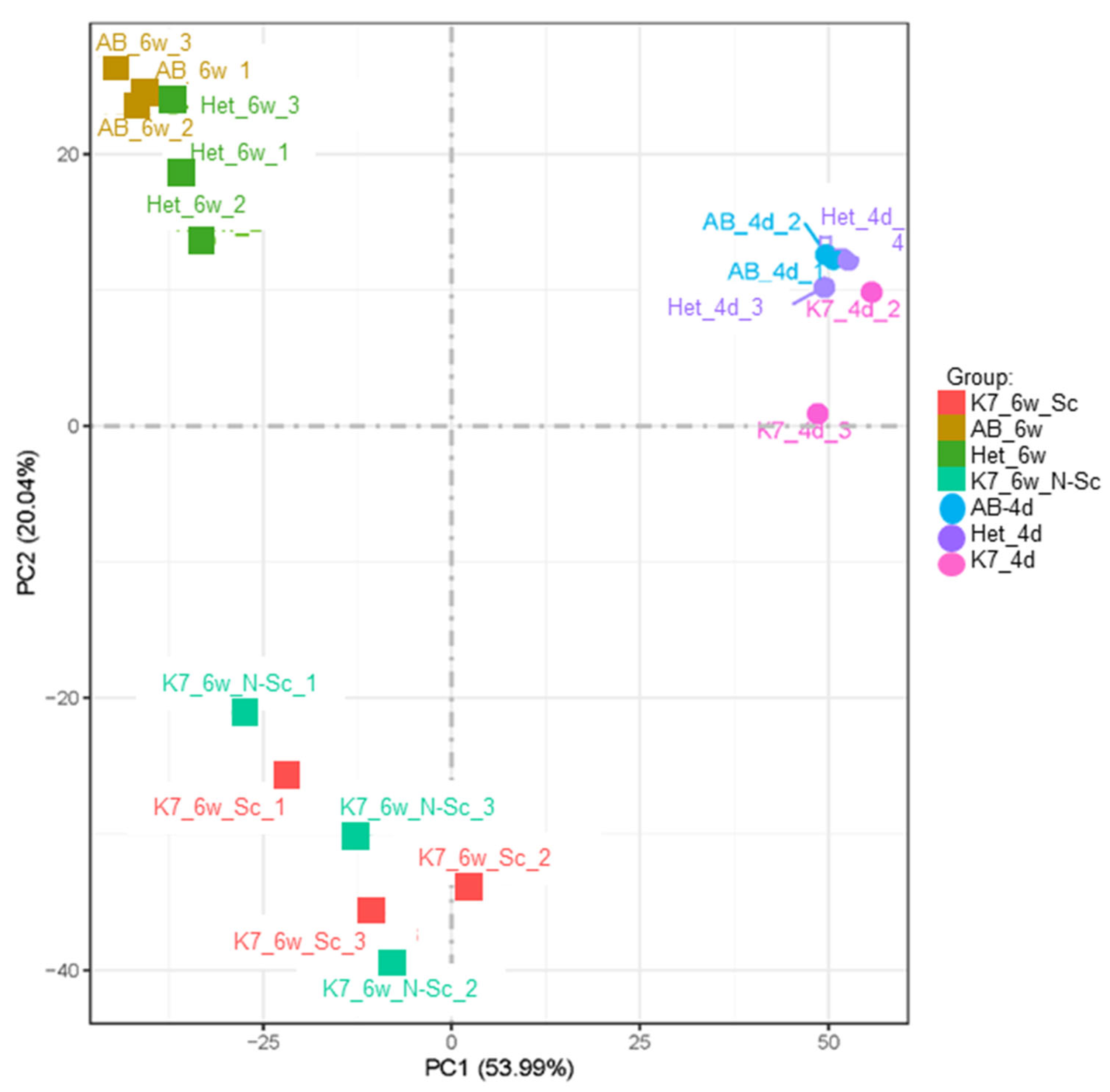
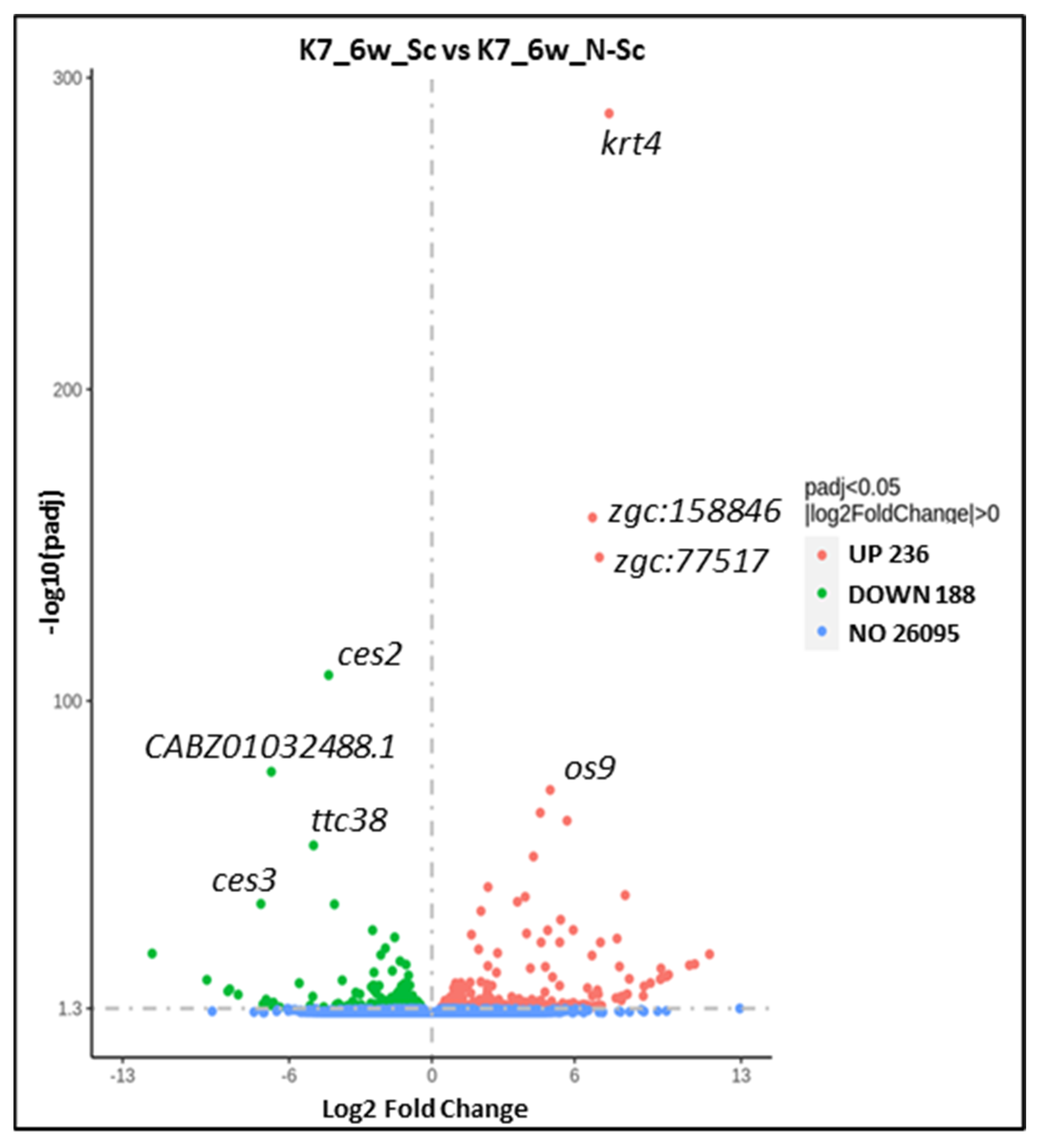
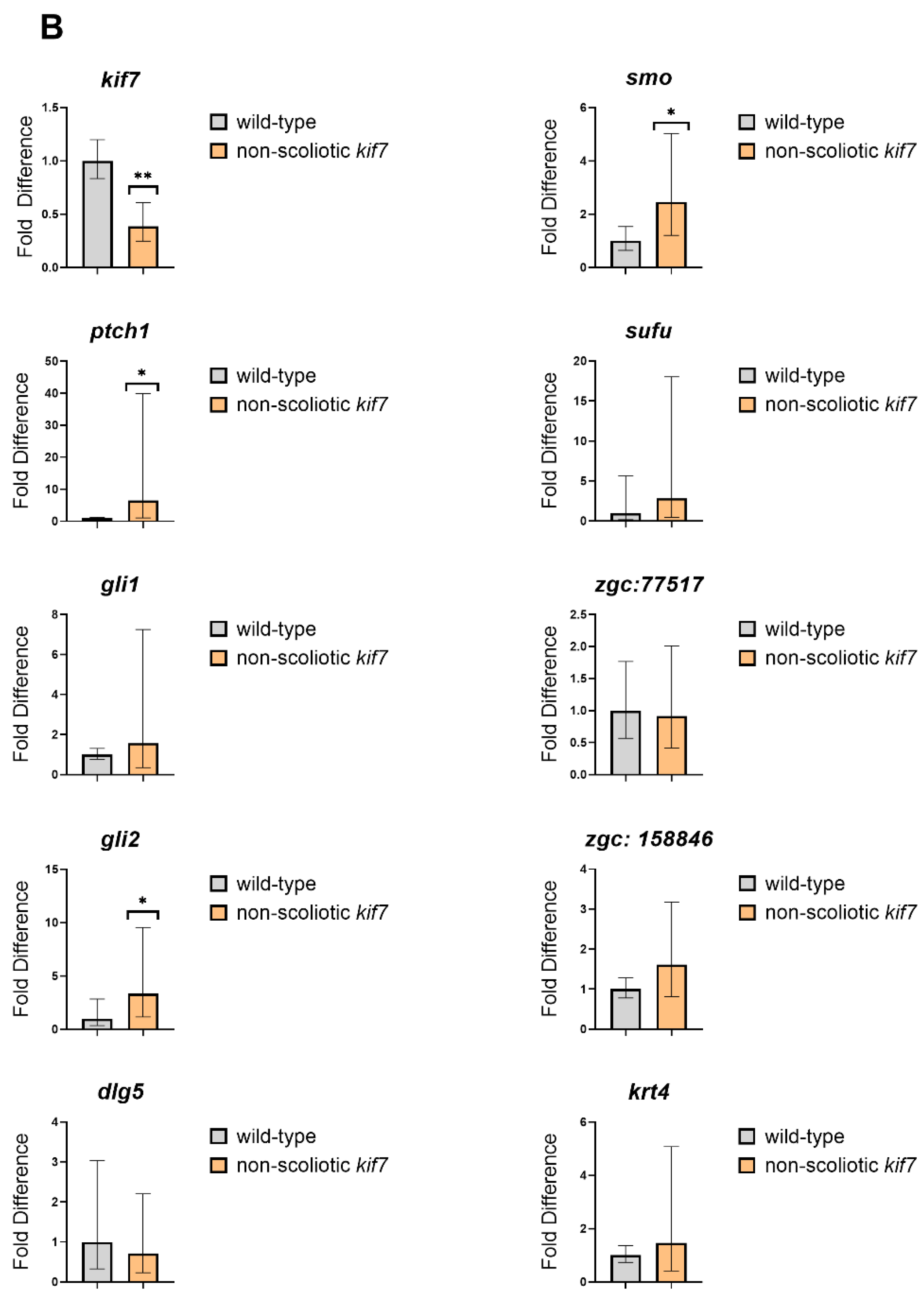
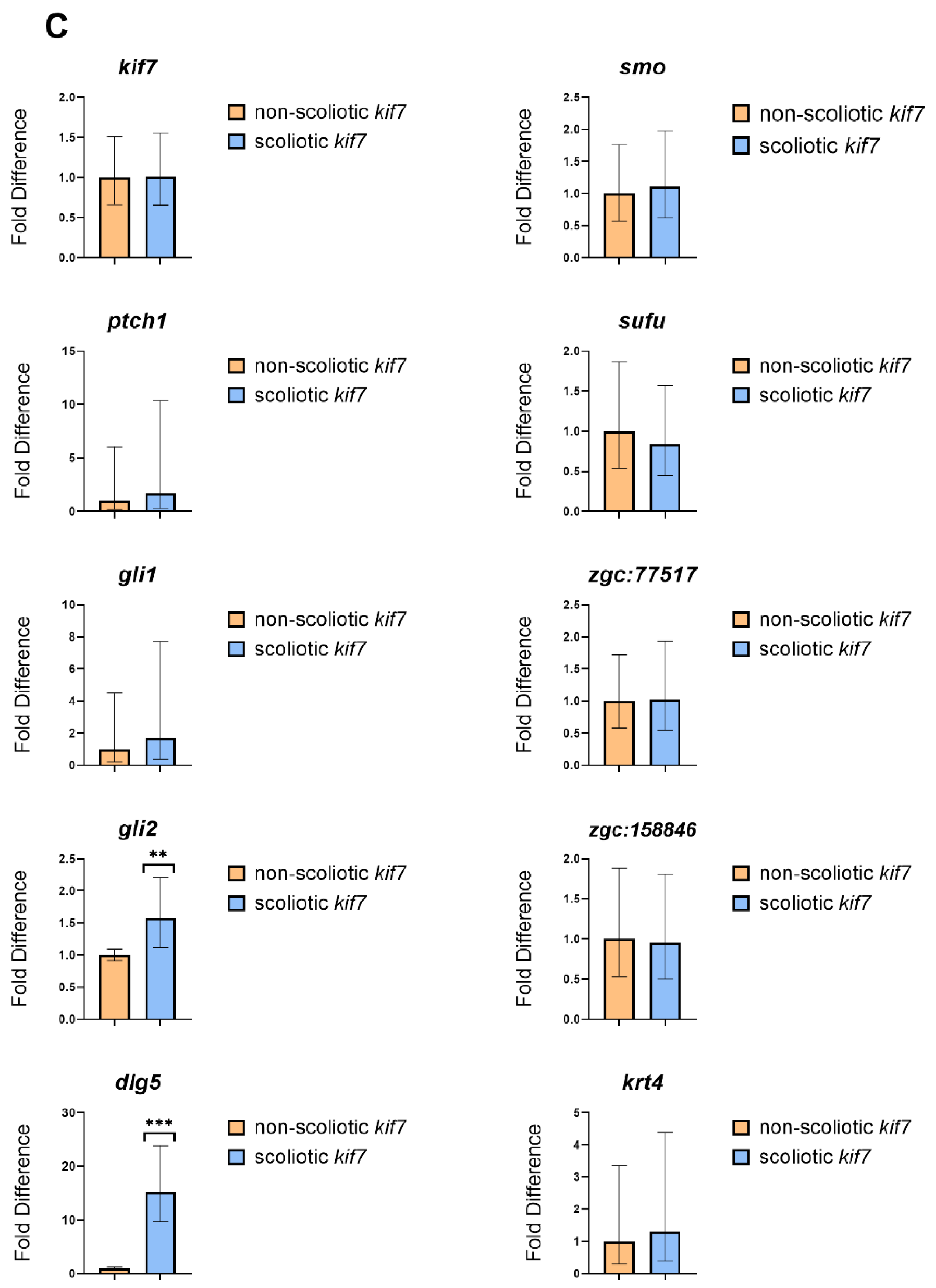
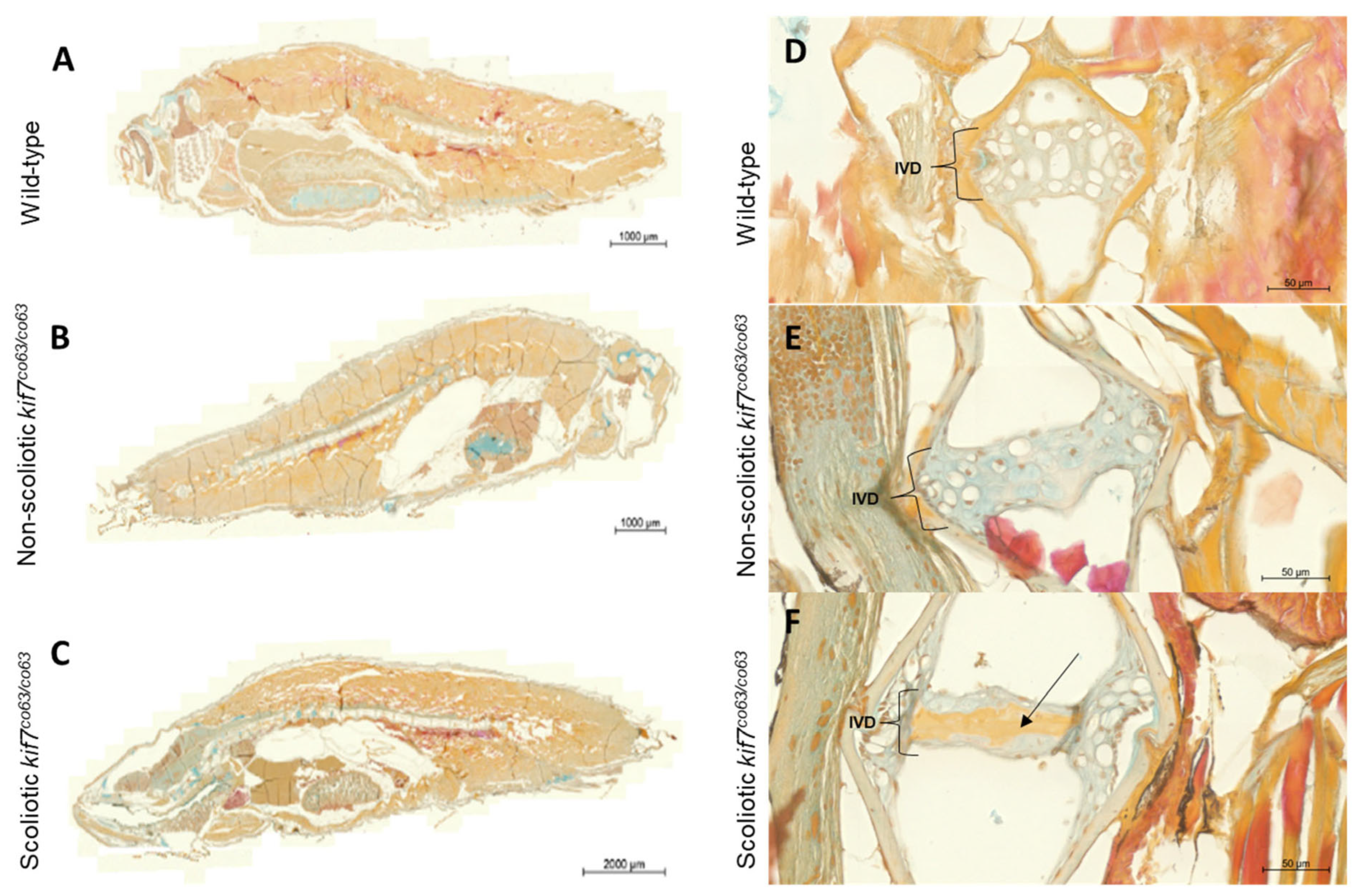
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, N.H.; Schwab, D.L.; Sponseller, P.D.; Manolio, T.A.; Pugh, E.W.; Wilson, A.P. Characterization of Idiopathic Scoliosis in a Clinically Well-Defined Population. Clin. Orthop. Relat. Res. 2001, 392, 349–357. [Google Scholar] [CrossRef]
- Roach, J.W. Adolescent idiopathic scoliosis. Orthop. Clin. N. Am. 1999, 30, 353–365. [Google Scholar] [CrossRef]
- Asher, M.A.; Burton, D.C. Adolescent idiopathic scoliosis: Natural history and long term treatment effects. Scoliosis 2006, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.Y.; Qin, L.; Cheung, C.S.K.; Sher, A.H.L.; Lee, K.M.; Ng, S.W.E.; Guo, X. Generalized Low Areal and Volumetric Bone Mineral Density in Adolescent Idiopathic Scoliosis. J. Bone Miner. Res. 2000, 15, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.S.K.; Lee, W.T.K.; Tse, Y.K.; Lee, K.M.; Guo, X.; Qin, L.; Cheng, J.C.Y. Generalized osteopenia in adolescent idiopathic scoliosis—Association with abnormal pubertal growth, bone turnover, and calcium intake? Spine 2006, 31, 330–338. [Google Scholar] [CrossRef]
- Guo, X.; Chau, W.W.; Chan, Y.L.; Cheng, J.Y. Relative anterior spinal overgrowth in adolescent idiopathic scoliosis: Results of disproportionate endochondral-membranous bone growth. J. Bone Jt. Surg. Br. 2003, 85, 1026–1031. [Google Scholar] [CrossRef]
- Man, G.C.-W.; Wong, J.H.; Wang, W.W.-J.; Sun, G.-Q.; Yeung, B.H.-Y.; Ng, T.-B.; Lee, S.K.-M.; Ng, B.K.-W.; Qiu, Y.; Cheng, J.C.-Y. Abnormal melatonin receptor 1B expression in osteoblasts from girls with adolescent idiopathic scoliosis. J. Pineal Res. 2011, 50, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Blanco, G.; Coulton, G.R.; Biggin, A.; Grainge, C.; Moss, J.; Barrett, M.; Berquin, A.; Maréchal, G.; Skynner, M.; Van Mier, P.; et al. The kyphoscoliosis (ky) mouse is deficient in hypertrophic responses and is caused by a mutation in a novel muscle-specific protein. Hum. Mol. Genet. 2001, 10, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Latalski, M.; Danielewicz-Bromberek, A.; Fatyga, M.; Latalska, M.; Kröber, M.; Zwolak, P. Current insights into the aetiology of adolescent idiopathic scoliosis. Arch. Orthop. Trauma Surg. 2017, 137, 1327–1333. [Google Scholar] [CrossRef]
- Yagci, G.; Ayhan, C.; Yakut, Y. Effectiveness of basic body awareness therapy in adolescents with idiopathic scoliosis: A randomized controlled study1. J. Back Musculoskelet. Rehabil. 2018, 31, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Blecher, R.; Krief, S.; Galili, T.; Biton, I.E.; Stern, T.; Assaraf, E.; Levanon, D.; Appel, E.; Anekstein, Y.; Agar, G.; et al. The Proprioceptive System Masterminds Spinal Alignment: Insight into the Mechanism of Scoliosis. Dev. Cell 2017, 42, 388–399.e3. [Google Scholar] [CrossRef] [PubMed]
- Haumont, T.; Gauchard, G.C.; Lascombes, P.; Perrin, P.P. Postural Instability in Early-Stage Idiopathic Scoliosis in Adolescent Girls. Spine 2011, 36, E847–E854. [Google Scholar] [CrossRef] [PubMed]
- Missaoui, B.; Portero, P.; Bendaya, S.; Hanktie, O.; Thoumie, P. Posture and equilibrium in orthopedic and rheumatologic diseases. Neurophysiol. Clin. 2008, 38, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Burwell, R.G.; Aujla, R.K.; Grevitt, M.P.; Dangerfield, P.H.; Moulton, A.; Randell, T.L.; Anderson, S.I. Pathogenesis of adolescent idiopathic scoliosis in girls—A double neuro-osseous theory involving disharmony between two nervous systems, somatic and autonomic expressed in the spine and trunk: Possible dependency on sympathetic nervous system and hormones with implications for medical therapy. Scoliosis 2009, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.; MacEwen, G.D. Idiopathic scoliosis; A visio-vestibular disorder of the central nervous system. In Scoliosis, 1979; Zorab, P.A., Siegler, D., Cardiothoracic Institute (London, England), Eds.; Academic Press: London, UK; New York, NY, USA, 1980; Volume xvi, 289p. [Google Scholar]
- Hadley-Miller, N.; Mims, B.; Milewicz, D.M. The potential role of the elastic fiber system in adolescent idiopathic scoliosis. J. Bone Jt. Surg. 1994, 76, 1193–1206. [Google Scholar] [CrossRef]
- Clough, M.; Justice, C.M.; Marosy, B.; Miller, N.H. Males with familial idiopathic scoliosis: A distinct phenotypic subgroup. Spine 2010, 35, 162–168. [Google Scholar] [CrossRef]
- Dunwoodie, S.L.; Kusumi, K. The Genetics and Development of Scoliosis; Springer International Publishing: Cham, Switzerland, 2018; Volume XII, 199p. [Google Scholar]
- Edery, P.; Margaritte-Jeannin, P.; Biot, B.; Labalme, A.; Bernard, J.-C.; Chastang, J.; Kassai, B.; Plais, M.-H.; Moldovan, F.; Clerget-Darpoux, F. New disease gene location and high genetic heterogeneity in idiopathic scoliosis. Eur. J. Hum. Genet. 2011, 19, 865–869. [Google Scholar] [CrossRef]
- Justice, C.M.; Bishop, K.; Carrington, B.; Mullikin, J.C.; Swindle, K.; Marosy, B.; Sood, R.; Miller, N.H.; Wilson, A.F. Evaluation of IRX Genes and Conserved Noncoding Elements in a Region on 5p13.3 Linked to Families with Familial Idiopathic Scoliosis and Kyphosis. G3 2016, 6, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Boswell, C.W.; Ciruna, B. Understanding Idiopathic Scoliosis: A New Zebrafish School of Thought. Trends Genet. 2017, 33, 183–196. [Google Scholar] [CrossRef]
- Hayes, M.; Gao, X.; Yu, L.X.; Paria, N.; Henkelman, R.M.; Wise, C.A.; Ciruna, B. ptk7 mutant zebrafish models of congenital and idiopathic scoliosis implicate dysregulated Wnt signalling in disease. Nat. Commun. 2014, 5, 4777. [Google Scholar] [CrossRef]
- Grimes, D.T.; Boswell, C.W.; Morante, N.F.C.; Henkelman, R.M.; Burdine, R.D.; Ciruna, B. Zebrafish models of idiopathic scoliosis link cerebrospinal fluid flow defects to spine curvature. Science 2016, 352, 1341–1344. [Google Scholar] [CrossRef] [PubMed]
- Patten, S.; Margaritte-Jeannin, P.; Bernard, J.-C.; Alix, E.; Labalme, A.; Besson, A.; Girard, S.; Fendri, K.; Fraisse, N.; Biot, B.; et al. Functional variants of POC5 identified in patients with idiopathic scoliosis. J. Clin. Investig. 2015, 125, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Buchan, J.G.; Gray, R.S.; Gansner, J.M.; Alvarado, D.M.; Burgert, L.; Gitlin, J.D.; Gurnett, C.A.; Goldsmith, M.I. Kinesin family member 6 (kif6) is necessary for spine development in zebrafish. Dev. Dyn. 2014, 243, 1646–1657. [Google Scholar] [CrossRef] [PubMed]
- Cantaut-Belarif, Y.; Sternberg, J.R.; Thouvenin, O.; Wyart, C.; Bardet, P.-L. The Reissner Fiber in the Cerebrospinal Fluid Controls Morphogenesis of the Body Axis. Curr. Biol. 2018, 28, 2479–2486.e4. [Google Scholar] [CrossRef] [PubMed]
- Troutwine, B.R.; Gontarz, P.; Konjikusic, M.J.; Minowa, R.; Monstad-Rios, A.; Sepich, D.S.; Kwon, R.Y.; Solnica-Krezel, L.; Gray, R.S. The Reissner Fiber Is Highly Dynamic In Vivo and Controls Morphogenesis of the Spine. Curr. Biol. 2020, 30, 2353–2362.e3. [Google Scholar] [CrossRef]
- Rose, C.D.; Pompili, D.; Henke, K.; Van Gennip, J.L.; Meyer-Miner, A.; Rana, R.; Gobron, S.; Harris, M.P.; Nitz, M.; Ciruna, B. SCO-Spondin Defects and Neuroinflammation Are Conserved Mechanisms Driving Spinal Deformity across Genetic Models of Idiopathic Scoliosis. Curr. Biol. 2020, 30, 2363–2373.e6. [Google Scholar] [CrossRef]
- Catanzariti, J.F.; D’hulster-Hocquet, L.; Le Berre, M.; Delaplace, C.; Boukelifa, M.; Thevenon, A. Adolescent idiopathic scoliosis (AIS), cerebrospinal fluid (CSF) flow and ciliopathy. Ann. Phys. Rehabil. Med. 2017, 60, e80–e81. [Google Scholar] [CrossRef]
- Sternberg, J.R.; Prendergast, A.E.; Brosse, L.; Cantaut-Belarif, Y.; Thouvenin, O.; Orts-Del’immagine, A.; Castillo, L.; Djenoune, L.; Kurisu, S.; McDearmid, J.R.; et al. Pkd2l1 is required for mechanoception in cerebrospinal fluid-contacting neurons and maintenance of spine curvature. Nat. Commun. 2018, 9, 3804. [Google Scholar] [CrossRef]
- Van Gennip, J.L.M.; Boswell, C.W.; Ciruna, B. Neuroinflammatory signals drive spinal curve formation in zebrafish models of idiopathic scoliosis. Sci. Adv. 2018, 4, eaav1781. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, S.; Chen, Z.; Chong, Y.L.; Xie, H.; Feng, D.; Wu, X.; Song, D.Z.; Roy, S.; Zhao, C. Cilia-driven cerebrospinal fluid flow directs expression of urotensin neuropeptides to straighten the vertebrate body axis. Nat. Genet. 2018, 50, 1666–1673. [Google Scholar] [CrossRef]
- Terhune, E.A.; Cuevas, M.T.; Monley, A.M.; Wethey, C.I.; Chen, X.; Cattell, M.V.; Bayrak, M.N.; Bland, M.R.; Sutphin, B.; Trahan, G.D.; et al. Mutations in KIF7 implicated in idiopathic scoliosis in humans and axial curvatures in zebrafish. Hum. Mutat. 2020, 42, 392–407. [Google Scholar] [CrossRef]
- He, M.; Subramanian, R.; Bangs, F.; Omelchenko, T.; Liem, K.F., Jr.; Kapoor, T.M.; Anderson, K.V. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat. Cell Biol. 2014, 16, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, D.; Dahia, C.L. Role of Sonic Hedgehog Signaling Pathway in Intervertebral Disc Formation and Maintenance. Curr. Mol. Biol. Rep. 2018, 4, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Ben, J.; Zhao, Z.; Lee, R.T.H.; Niah, W.; Ng, A.S.M.; Iyu, A.; Yu, W.; Elworthy, S.; van Eeden, F.J.M.; et al. Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo. PLoS Genet. 2013, 9, e1003955. [Google Scholar] [CrossRef] [PubMed]
- Brancati, F.; Dallapiccola, B.; Valente, E.M. Joubert Syndrome and related disorders. Orphanet J. Rare Dis. 2010, 5, 20. [Google Scholar] [CrossRef]
- Bachmann-Gagescu, R.; Dempsey, J.C.; Phelps, I.G.; O’Roak, B.J.; Knutzen, D.M.; Rue, T.C.; Ishak, G.E.; Isabella, C.R.; Gorden, N.; Adkins, J.; et al. Joubert syndrome: A model for untangling recessive disorders with extreme genetic heterogeneity. J. Med. Genet. 2015, 52, 514–522. [Google Scholar] [CrossRef]
- Elhassanien, A.F.; Alghaiaty, H.A.-A. Joubert syndrome: Clinical and radiological characteristics of nine patients. Ann. Indian Acad. Neurol. 2013, 16, 239–244. [Google Scholar] [CrossRef]
- Haeussler, M.; Schönig, K.; Eckert, H.; Eschstruth, A.; Mianné, J.; Renaud, J.-B.; Schneider-Maunoury, S.; Shkumatava, A.; Teboul, L.; Kent, J.; et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016, 17, 148. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Dane, E.T.; Herman, D.L. Haematoxylin-Phloxine-Alcian Blue-Orange G Differential Staining of Prekeratin, Keratin and Mucin. Stain. Technol. 1963, 38, 97–101. [Google Scholar] [CrossRef]
- Cohen, M.; Kicheva, A.; Ribeiro, A.; Blassberg, R.; Page, K.M.; Barnes, C.P.; Briscoe, J. Ptch1 and Gli regulate Shh signalling dynamics via multiple mechanisms. Nat. Commun. 2015, 6, 6709. [Google Scholar] [CrossRef]
- Sasai, N.; Toriyama, M.; Kondo, T. Hedgehog Signal and Genetic Disorders. Front. Genet. 2019, 10, 1103. [Google Scholar] [CrossRef]
- Van Dam, T.J.; Wheway, G.; Slaats, G.G.; Huynen, M.A.; Giles, R.H. The SYSCILIA gold standard (SCGSv1) of known ciliary components and its applications within a systems biology consortium. Cilia 2013, 2, 7. [Google Scholar] [CrossRef]
- The Genotype-Tissue Expression (GTEx) Project KIF7. Available online: https://www.gtexportal.org/home/gene/KIF7 (accessed on 21 April 2023).
- Tay, S.Y.; Ingham, P.W.; Roy, S. A homologue of the Drosophila kinesin-like protein Costal2 regulates Hedgehog signal transduction in the vertebrate embryo. Development 2005, 132, 625–634. [Google Scholar] [CrossRef]
- Liem, K.F., Jr.; He, M.; Ocbina, P.J.R.; Anderson, K.V. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 13377–13382. [Google Scholar] [CrossRef]
- Chong, Y.C.; Mann, R.K.; Zhao, C.; Kato, M.; Beachy, P.A. Bifurcating action of Smoothened in Hedgehog signaling is mediated by Dlg5. Genes Dev. 2015, 29, 262–276. [Google Scholar] [CrossRef]
- Wang, S.-H.J.; Celic, I.; Choi, S.-Y.; Riccomagno, M.; Wang, Q.; Sun, L.O.; Mitchell, S.P.; Vasioukhin, V.; Huganir, R.L.; Kolodkin, A.L. Dlg5 regulates dendritic spine formation and synaptogenesis by controlling subcellular N-cadherin localization. J. Neurosci. 2014, 34, 12745–12761. [Google Scholar] [CrossRef]
- Kundu, S.; Nandhu, M.S.; Longo, S.L.; Longo, J.A.; Rai, S.; Chin, L.S.; E Richardson, T.; Viapiano, M.S. The scaffolding protein DLG5 promotes glioblastoma growth by controlling Sonic Hedgehog signaling in tumor stem cells. Neuro-Oncol. 2022, 24, 1230–1242. [Google Scholar] [CrossRef]
- Everaert, C.; Luypaert, M.; Maag, J.L.; Cheng, Q.X.; Dinger, M.E.; Hellemans, J.; Mestdagh, P. Benchmarking of RNA-sequencing analysis workflows using whole-transcriptome RT-qPCR expression data. Sci. Rep. 2017, 7, 1559. [Google Scholar] [CrossRef]
- Chan, K.Y.; Yan, C.-C.S.; Roan, H.-Y.; Hsu, S.-C.; Tseng, T.-L.; Hsiao, C.-D.; Hsu, C.-P.; Chen, C.-H. Skin cells undergo asynthetic fission to expand body surfaces in zebrafish. Nature 2022, 605, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Kim, J.E.; Plikus, M.V. Keratinocytes cut corners on the cell cycle for the sake of skin barrier integrity. Dev. Cell 2022, 57, 1437–1438. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.H.; Asharani, P.V.; Carney, T.J. Basal Keratinocytes Contribute to All Strata of the Adult Zebrafish Epidermis. PLoS ONE 2014, 9, e84858. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Nieuwenhuis, E.; Nien, W.; Zhang, X.; Zhang, J.; Puviindran, V.; Wainwright, B.; Kim, P.C.W.; Hui, C.-C. Kif7 regulates Gli2 through Sufu-dependent and -independent functions during skin development and tumorigenesis. Development 2012, 139, 4152–4161. [Google Scholar] [CrossRef]
- Chen, S.; Su, T.; Zhang, Y.; Lee, A.; He, J.; Ge, Q.; Wang, L.; Si, J.; Zhuo, W.; Wang, L. Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7. Gut Microbes 2020, 11, 511–525. [Google Scholar] [CrossRef]
- An, Q.; Liu, T.; Wang, M.Y.; Yang, Y.J.; Zhang, Z.D.; Liu, Z.J.; Yang, B. KRT7 promotes epithelial-mesenchymal transition in ovarian cancer via the TGF-beta/Smad2/3 signaling pathway. Oncol. Rep. 2021, 45, 481–492. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Z.; Guan, T.; Zhou, Y.; Ge, L.; Zhang, H.; Wu, Y.; Jiang, G.-M.; He, W.; Li, J.; et al. N6-Methyladenosine Regulates mRNA Stability and Translation Efficiency of KRT7 to Promote Breast Cancer Lung Metastasis. Cancer Res. 2021, 81, 2847–2860. [Google Scholar] [CrossRef]
- O’Neill, A.; Williams, M.W.; Resneck, W.G.; Milner, D.J.; Capetanaki, Y.; Bloch, R.J. Sarcolemmal Organization in Skeletal Muscle Lacking Desmin: Evidence for Cytokeratins Associated with the Membrane Skeleton at Costameres. Mol. Biol. Cell 2002, 13, 2347–2359. [Google Scholar] [CrossRef]
- Stone, M.R.; O’Neill, A.; Catino, D.; Bloch, R.J. Specific Interaction of the Actin-binding Domain of Dystrophin with Intermediate Filaments Containing Keratin 19. Mol. Biol. Cell 2005, 16, 4280–4293. [Google Scholar] [CrossRef]
- Richardson, S.M.; Ludwinski, F.E.; Gnanalingham, K.K.; Atkinson, R.A.; Freemont, A.J.; Hoyland, J.A. Notochordal and nucleus pulposus marker expression is maintained by sub-populations of adult human nucleus pulposus cells through aging and degeneration. Sci. Rep. 2017, 7, 1501. [Google Scholar] [CrossRef]
- Mohanty, S.; Pinelli, R.; Dahia, C.L. Characterization of Krt19 (CreERT) allele for targeting the nucleus pulposus cells in the postnatal mouse intervertebral disc. J. Cell. Physiol. 2020, 235, 128–140. [Google Scholar] [CrossRef]
- Kague, E.; Turci, F.; Newman, E.; Yang, Y.; Brown, K.R.; Aglan, M.S.; Otaify, G.A.; Temtamy, S.A.; Ruiz-Perez, V.L.; Cross, S.; et al. 3D assessment of intervertebral disc degeneration in zebrafish identifies changes in bone density that prime disc disease. Bone Res. 2021, 9, 39. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference sequence (RefSeq) database at NCBI: Current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef]


| Initial | Denature | Anneal | Extend | Final | |
|---|---|---|---|---|---|
| Temp | 95 | 95 | 58 | 72 | 72 |
| Time | 3 min | 30 s | 30 s | 30 s | 20 min |
| 34× | |||||
| Fold Change | Log2 Fold Change | p Value | p Adjusted | Gene Name | Predicted Human Ortholog |
|---|---|---|---|---|---|
| 0.0494 | −4.34 | 8.55 × 10−113 | 5.20 × 10−109 | ces2 | CES2 |
| 0.0093 | −6.75 | 1.12 × 10−81 | 5.44 × 10−78 | CABZ01032488.1 | Unknown |
| 0.0317 | −4.98 | 8.66 × 10−58 | 2.34 × 10−54 | ttc38 | TTC38 |
| 0.0068 | −7.19 | 8.69 × 10−39 | 1.41 × 10−35 | ces3 | CES3 |
| 0.0583 | −4.10 | 1.23 × 10−38 | 1.88 × 10−35 | si:dkey-167k11.5 | CCDC134 |
| 0.1780 | −2.49 | 3.19 × 10−30 | 3.89 × 10−27 | pip4p1a | PIP4P1 |
| 0.3392 | −1.56 | 7.42 × 10−28 | 7.53 × 10−25 | ctsd | CTSD |
| 0.2588 | −1.95 | 2.89 × 10−24 | 2.43 × 10−21 | si:ch211-195h23.3 | CARD8 |
| 0.0003 | −11.76 | 1.63 × 10−22 | 1.24 × 10−19 | si:dkeyp-73d8.9 | Unknown |
| 0.2253 | −2.15 | 4.62 × 10−22 | 3.31 × 10−19 | lig1 | LIG1 |
| 174.85 | +7.45 | 1.14 × 10−293 | 2.79 × 10−289 | krt4 | KRT8 |
| 107.63 | +6.75 | 1.13 × 10−163 | 1.37 × 10−159 | zgc:158846 (krtt2c6) | KRT7,8,9 |
| 131.60 | +7.04 | 1.00 × 10−150 | 8.13 × 10−147 | zgc:77517 (krtt1c6) | KRT18 |
| 31.34 | +4.97 | 9.44 × 10−76 | 3.83 × 10−72 | os9 | OS9 |
| 23.59 | +4.56 | 2.27 × 10−68 | 7.90 × 10−65 | si:ch73-308m11.1 | GIMAP8 |
| 51.27 | +5.68 | 9.23 × 10−66 | 2.81 × 10−62 | si:ch211-11p18.6 | Unknown |
| 19.29 | +4.27 | 3.63 × 10−54 | 8.84 × 10−51 | CR774195.1 | Unknown |
| 5.13 | +2.36 | 2.49 × 10−44 | 5.52 × 10−41 | tmem97 | TMEM97 |
| 280.14 | +8.13 | 1.12 × 10−41 | 2.27 × 10−38 | myl6 | MYL6 |
| 15.14 | +3.92 | 4.14 × 10−41 | 7.77 × 10−38 | BX322618.1 | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas, M.; Terhune, E.; Wethey, C.; James, M.; Netsanet, R.; Grofova, D.; Monley, A.; Hadley Miller, N. Cytoskeletal Keratins Are Overexpressed in a Zebrafish Model of Idiopathic Scoliosis. Genes 2023, 14, 1058. https://doi.org/10.3390/genes14051058
Cuevas M, Terhune E, Wethey C, James M, Netsanet R, Grofova D, Monley A, Hadley Miller N. Cytoskeletal Keratins Are Overexpressed in a Zebrafish Model of Idiopathic Scoliosis. Genes. 2023; 14(5):1058. https://doi.org/10.3390/genes14051058
Chicago/Turabian StyleCuevas, Melissa, Elizabeth Terhune, Cambria Wethey, MkpoutoAbasi James, Rahwa Netsanet, Denisa Grofova, Anna Monley, and Nancy Hadley Miller. 2023. "Cytoskeletal Keratins Are Overexpressed in a Zebrafish Model of Idiopathic Scoliosis" Genes 14, no. 5: 1058. https://doi.org/10.3390/genes14051058
APA StyleCuevas, M., Terhune, E., Wethey, C., James, M., Netsanet, R., Grofova, D., Monley, A., & Hadley Miller, N. (2023). Cytoskeletal Keratins Are Overexpressed in a Zebrafish Model of Idiopathic Scoliosis. Genes, 14(5), 1058. https://doi.org/10.3390/genes14051058






