phox2ba: The Potential Genetic Link behind the Overlap in the Symptomatology between CHARGE and Central Congenital Hypoventilation Syndromes
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry and Genetic Lines
2.2. Genotyping Mutant Lines
2.3. In Situ Hybridization and Correlation with Genotype
2.4. Morpholino Injection
2.5. Cloning and Expression of phox2ba-sfGFP Fusion
2.6. Selection of Anesthetic Concentration
2.7. Opercular Beat Analysis
2.8. Heart Rate Analysis
2.9. Statistical Analysis
3. Results
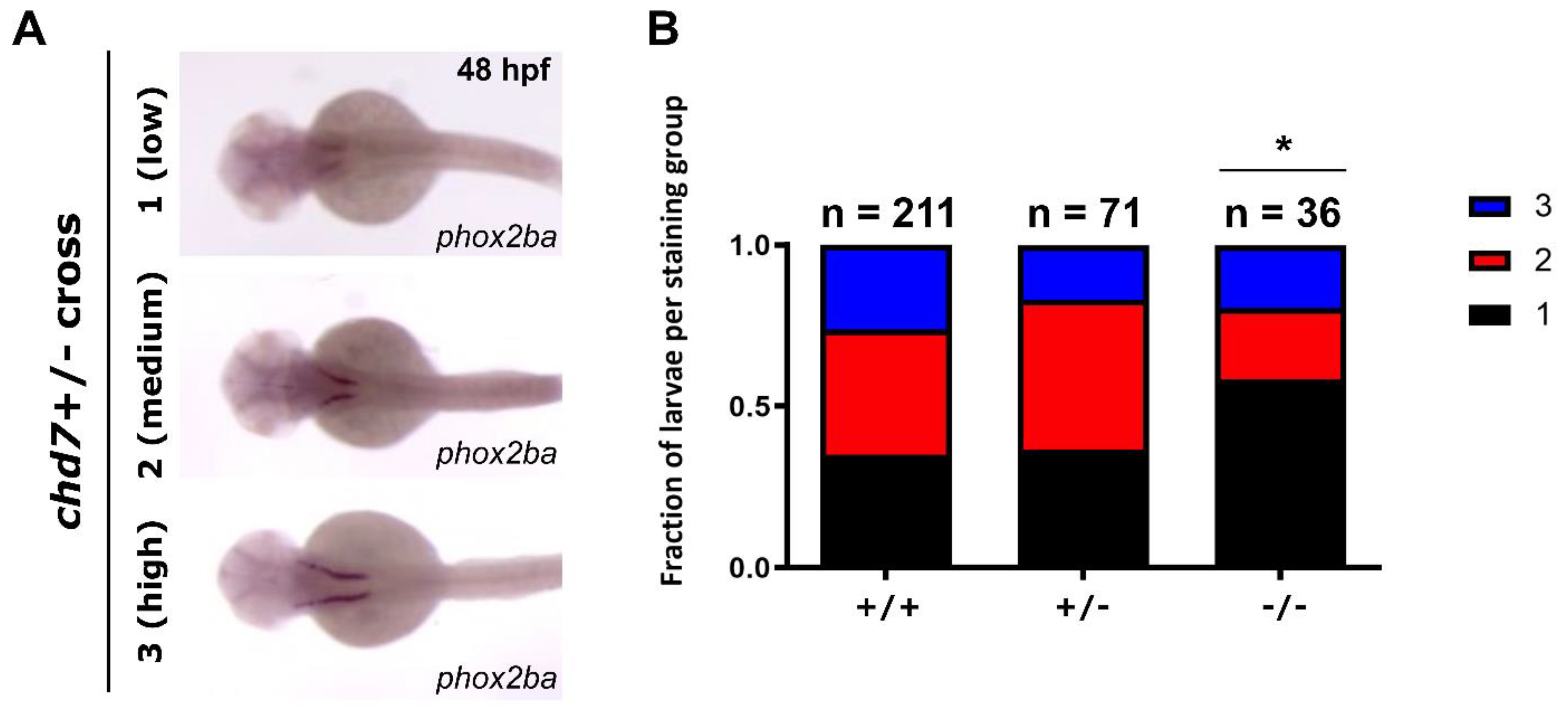
3.1. phox2ba Expression Is Reduced in chd7 Mutants
3.2. Morpholino-Mediated Knockdown of phox2ba Mimics the Decreased Heart Rates Observed in chd7−/− Larvae
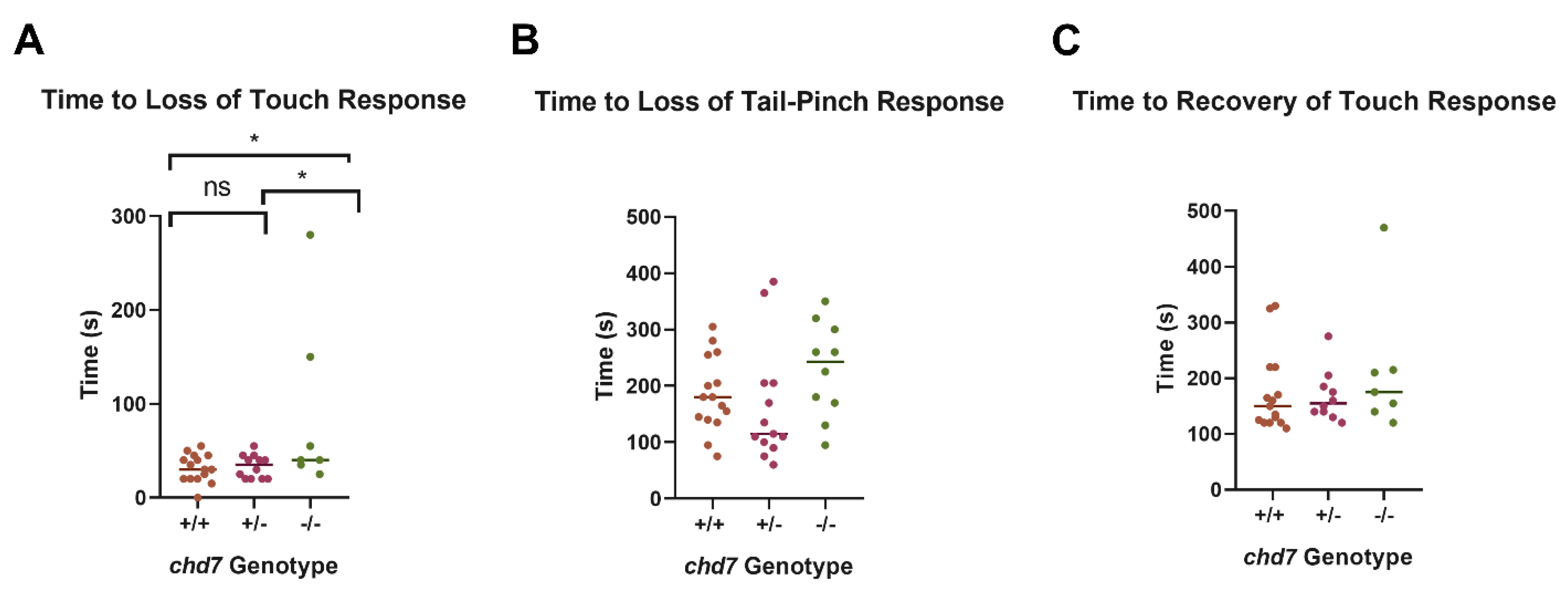
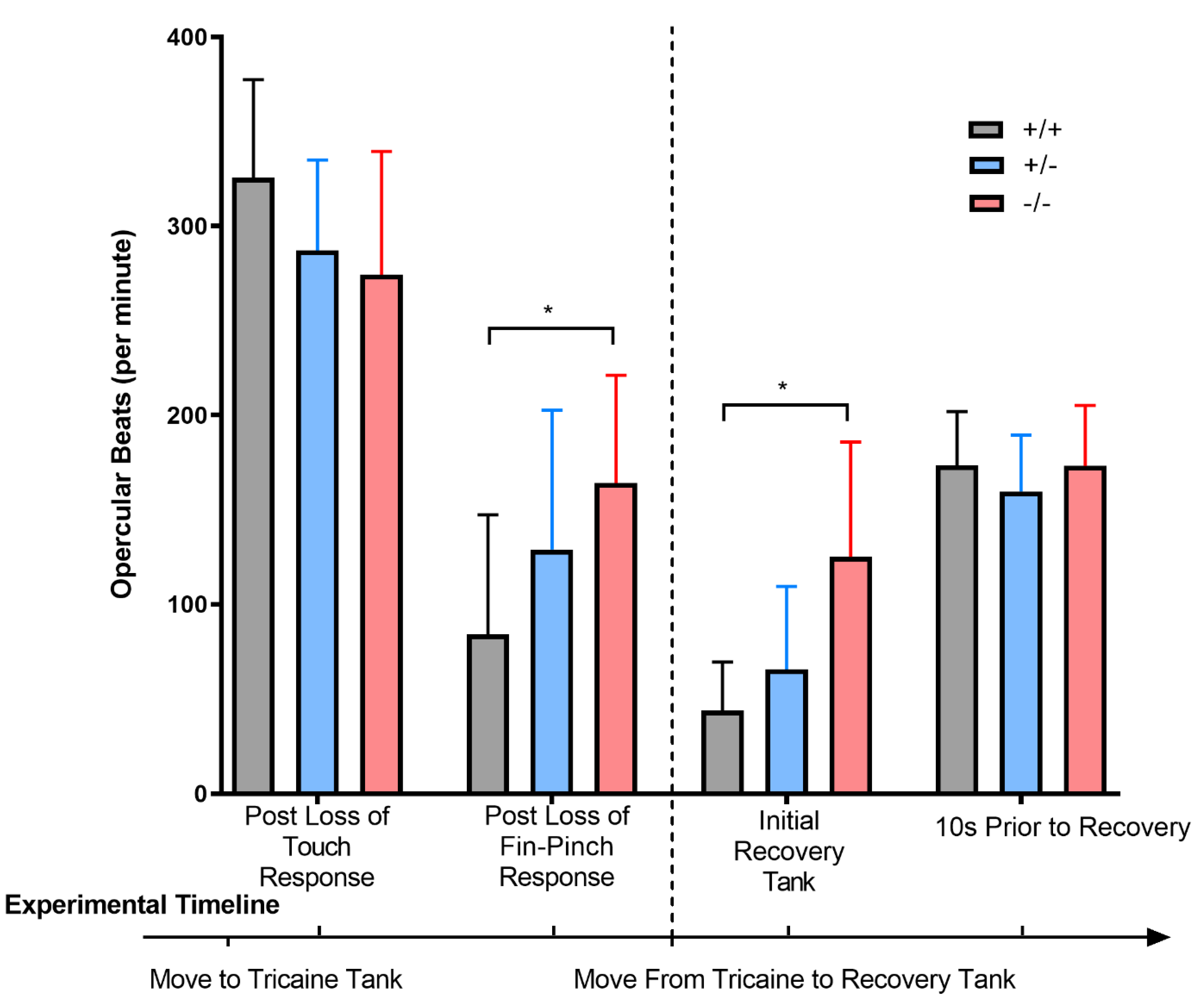
3.3. Chd7 Mutant Adults Exhibit Differential Response to Anesthesia and Opercular Rates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pagon, R.A.; Graham, J.M., Jr.; Zonana, J.; Yong, S.-L. Coloboma, Congenital Heart Disease, and Choanal Atresia with Multiple Anomalies: CHARGE Association. J Pediatr. 1981, 99, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.; Bergman, J.E.H.; Swertz, M.A.; Tranebjaerg, L.; Lodahl, M.; Schoots, J.; Hofstra, R.M.W.; van Ravenswaaij-Arts, C.M.A.; Hoefsloot, L.H. Mutation Update on the CHD7 Gene Involved in CHARGE Syndrome. Hum. Mutat. 2012, 33, 1149–1160. [Google Scholar] [CrossRef]
- Vissers, L.E.L.M.; van Ravenswaaij, C.M.A.; Admiraal, R.; Hurst, J.A.; de Vries, B.B.A.; Janssen, I.M.; van der Vliet, W.A.; Huys, E.H.L.P.G.; de Jong, P.J.; Hamel, B.C.J.; et al. Mutations in a New Member of the Chromodomain Gene Family Cause CHARGE Syndrome. Nat. Genet. 2004, 36, 955–957. [Google Scholar] [CrossRef] [PubMed]
- Blake, K.D.; Prasad, C. CHARGE Syndrome. Orphanet J. Rare Dis. 2006, 1, 34. [Google Scholar] [CrossRef]
- Nagashimada, M.; Ohta, H.; Li, C.; Nakao, K.; Uesaka, T.; Brunet, J.F.; Amiel, J.; Trochet, D.; Wakayama, T.; Enomoto, H. Autonomic Neurocristopathy-Associated Mutations in PHOX2B Dysregulate Sox10 Expression. J. Clin. Investig. 2012, 122, 3145–3158. [Google Scholar] [CrossRef] [PubMed]
- Blake, K.; MacCuspie, J.; Hartshorne, T.S.; Roy, M.; Davenport, S.L.H.; Corsten, G. Postoperative Airway Events of Individuals with CHARGE Syndrome. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 219–226. [Google Scholar] [CrossRef]
- Trider, C.L.; Blake, K. Anaesthesia Issues in CHARGE Syndrome—What Are the Risks? 2013. Available online: https://www.sense.org.uk/information-and-advice/conditions/charge-syndrome/ (accessed on 11 October 2022).
- Bishara, J.; Keens, T.G.; Perez, I.A. The Genetics of Congenital Central Hypoventilation Syndrome: Clinical Implications. Appl. Clin. Genet. 2018, 11, 135–144. [Google Scholar] [CrossRef]
- Weese-Mayer, D.E.; Berry-Kravis, E.M.; Ceccherini, I.; Keens, T.G.; Loghmanee, D.A.; Trang, H. An Official ATS Clinical Policy Statement: Congenital Central Hypoventilation Syndrome: Genetic Basis, Diagnosis, and Management. Am. J. Respir. Crit. Care Med. 2010, 181, 626–644. [Google Scholar] [CrossRef]
- Chang, G.Y.; Salazar, T.; Karnwal, A.; Kun, S.S.; Ellashek, J.; Shin, C.E.; McComb, J.G.; Keens, T.G.; Perez, I.A. Perioperative Outcomes and the Effects of Anesthesia in Congenital Central Hypoventilation Patients. Sleep Breath. 2022. [Google Scholar] [CrossRef]
- Santoriello, C.; Zon, L.I. Science in Medicine Hooked! Modeling Human Disease in Zebrafish. Sci. Med. 2012, 122, 2337–2343. [Google Scholar]
- Lele, Z.; Krone, P.H. The Zebrafish as a Model System in Developmental, Toxicological and Transgenic Research. Biotechnol. Adv. 1996, 14, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Lieschke, G.J.; Currie, P.D. Animal Models of Human Disease: Zebrafish Swim into View. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, D.; Bronner-Fraser, M. Gene-Regulatory Interactions in Neural Crest Evolution and Development. Dev. Cell 2004, 7, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Theveneau, E.; Mayor, R. Neural Crest Delamination and Migration: From Epithelium-to-Mesenchyme Transition to Collective Cell Migration. Dev. Biol. 2012, 366, 34–54. [Google Scholar] [CrossRef]
- Asad, Z.; Pandey, A.; Babu, A.; Sun, Y.; Shevade, K.; Kapoor, S.; Ullah, I.; Ranjan, S.; Scaria, V.; Bajpai, R.; et al. Rescue of Neural Crest-Derived Phenotypes in a Zebrafish CHARGE Model by Sox10 Downregulation. Hum. Mol. Genet. 2016, 25, 3539–3554. [Google Scholar] [CrossRef]
- Patten, S.A.; Jacobs-McDaniels, N.L.; Zaouter, C.; Drapeau, P.; Albertson, R.C.; Moldovan, F. Role of Chd7 in Zebrafish: A Model for CHARGE Syndrome. PLoS ONE 2012, 7, e31650. [Google Scholar] [CrossRef]
- Prykhozhij, S.V.; Steele, S.L.; Razaghi, B.; Berman, J.N. A Rapid and Effective Method for Screening, Sequencing and Reporter Verification of Engineered Frameshift Mutations in Zebrafish. Dis. Model. Mech. 2017, 10, 811–822. [Google Scholar] [CrossRef]
- Cloney, K.; Steele, S.L.; Stoyek, M.R.; Croll, R.P.; Smith, F.M.; Prykhozhij, S.V.; Brown, M.M.; Midgen, C.; Blake, K.; Berman, J.N. Etiology and Functional Validation of Gastrointestinal Motility Dysfunction in a Zebrafish Model of CHARGE Syndrome. FEBS J. 2018, 285, 2125–2140. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book; University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- Chen, J.I.; Zhang, X.I.; Wang, T.I.; Li, Z.H.; Guan, G.U.; Hong, Y.U. Efficient Detection, Quantification and Enrichment of Subtle Allelic Alterations. DNA Res. 2012, 19, 423–433. [Google Scholar] [CrossRef]
- Bennett, C.M.; Kanki, J.P.; Rhodes, J.; Liu, T.X.; Paw, B.H.; Kieran, M.W.; Langenau, D.M.; Delahaye-brown, A.; Zon, L.I.; Fleming, M.D.; et al. Myelopoiesis in the Zebrafish, Danio Rerio. Blood 2001, 98, 643–651. [Google Scholar] [CrossRef]
- Pei, D.; Luther, W.; Wang, W.; Paw, B.H.; Stewart, R.A.; George, R.E. Distinct Neuroblastoma-Associated Alterations of PHOX2B Impair Sympathetic Neuronal Differentiation in Zebrafish Models. PLoS Genet 2013, 9, 1003533. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; Leblanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent Adult Zebrafish as a Tool for In Vivo Transplantation Analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Collymore, C.; Tolwani, A.; Lieggi, C.; Rasmussen, S. Efficacy and Safety of 5 Anesthetics in Adult Zebrafish (Danio Rerio). J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 198–203. [Google Scholar] [PubMed]
- Taylor, J.S.; Braasch, I.; Frickey, T.; Meyer, A.; Van de Peer, Y. Genome Duplication, a Trait Shared by 22,000 Species of Ray-Finned Fish. Genome Res 2003, 13, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Gebhardt, M.; Stewart, A.M.; Cachat, J.M.; Brimmer, M.; Chawla, J.S.; Craddock, C.; Kyzar, E.J.; Roth, A.; Landsman, S.; et al. Towards a Comprehensive Catalog of Zebrafish Behavior 1.0 and Beyond. Zebrafish 2013, 10, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Bergman, J.E.H.; Janssen, N.; Hoefsloot, L.H.; Jongmans, M.C.J.; Hofstra, R.M.W.; van Ravenswaaij-Arts, C.M.A. CHD7 Mutations and CHARGE Syndrome: The Clinical Implications of an Expanding Phenotype. J. Med. Genet. 2011, 48, 334–342. [Google Scholar] [CrossRef]
- Weese-Mayer, D.; Shannon, D.; Keens, T.; Silvestri, J. Idiopathic Congenital Central Hypoventilation Syndrome: Diagnosis and Management. Am. J. Respir. Crit. Care Med. 1999, 160, 368–373. [Google Scholar]
- Dobbelsteyn, C.; Peacocke, S.D.; Blake, K.; Crist, W.; Rashid, M. Feeding Difficulties in Children with CHARGE Syndrome: Prevalence, Risk Factors, and Prognosis. Dysphagia 2008, 23, 127–135. [Google Scholar] [CrossRef]
- Riedijk, M.A.; König-Jung, A.M.; Van Woensel, J.B.M.; Kruisinga, F.H. A Neonate with Marked Prolonged Mixed Apneas and CHARGE Syndrome: A Case Report. J. Med. Case Rep. 2018, 12, 10–13. [Google Scholar] [CrossRef]
- Kasi, A.S.; Li, H.; Harford, K.L.; Lam, H.V.; Mao, C.; Landry, A.M.; Mitchell, S.G.; Clifton, M.S.; Leu, R.M. Congenital Central Hypoventilation Syndrome: Optimizing Care with a Multidisciplinary Approach. J. Multidiscip. Healthc. 2022, 15, 455–469. [Google Scholar] [CrossRef]
- Carter, K.M.; Woodley, C.M.; Brown, R.S. A Review of Tricaine Methanesulfonate for Anesthesia of Fish. Rev. Fish. Biol. Fish. 2011, 21, 51–59. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacLean, J.E.; Wertman, J.N.; Prykhozhij, S.V.; Chedrawe, E.; Langley, S.; Steele, S.L.; Ban, K.; Blake, K.; Berman, J.N. phox2ba: The Potential Genetic Link behind the Overlap in the Symptomatology between CHARGE and Central Congenital Hypoventilation Syndromes. Genes 2023, 14, 1086. https://doi.org/10.3390/genes14051086
MacLean JE, Wertman JN, Prykhozhij SV, Chedrawe E, Langley S, Steele SL, Ban K, Blake K, Berman JN. phox2ba: The Potential Genetic Link behind the Overlap in the Symptomatology between CHARGE and Central Congenital Hypoventilation Syndromes. Genes. 2023; 14(5):1086. https://doi.org/10.3390/genes14051086
Chicago/Turabian StyleMacLean, Jessica E., Jaime N. Wertman, Sergey V. Prykhozhij, Emily Chedrawe, Stewart Langley, Shelby L. Steele, Kevin Ban, Kim Blake, and Jason N. Berman. 2023. "phox2ba: The Potential Genetic Link behind the Overlap in the Symptomatology between CHARGE and Central Congenital Hypoventilation Syndromes" Genes 14, no. 5: 1086. https://doi.org/10.3390/genes14051086
APA StyleMacLean, J. E., Wertman, J. N., Prykhozhij, S. V., Chedrawe, E., Langley, S., Steele, S. L., Ban, K., Blake, K., & Berman, J. N. (2023). phox2ba: The Potential Genetic Link behind the Overlap in the Symptomatology between CHARGE and Central Congenital Hypoventilation Syndromes. Genes, 14(5), 1086. https://doi.org/10.3390/genes14051086





