Crosstalk between miRNAs and DNA Methylation in Cancer
Abstract
:1. Introduction
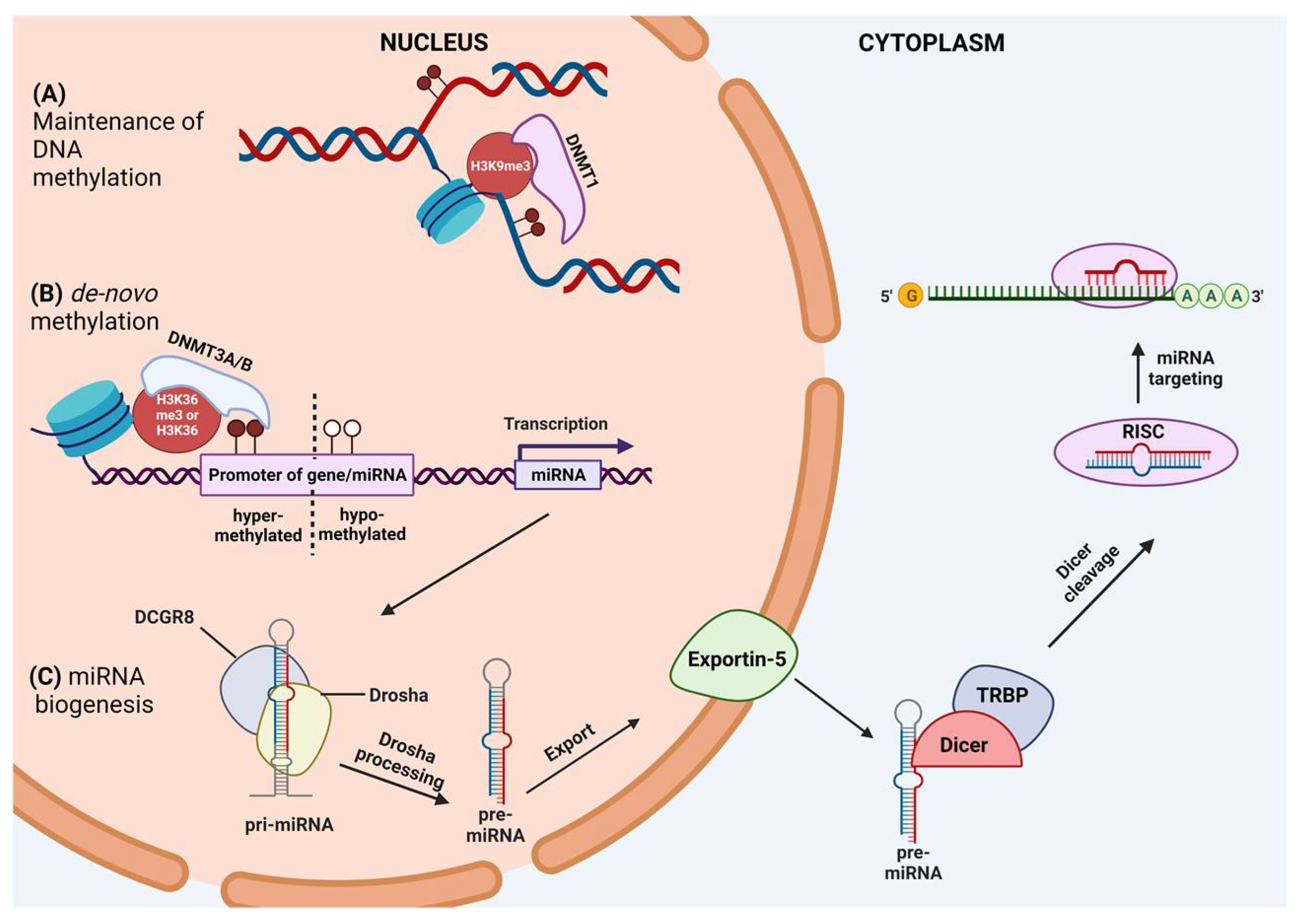
2. Mechanisms of DNA Methylation
3. miRNA: Biogenesis and Function
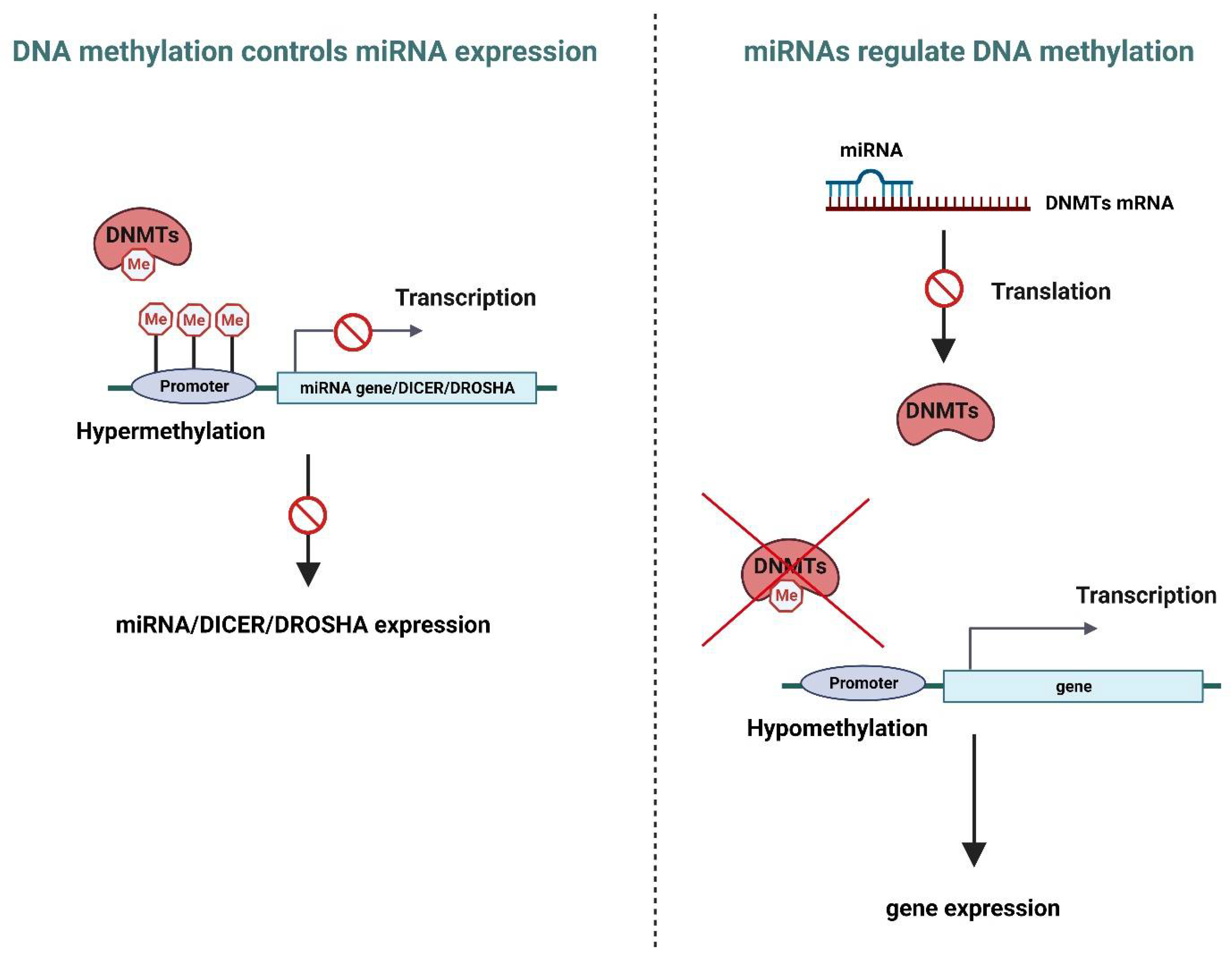
4. Crosstalk between miRNA and DNA Methylation in Human Cancer
- Epigenetic control of miRNA expression (Figure 2, left)
- miRNA as regulators of DNA methylation (Figure 2, right)
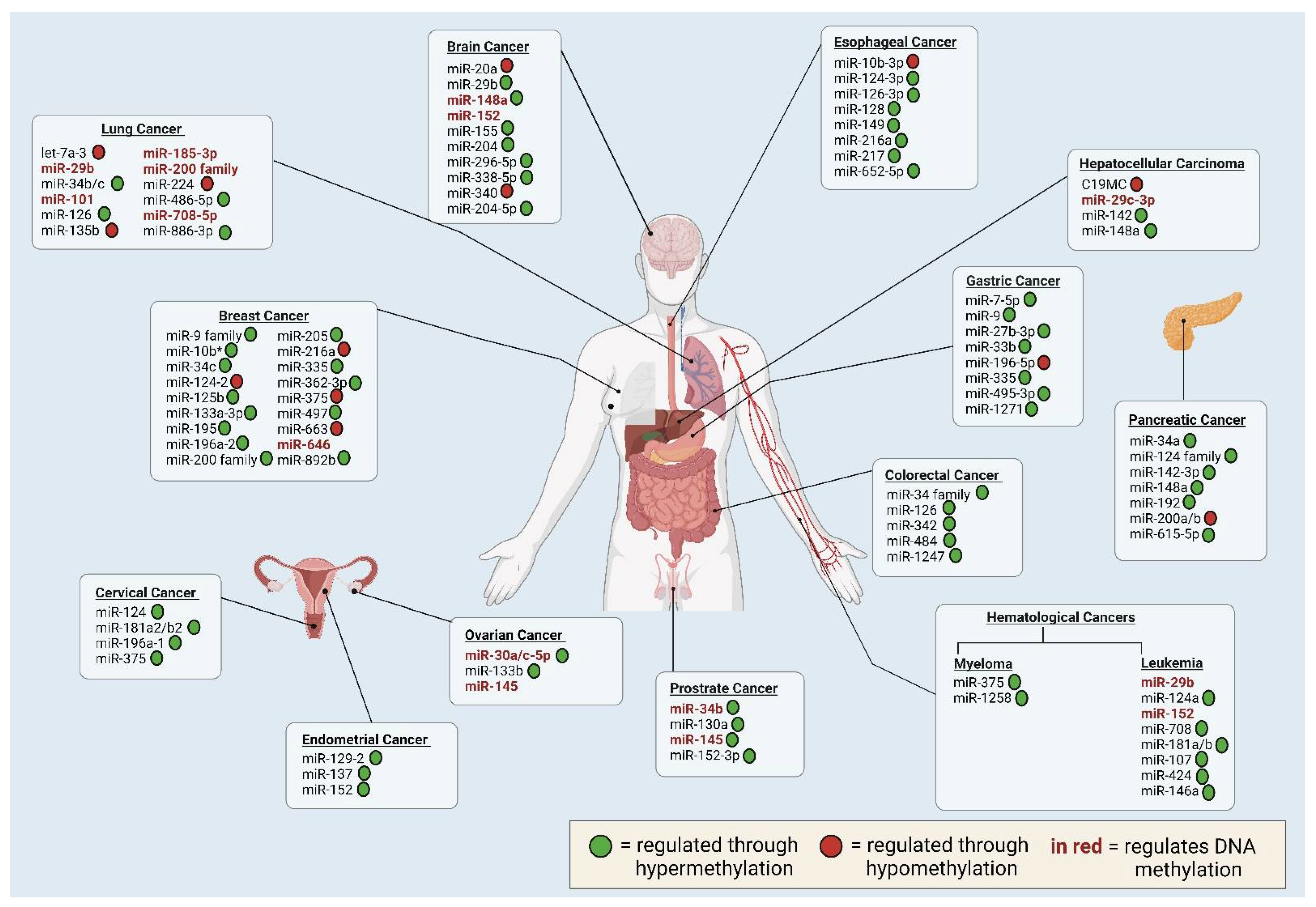
4.1. Lung Cancer
4.2. Breast Cancer
4.3. Brain Cancer
4.4. Hematologic Cancers
4.5. Gastrointestinal Cancers
4.5.1. Esophageal Cancers
4.5.2. Gastric Cancer
4.5.3. Hepatocellular Carcinoma
4.5.4. Pancreatic Cancer
4.5.5. Colorectal Cancers
4.6. Gynecological Cancers
4.6.1. Cervical cancer
4.6.2. Ovarian Cancer
4.6.3. Endometrial Cancer
4.7. Prostate Cancer
5. The Use of Epigenetics as Biomarkers
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Waddington, C.H. The epigenotype. 1942. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, B. Epigenetics: The science of change. Environ. Health Perspect. 2006, 114, A160–A167. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Grant, P.A. The role of DNA methylation and histone modifications in transcriptional regulation in humans. Subcell. Biochem. 2013, 61, 289–317. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kelly, T.K.; Jones, P.A. Epigenetics in cancer. Carcinogenesis 2010, 31, 27–36. [Google Scholar] [CrossRef]
- Sandoval, J.; Esteller, M. Cancer epigenomics: Beyond genomics. Curr. Opin. Genet. Dev. 2012, 22, 50–55. [Google Scholar] [CrossRef]
- Wang, S.; Wu, W.; Claret, F.X. Mutual regulation of microRNAs and DNA methylation in human cancers. Epigenetics 2017, 12, 187–197. [Google Scholar] [CrossRef]
- Nishiyama, A.; Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021, 37, 1012–1027. [Google Scholar] [CrossRef]
- Spada, F.; Haemmer, A.; Kuch, D.; Rothbauer, U.; Schermelleh, L.; Kremmer, E.; Carell, T.; Langst, G.; Leonhardt, H. DNMT1 but not its interaction with the replication machinery is required for maintenance of DNA methylation in human cells. J. Cell Biol. 2007, 176, 565–571. [Google Scholar] [CrossRef]
- Easwaran, H.P.; Schermelleh, L.; Leonhardt, H.; Cardoso, M.C. Replication-independent chromatin loading of Dnmt1 during G2 and M phases. EMBO Rep. 2004, 5, 1181–1186. [Google Scholar] [CrossRef]
- Bostick, M.; Kim, J.K.; Esteve, P.O.; Clark, A.; Pradhan, S.; Jacobsen, S.E. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science 2007, 317, 1760–1764. [Google Scholar] [CrossRef]
- Nady, N.; Lemak, A.; Walker, J.R.; Avvakumov, G.V.; Kareta, M.S.; Achour, M.; Xue, S.; Duan, S.; Allali-Hassani, A.; Zuo, X.; et al. Recognition of multivalent histone states associated with heterochromatin by UHRF1 protein. J. Biol. Chem. 2011, 286, 24300–24311. [Google Scholar] [CrossRef]
- Nishiyama, A.; Yamaguchi, L.; Sharif, J.; Johmura, Y.; Kawamura, T.; Nakanishi, K.; Shimamura, S.; Arita, K.; Kodama, T.; Ishikawa, F.; et al. Uhrf1-dependent H3K23 ubiquitylation couples maintenance DNA methylation and replication. Nature 2013, 502, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Xie, S.; Li, E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat. Genet. 1998, 19, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Piunti, A.; Shilatifard, A. Epigenetic balance of gene expression by Polycomb and COMPASS families. Science 2016, 352, aad9780. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Li, J.; Ding, Z.; Xiao, J.; Yin, X.; He, S.; Shi, P.; Dong, L.; Li, G.; et al. Structural insight into autoinhibition and histone H3-induced activation of DNMT3A. Nature 2015, 517, 640–644. [Google Scholar] [CrossRef]
- Dhayalan, A.; Rajavelu, A.; Rathert, P.; Tamas, R.; Jurkowska, R.Z.; Ragozin, S.; Jeltsch, A. The Dnmt3a PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. J. Biol. Chem. 2010, 285, 26114–26120. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y. Reversing DNA methylation: Mechanisms, genomics, and biological functions. Cell 2014, 156, 45–68. [Google Scholar] [CrossRef]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Maiti, A.; Drohat, A.C. Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: Potential implications for active demethylation of CpG sites. J. Biol. Chem. 2011, 286, 35334–35338. [Google Scholar] [CrossRef]
- Messerschmidt, D.M.; Knowles, B.B.; Solter, D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 2014, 28, 812–828. [Google Scholar] [CrossRef]
- Altun, G.; Loring, J.F.; Laurent, L.C. DNA methylation in embryonic stem cells. J. Cell. Biochem. 2010, 109, 1–6. [Google Scholar] [CrossRef]
- Iwamoto, K.; Bundo, M.; Ueda, J.; Oldham, M.C.; Ukai, W.; Hashimoto, E.; Saito, T.; Geschwind, D.H.; Kato, T. Neurons show distinctive DNA methylation profile and higher interindividual variations compared with non-neurons. Genome Res. 2011, 21, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Maatouk, D.M.; Kellam, L.D.; Mann, M.R.; Lei, H.; Li, E.; Bartolomei, M.S.; Resnick, J.L. DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development 2006, 133, 3411–3418. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Fu, H.; Wu, Y.; Zheng, X. Function of lncRNAs and approaches to lncRNA-protein interactions. Sci. China Life Sci. 2013, 56, 876–885. [Google Scholar] [CrossRef]
- Kwon, S.C.; Nguyen, T.A.; Choi, Y.G.; Jo, M.H.; Hohng, S.; Kim, V.N.; Woo, J.S. Structure of Human DROSHA. Cell 2016, 164, 81–90. [Google Scholar] [CrossRef]
- Herbert, K.M.; Sarkar, S.K.; Mills, M.; Delgado De la Herran, H.C.; Neuman, K.C.; Steitz, J.A. A heterotrimer model of the complete Microprocessor complex revealed by single-molecule subunit counting. RNA 2016, 22, 175–183. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Jo, M.H.; Choi, Y.G.; Park, J.; Kwon, S.C.; Hohng, S.; Kim, V.N.; Woo, J.S. Functional Anatomy of the Human Microprocessor. Cell 2015, 161, 1374–1387. [Google Scholar] [CrossRef]
- Ballarino, M.; Pagano, F.; Girardi, E.; Morlando, M.; Cacchiarelli, D.; Marchioni, M.; Proudfoot, N.J.; Bozzoni, I. Coupled RNA processing and transcription of intergenic primary microRNAs. Mol. Cell. Biol. 2009, 29, 5632–5638. [Google Scholar] [CrossRef]
- Lund, E.; Guttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear export of microRNA precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Leitao, A.L.; Enguita, F.J. A Structural View of miRNA Biogenesis and Function. Noncoding RNA 2022, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Huntzinger, E.; Izaurralde, E. Gene silencing by microRNAs: Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 2011, 12, 99–110. [Google Scholar] [CrossRef]
- Helwak, A.; Kudla, G.; Dudnakova, T.; Tollervey, D. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 2013, 153, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Hafner, M.; Landthaler, M.; Burger, L.; Khorshid, M.; Hausser, J.; Berninger, P.; Rothballer, A.; Ascano, M., Jr.; Jungkamp, A.C.; Munschauer, M.; et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 2010, 141, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B.P.; Shih, I.H.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of mammalian microRNA targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef]
- Acunzo, M.; Romano, G.; Nigita, G.; Veneziano, D.; Fattore, L.; Lagana, A.; Zanesi, N.; Fadda, P.; Fassan, M.; Rizzotto, L.; et al. Selective targeting of point-mutated KRAS through artificial microRNAs. Proc. Natl. Acad. Sci. USA 2017, 114, E4203–E4212. [Google Scholar] [CrossRef] [PubMed]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333. [Google Scholar] [CrossRef]
- Behm-Ansmant, I.; Rehwinkel, J.; Doerks, T.; Stark, A.; Bork, P.; Izaurralde, E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 2006, 20, 1885–1898. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.E.; Truffault, V.; Boland, A.; Huntzinger, E.; Chang, C.T.; Haas, G.; Weichenrieder, O.; Coles, M.; Izaurralde, E. A direct interaction between DCP1 and XRN1 couples mRNA decapping to 5′ exonucleolytic degradation. Nat. Struct. Mol. Biol. 2012, 19, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar] [PubMed]
- Romano, G.; Veneziano, D.; Acunzo, M.; Croce, C.M. Small non-coding RNA and cancer. Carcinogenesis 2017, 38, 485–491. [Google Scholar] [CrossRef]
- Hayes, J.; Peruzzi, P.P.; Lawler, S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol. Med. 2014, 20, 460–469. [Google Scholar] [CrossRef]
- Li, S.; Chowdhury, R.; Liu, F.; Chou, A.P.; Li, T.; Mody, R.R.; Lou, J.J.; Chen, W.; Reiss, J.; Soto, H.; et al. Tumor-suppressive miR148a is silenced by CpG island hypermethylation in IDH1-mutant gliomas. Clin. Cancer Res. 2014, 20, 5808–5822. [Google Scholar] [CrossRef]
- Glaich, O.; Parikh, S.; Bell, R.E.; Mekahel, K.; Donyo, M.; Leader, Y.; Shayevitch, R.; Sheinboim, D.; Yannai, S.; Hollander, D.; et al. DNA methylation directs microRNA biogenesis in mammalian cells. Nat. Commun. 2019, 10, 5657. [Google Scholar] [CrossRef]
- Morales, S.; Monzo, M.; Navarro, A. Epigenetic regulation mechanisms of microRNA expression. Biomol. Concepts 2017, 8, 203–212. [Google Scholar] [CrossRef]
- Heydarzadeh, S.; Ranjbar, M.; Karimi, F.; Seif, F.; Alivand, M.R. Overview of host miRNA properties and their association with epigenetics, long non-coding RNAs, and Xeno-infectious factors. Cell Biosci. 2021, 11, 43. [Google Scholar] [CrossRef]
- Fabbri, M.; Garzon, R.; Cimmino, A.; Liu, Z.; Zanesi, N.; Callegari, E.; Liu, S.; Alder, H.; Costinean, S.; Fernandez-Cymering, C.; et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc. Natl. Acad. Sci. USA 2007, 104, 15805–15810. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.L.; Lehmann, U. DNA methylation, microRNAs, and their crosstalk as potential biomarkers in hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 7894–7913. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Maruyama, R.; Yamamoto, E.; Kai, M. DNA methylation and microRNA dysregulation in cancer. Mol. Oncol. 2012, 6, 567–578. [Google Scholar] [CrossRef]
- Karimzadeh, M.R.; Pourdavoud, P.; Ehtesham, N.; Qadbeigi, M.; Asl, M.M.; Alani, B.; Mosallaei, M.; Pakzad, B. Regulation of DNA methylation machinery by epi-miRNAs in human cancer: Emerging new targets in cancer therapy. Cancer Gene. 2021, 28, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Xia, L.; Cai, Z.; Liang, L.; Chen, Y.; Meng, J.; Wang, Z. Interaction Between microRNA and DNA Methylation in Atherosclerosis. DNA Cell Biol. 2021, 40, 101–115. [Google Scholar] [CrossRef]
- Van den Hove, D.L.; Kompotis, K.; Lardenoije, R.; Kenis, G.; Mill, J.; Steinbusch, H.W.; Lesch, K.P.; Fitzsimons, C.P.; De Strooper, B.; Rutten, B.P. Epigenetically regulated microRNAs in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Sankrityayan, H.; Kulkarni, Y.A.; Gaikwad, A.B. Diabetic nephropathy: The regulatory interplay between epigenetics and microRNAs. Pharm. Res. 2019, 141, 574–585. [Google Scholar] [CrossRef]
- Colpaert, R.M.W.; Calore, M. Epigenetics and microRNAs in cardiovascular diseases. Genomics 2021, 113, 540–551. [Google Scholar] [CrossRef]
- Wang, Y.S.; Chou, W.W.; Chen, K.C.; Cheng, H.Y.; Lin, R.T.; Juo, S.H. MicroRNA-152 mediates DNMT1-regulated DNA methylation in the estrogen receptor α gene. PLoS ONE 2012, 7, e30635. [Google Scholar] [CrossRef]
- Saito, Y.; Saito, H.; Liang, G.; Friedman, J.M. Epigenetic alterations and microRNA misexpression in cancer and autoimmune diseases: A critical review. Clin. Rev. Allergy Immunol. 2014, 47, 128–135. [Google Scholar] [CrossRef]
- Pan, W.; Zhu, S.; Yuan, M.; Cui, H.; Wang, L.; Luo, X.; Li, J.; Zhou, H.; Tang, Y.; Shen, N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4+ T cells by directly and indirectly targeting DNA methyltransferase 1. J. Immunol. 2010, 184, 6773–6781. [Google Scholar] [CrossRef]
- Niederer, F.; Trenkmann, M.; Ospelt, C.; Karouzakis, E.; Neidhart, M.; Stanczyk, J.; Kolling, C.; Gay, R.E.; Detmar, M.; Gay, S.; et al. Down-regulation of microRNA-34a* in rheumatoid arthritis synovial fibroblasts promotes apoptosis resistance. Arthritis Rheum. 2012, 64, 1771–1779. [Google Scholar] [CrossRef]
- Das, S.; Foley, N.; Bryan, K.; Watters, K.M.; Bray, I.; Murphy, D.M.; Buckley, P.G.; Stallings, R.L. MicroRNA mediates DNA demethylation events triggered by retinoic acid during neuroblastoma cell differentiation. Cancer Res. 2010, 70, 7874–7881. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tian, X.; Zhang, J.; Huang, Y.; Lin, X.; Chen, L.; Zhang, S. Regulation of human glioma cell apoptosis and invasion by miR-152-3p through targeting DNMT1 and regulating NF2: MiR-152-3p regulate glioma cell apoptosis and invasion. J. Exp. Clin. Cancer Res. 2017, 36, 100. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Chen, J.; Song, W.; Bai, Y. miR-646/TET1 mediated demethylation of IRX1 promoter upregulates HIST2H2BE and promotes the progression of invasive ductal carcinoma. Genomics 2021, 113, 1469–1481. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Liu, J.; Li, X.; Xiao, G.; Wang, H.; Yang, G.; Li, Y.; Tang, S.C.; Qin, S.; Du, N.; et al. MYC and DNMT3A-mediated DNA methylation represses microRNA-200b in triple negative breast cancer. J. Cell. Mol. Med. 2018, 22, 6262–6274. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wu, X.; Chen, T.; Luo, Z.; Hu, X. Downregulation of DNMT3A by miR-708-5p Inhibits Lung Cancer Stem Cell-like Phenotypes through Repressing Wnt/β-catenin Signaling. Clin. Cancer Res. 2018, 24, 1748–1760. [Google Scholar] [CrossRef]
- Li, G.; Zhao, J.; Peng, X.; Liang, J.; Deng, X.; Chen, Y. The mechanism involved in the loss of PTEN expression in NSCLC tumor cells. Biochem. Biophys. Res. Commun. 2012, 418, 547–552. [Google Scholar] [CrossRef]
- Yan, F.; Shen, N.; Pang, J.; Xie, D.; Deng, B.; Molina, J.R.; Yang, P.; Liu, S. Restoration of miR-101 suppresses lung tumorigenesis through inhibition of DNMT3a-dependent DNA methylation. Cell Death Dis. 2014, 5, e1413. [Google Scholar] [CrossRef]
- Han, X.; Wu, J.; Zhang, Y.; Song, J.; Shi, Z.; Chang, H. LINC00518 Promotes Cell Proliferation by Regulating the Cell Cycle of Lung Adenocarcinoma Through miR-185-3p Targeting MECP2. Front. Oncol. 2021, 11, 646559. [Google Scholar] [CrossRef]
- Pei, Y.F.; Xu, X.N.; Wang, Z.F.; Wang, F.W.; Wu, W.D.; Geng, J.F.; Liu, X.Q. Methyl-CpG Binding Domain Protein 2 Inhibits the Malignant Characteristic of Lung Adenocarcinoma through the Epigenetic Modulation of 10 to 11 Translocation 1 and miR-200s. Am. J. Pathol. 2019, 189, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhen, S.; Ye, Z.; Lu, J.; Wang, L.; Li, P.; Li, J.; Zheng, X.; Li, H.; Chen, W.; et al. A Feedback Loop Between miR-30a/c-5p and DNMT1 Mediates Cisplatin Resistance in Ovarian Cancer Cells. Cell Physiol. Biochem. 2017, 41, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, S.; Zou, Y.; Wu, L.; Pei, M.; Jiang, Y. miR-145 promotes miR-133b expression through c-myc and DNMT3A-mediated methylation in ovarian cancer cells. J. Cell. Physiol. 2020, 235, 4291–4301. [Google Scholar] [CrossRef] [PubMed]
- Majid, S.; Dar, A.A.; Saini, S.; Shahryari, V.; Arora, S.; Zaman, M.S.; Chang, I.; Yamamura, S.; Tanaka, Y.; Chiyomaru, T.; et al. miRNA-34b inhibits prostate cancer through demethylation, active chromatin modifications, and AKT pathways. Clin. Cancer Res. 2013, 19, 73–84. [Google Scholar] [CrossRef]
- Xue, G.; Ren, Z.; Chen, Y.; Zhu, J.; Du, Y.; Pan, D.; Li, X.; Hu, B. A feedback regulation between miR-145 and DNA methyltransferase 3b in prostate cancer cell and their responses to irradiation. Cancer Lett. 2015, 361, 121–127. [Google Scholar] [CrossRef]
- Garzon, R.; Liu, S.; Fabbri, M.; Liu, Z.; Heaphy, C.E.; Callegari, E.; Schwind, S.; Pang, J.; Yu, J.; Muthusamy, N.; et al. MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1. Blood 2009, 113, 6411–6418. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, W.; Wu, Z.; Liu, Y.; Shi, Y.; Gong, J.; Shen, W.; Liu, C. miR-29c-3p regulates DNMT3B and LATS1 methylation to inhibit tumor progression in hepatocellular carcinoma. Cell Death Dis. 2019, 10, 48. [Google Scholar] [CrossRef]
- Das, S.; Bryan, K.; Buckley, P.G.; Piskareva, O.; Bray, I.M.; Foley, N.; Ryan, J.; Lynch, J.; Creevey, L.; Fay, J.; et al. Modulation of neuroblastoma disease pathogenesis by an extensive network of epigenetically regulated microRNAs. Oncogene 2013, 32, 2927–2936. [Google Scholar] [CrossRef]
- Hu, S.; Yao, Y.; Hu, X.; Zhu, Y. LncRNA DCST1-AS1 downregulates miR-29b through methylation in glioblastoma (GBM) to promote cancer cell proliferation. Clin. Transl. Oncol. 2020, 22, 2230–2235. [Google Scholar] [CrossRef]
- Huang, G.H.; Du, L.; Li, N.; Zhang, Y.; Xiang, Y.; Tang, J.H.; Xia, S.; Zhang, E.E.; Lv, S.Q. Methylation-mediated miR-155-FAM133A axis contributes to the attenuated invasion and migration of IDH mutant gliomas. Cancer Lett. 2018, 432, 93–102. [Google Scholar] [CrossRef]
- Jiang, H.; Ge, R.; Chen, S.; Huang, L.; Mao, J.; Sheng, L. miRNA-204-5p acts as tumor suppressor to influence the invasion and migration of astrocytoma by targeting ezrin and is downregulated by DNA methylation. Bioengineered 2021, 12, 9301–9312. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bertoni, H.; Lal, B.; Michelson, N.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; Li, Y.; Laterra, J. Epigenetic modulation of a miR-296-5p:HMGA1 axis regulates Sox2 expression and glioblastoma stem cells. Oncogene 2016, 35, 4903–4913. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huo, C.; Yin, J.; Tian, L.; Ma, L.; Wang, D. Hypermethylation of the Promoter of miR-338-5p Mediates Aberrant Expression of ETS-1 and Is Correlated with Disease Severity of Astrocytoma Patients. Front. Oncol. 2021, 11, 773644. [Google Scholar] [CrossRef]
- Ying, Z.; Li, Y.; Wu, J.; Zhu, X.; Yang, Y.; Tian, H.; Li, W.; Hu, B.; Cheng, S.Y.; Li, M. Loss of miR-204 expression enhances glioma migration and stem cell-like phenotype. Cancer Res. 2013, 73, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wan, Y.; Xie, D.; Wang, Y.; Wei, J.; Yan, Q.; Lu, P.; Mo, L.; Xie, J.; Yang, S.; et al. DNMT1 mediates chemosensitivity by reducing methylation of miRNA-20a promoter in glioma cells. Exp. Mol. Med. 2015, 47, e182. [Google Scholar] [CrossRef]
- Agirre, X.; Vilas-Zornoza, A.; Jimenez-Velasco, A.; Martin-Subero, J.I.; Cordeu, L.; Garate, L.; San Jose-Eneriz, E.; Abizanda, G.; Rodriguez-Otero, P.; Fortes, P.; et al. Epigenetic silencing of the tumor suppressor microRNA Hsa-miR-124a regulates CDK6 expression and confers a poor prognosis in acute lymphoblastic leukemia. Cancer Res. 2009, 69, 4443–4453. [Google Scholar] [CrossRef]
- Baer, C.; Oakes, C.C.; Ruppert, A.S.; Claus, R.; Kim-Wanner, S.Z.; Mertens, D.; Zenz, T.; Stilgenbauer, S.; Byrd, J.C.; Plass, C. Epigenetic silencing of miR-708 enhances NF-kappaB signaling in chronic lymphocytic leukemia. Int. J. Cancer 2015, 137, 1352–1361. [Google Scholar] [CrossRef]
- Pallasch, C.P.; Patz, M.; Park, Y.J.; Hagist, S.; Eggle, D.; Claus, R.; Debey-Pascher, S.; Schulz, A.; Frenzel, L.P.; Claasen, J.; et al. miRNA deregulation by epigenetic silencing disrupts suppression of the oncogene PLAG1 in chronic lymphocytic leukemia. Blood 2009, 114, 3255–3264. [Google Scholar] [CrossRef]
- Paik, J.H.; Jang, J.Y.; Jeon, Y.K.; Kim, W.Y.; Kim, T.M.; Heo, D.S.; Kim, C.W. MicroRNA-146a downregulates NFkappaB activity via targeting TRAF6 and functions as a tumor suppressor having strong prognostic implications in NK/T cell lymphoma. Clin. Cancer Res. 2011, 17, 4761–4771. [Google Scholar] [CrossRef]
- Wang, L.Q.; Kumar, S.; Calin, G.A.; Li, Z.; Chim, C.S. Frequent methylation of the tumour suppressor miR-1258 targeting PDL1: Implication in multiple myeloma-specific cytotoxicity and prognostification. Br. J. Haematol. 2020, 190, 249–261. [Google Scholar] [CrossRef]
- Tatekawa, S.; Chinen, Y.; Ri, M.; Narita, T.; Shimura, Y.; Matsumura-Kimoto, Y.; Tsukamoto, T.; Kobayashi, T.; Kawata, E.; Uoshima, N.; et al. Epigenetic repression of miR-375 is the dominant mechanism for constitutive activation of the PDPK1/RPS6KA3 signalling axis in multiple myeloma. Br. J. Haematol. 2017, 178, 534–546. [Google Scholar] [CrossRef]
- Hsu, P.Y.; Deatherage, D.E.; Rodriguez, B.A.; Liyanarachchi, S.; Weng, Y.I.; Zuo, T.; Liu, J.; Cheng, A.S.; Huang, T.H. Xenoestrogen-induced epigenetic repression of microRNA-9-3 in breast epithelial cells. Cancer Res. 2009, 69, 5936–5945. [Google Scholar] [CrossRef]
- Lehmann, U.; Hasemeier, B.; Christgen, M.; Muller, M.; Romermann, D.; Langer, F.; Kreipe, H. Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J. Pathol. 2008, 214, 17–24. [Google Scholar] [CrossRef]
- Kim, B.G.; Gao, M.Q.; Kang, S.; Choi, Y.P.; Lee, J.H.; Kim, J.E.; Han, H.H.; Mun, S.G.; Cho, N.H. Mechanical compression induces VEGFA overexpression in breast cancer via DNMT3A-dependent miR-9 downregulation. Cell Death Dis. 2017, 8, e2646. [Google Scholar] [CrossRef] [PubMed]
- Biagioni, F.; Bossel Ben-Moshe, N.; Fontemaggi, G.; Canu, V.; Mori, F.; Antoniani, B.; Di Benedetto, A.; Santoro, R.; Germoni, S.; De Angelis, F.; et al. miR-10b*, a master inhibitor of the cell cycle, is down-regulated in human breast tumours. EMBO Mol. Med. 2012, 4, 1214–1229. [Google Scholar] [CrossRef]
- Yu, F.; Jiao, Y.; Zhu, Y.; Wang, Y.; Zhu, J.; Cui, X.; Liu, Y.; He, Y.; Park, E.Y.; Zhang, H.; et al. MicroRNA 34c gene down-regulation via DNA methylation promotes self-renewal and epithelial-mesenchymal transition in breast tumor-initiating cells. J. Biol. Chem. 2012, 287, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Oltra, S.S.; Pena-Chilet, M.; Vidal-Tomas, V.; Flower, K.; Martinez, M.T.; Alonso, E.; Burgues, O.; Lluch, A.; Flanagan, J.M.; Ribas, G. Methylation deregulation of miRNA promoters identifies miR124-2 as a survival biomarker in Breast Cancer in very young women. Sci. Rep. 2018, 8, 14373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, L.X.; Wu, Q.N.; Du, Z.M.; Chen, J.; Liao, D.Z.; Huang, M.Y.; Hou, J.H.; Wu, Q.L.; Zeng, M.S.; et al. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 2011, 71, 3552–3562. [Google Scholar] [CrossRef]
- Shi, W.; Tang, T.; Li, X.; Deng, S.; Li, R.; Wang, Y.; Wang, Y.; Xia, T.; Zhang, Y.; Zen, K.; et al. Methylation-mediated silencing of miR-133a-3p promotes breast cancer cell migration and stemness via miR-133a-3p/MAML1/DNMT3A positive feedback loop. J. Exp. Clin. Cancer Res. 2019, 38, 429. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Liu, C.; Chen, X.; Qi, Y.; Jiang, Y.; Zou, C.; Zhang, X.; Liu, S.; Wang, X.; et al. Analysis of MiR-195 and MiR-497 expression, regulation and role in breast cancer. Clin. Cancer Res. 2011, 17, 1722–1730. [Google Scholar] [CrossRef]
- Hoffman, A.E.; Zheng, T.; Yi, C.; Leaderer, D.; Weidhaas, J.; Slack, F.; Zhang, Y.; Paranjape, T.; Zhu, Y. microRNA miR-196a-2 and breast cancer: A genetic and epigenetic association study and functional analysis. Cancer Res. 2009, 69, 5970–5977. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Wright, J.A.; Attema, J.L.; Gregory, P.A.; Bert, A.G.; Smith, E.; Thomas, D.; Lopez, A.F.; Drew, P.A.; Khew-Goodall, Y.; et al. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state. J. Cell Sci. 2013, 126, 2256–2266. [Google Scholar] [CrossRef] [PubMed]
- Wee, E.J.; Peters, K.; Nair, S.S.; Hulf, T.; Stein, S.; Wagner, S.; Bailey, P.; Lee, S.Y.; Qu, W.J.; Brewster, B.; et al. Mapping the regulatory sequences controlling 93 breast cancer-associated miRNA genes leads to the identification of two functional promoters of the Hsa-mir-200b cluster, methylation of which is associated with metastasis or hormone receptor status in advanced breast cancer. Oncogene 2012, 31, 4182–4195. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, M.K.; Park, J.H.; Lee, H.J.; Shin, D.H.; Kang, Y.; Lee, C.H.; Kong, G. Loss of the polycomb protein Mel-18 enhances the epithelial-mesenchymal transition by ZEB1 and ZEB2 expression through the downregulation of miR-205 in breast cancer. Oncogene 2014, 33, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Wang, C.; Chang, D.; Zhu, X.; Sai, C.; Pei, J. Limonin attenuates the stemness of breast cancer cells via suppressing MIR216A methylation. Biomed. Pharm. 2019, 112, 108699. [Google Scholar] [CrossRef] [PubMed]
- Png, K.J.; Yoshida, M.; Zhang, X.H.; Shu, W.; Lee, H.; Rimner, A.; Chan, T.A.; Comen, E.; Andrade, V.P.; Kim, S.W.; et al. MicroRNA-335 inhibits tumor reinitiation and is silenced through genetic and epigenetic mechanisms in human breast cancer. Genes Dev. 2011, 25, 226–231. [Google Scholar] [CrossRef]
- Kang, H.; Kim, C.; Lee, H.; Rho, J.G.; Seo, J.W.; Nam, J.W.; Song, W.K.; Nam, S.W.; Kim, W.; Lee, E.K. Downregulation of microRNA-362-3p and microRNA-329 promotes tumor progression in human breast cancer. Cell Death Differ. 2016, 23, 484–495. [Google Scholar] [CrossRef]
- de Souza Rocha Simonini, P.; Breiling, A.; Gupta, N.; Malekpour, M.; Youns, M.; Omranipour, R.; Malekpour, F.; Volinia, S.; Croce, C.M.; Najmabadi, H.; et al. Epigenetically deregulated microRNA-375 is involved in a positive feedback loop with estrogen receptor α in breast cancer cells. Cancer Res. 2010, 70, 9175–9184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bai, Z.; Chai, D.; Gao, Y.; Li, T.; Ma, Y.; Li, J. DNA methyltransferase 1 inhibits microRNA-497 and elevates GPRC5A expression to promote chemotherapy resistance and metastasis in breast cancer. Cancer Cell Int. 2022, 22, 112. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Li, H.; Ma, X.; Lian, B.; He, J.; Gao, Y.; Li, J. Methylation-Mediated Silencing of MicroRNA-497 Promotes Breast Cancer Progression Through Up-Regulation of Mucin1. Front. Oncol. 2020, 10, 552099. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Li, S.; Cui, X.; Lv, X.; Jiao, Y.; Yu, F.; Yao, H.; Song, E.; Chen, Y.; Wang, M.; et al. The overexpression of hypomethylated miR-663 induces chemotherapy resistance in human breast cancer cells by targeting heparin sulfate proteoglycan 2 (HSPG2). J. Biol. Chem. 2013, 288, 10973–10985. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yu, L.; Zhang, X.; Lei, F.; Wang, L.; Liu, X.; Wu, S.; Zhu, J.; Wu, G.; Cao, L.; et al. miR-892b Silencing Activates NF-kappaB and Promotes Aggressiveness in Breast Cancer. Cancer Res. 2016, 76, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Grady, W.M.; Parkin, R.K.; Mitchell, P.S.; Lee, J.H.; Kim, Y.H.; Tsuchiya, K.D.; Washington, M.K.; Paraskeva, C.; Willson, J.K.; Kaz, A.M.; et al. Epigenetic silencing of the intronic microRNA hsa-miR-342 and its host gene EVL in colorectal cancer. Oncogene 2008, 27, 3880–3888. [Google Scholar] [CrossRef] [PubMed]
- Toyota, M.; Suzuki, H.; Sasaki, Y.; Maruyama, R.; Imai, K.; Shinomura, Y.; Tokino, T. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res. 2008, 68, 4123–4132. [Google Scholar] [CrossRef]
- Kalimutho, M.; Di Cecilia, S.; Del Vecchio Blanco, G.; Roviello, F.; Sileri, P.; Cretella, M.; Formosa, A.; Corso, G.; Marrelli, D.; Pallone, F.; et al. Epigenetically silenced miR-34b/c as a novel faecal-based screening marker for colorectal cancer. Br. J. Cancer 2011, 104, 1770–1778. [Google Scholar] [CrossRef]
- Siemens, H.; Neumann, J.; Jackstadt, R.; Mansmann, U.; Horst, D.; Kirchner, T.; Hermeking, H. Detection of miR-34a promoter methylation in combination with elevated expression of c-Met and β-catenin predicts distant metastasis of colon cancer. Clin. Cancer Res. 2013, 19, 710–720. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, W.; Sakre, N.; DeVecchio, J.; Ferrandon, S.; Ting, A.H.; Bao, S.; Bissett, I.; Church, J.; Kalady, M.F. Epigenetically regulated miR-1247 functions as a novel tumour suppressor via MYCBP2 in methylator colon cancers. Br. J. Cancer 2018, 119, 1267–1277. [Google Scholar] [CrossRef]
- Mei, Q.; Xue, G.; Li, X.; Wu, Z.; Li, X.; Yan, H.; Guo, M.; Sun, S.; Han, W. Methylation-induced loss of miR-484 in microsatellite-unstable colorectal cancer promotes both viability and IL-8 production via CD137L. J. Pathol. 2015, 236, 165–174. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Xu, B.; Wang, B.; Wang, Z.; Liang, Y.; Zhou, J.; Hu, J.; Jiang, B. Epigenetic silencing of miR-126 contributes to tumor invasion and angiogenesis in colorectal cancer. Oncol. Rep. 2013, 30, 1976–1984. [Google Scholar] [CrossRef]
- Gao, P.; Wang, D.; Liu, M.; Chen, S.; Yang, Z.; Zhang, J.; Wang, H.; Niu, Y.; Wang, W.; Yang, J.; et al. DNA methylation-mediated repression of exosomal miR-652-5p expression promotes oesophageal squamous cell carcinoma aggressiveness by targeting PARG and VEGF pathways. PLoS Genet. 2020, 16, e1008592. [Google Scholar] [CrossRef]
- Lu, Y.F.; Yu, J.R.; Yang, Z.; Zhu, G.X.; Gao, P.; Wang, H.; Chen, S.Y.; Zhang, J.; Liu, M.Y.; Niu, Y.; et al. Promoter hypomethylation mediated upregulation of MicroRNA-10b-3p targets FOXO3 to promote the progression of esophageal squamous cell carcinoma (ESCC). J. Exp. Clin. Cancer Res. 2018, 37, 301. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Guo, T.; Guo, Y.; Liu, J.; Qu, F.; He, Y. Methylation-associated silencing of miR-128 promotes the development of esophageal cancer by targeting COX-2 in areas with a high incidence of esophageal cancer. Int. J. Oncol. 2019, 54, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gu, J.; Jiang, P.; Zheng, Y.; Liu, X.; Jiang, X.; Huang, E.; Xiong, S.; Xu, F.; Liu, G.; et al. DNMT1-microRNA126 epigenetic circuit contributes to esophageal squamous cell carcinoma growth via ADAM9-EGFR-AKT signaling. Clin. Cancer Res. 2015, 21, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.X.; Zhu, F.; Qi, L. Demethylated miR-216a Regulates High Mobility Group Box 3 Promoting Growth of Esophageal Cancer Cells Through Wnt/β-Catenin Pathway. Front. Oncol. 2021, 11, 622073. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, Z.; Zhu, Y.; Meng, L.; Liu, F.; Sang, M.; Wang, G. Hypermethylation-mediated inactivation of miR-124 predicts poor prognosis and promotes tumor growth at least partially through targeting EZH2/H3K27me3 in ESCC. Clin. Exp. Metastasis 2019, 36, 381–391. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Zhao, P.; Qiao, T.; Cao, Z.; Gao, F.; Liu, M.; Wu, S. DNA methyltransferase 3 beta regulates promoter methylation of microRNA-149 to augment esophageal squamous cell carcinoma development through the ring finger protein 2/Wnt/β-catenin axis. Bioengineered 2022, 13, 4010–4027. [Google Scholar] [CrossRef]
- Xi, S.; Inchauste, S.; Guo, H.; Shan, J.; Xiao, Z.; Xu, H.; Miettenen, M.; Zhang, M.R.; Hong, J.A.; Raiji, M.T.; et al. Cigarette smoke mediates epigenetic repression of miR-217 during esophageal adenocarcinogenesis. Oncogene 2015, 34, 5548–5559. [Google Scholar] [CrossRef]
- Botla, S.K.; Savant, S.; Jandaghi, P.; Bauer, A.S.; Mucke, O.; Moskalev, E.A.; Neoptolemos, J.P.; Costello, E.; Greenhalf, W.; Scarpa, A.; et al. Early Epigenetic Downregulation of microRNA-192 Expression Promotes Pancreatic Cancer Progression. Cancer Res. 2016, 76, 4149–4159. [Google Scholar] [CrossRef]
- Gao, W.; Gu, Y.; Li, Z.; Cai, H.; Peng, Q.; Tu, M.; Kondo, Y.; Shinjo, K.; Zhu, Y.; Zhang, J.; et al. miR-615-5p is epigenetically inactivated and functions as a tumor suppressor in pancreatic ductal adenocarcinoma. Oncogene 2015, 34, 1629–1640. [Google Scholar] [CrossRef]
- Godfrey, J.D.; Morton, J.P.; Wilczynska, A.; Sansom, O.J.; Bushell, M.D. MiR-142-3p is downregulated in aggressive p53 mutant mouse models of pancreatic ductal adenocarcinoma by hypermethylation of its locus. Cell Death Dis. 2018, 9, 644. [Google Scholar] [CrossRef]
- Sun, Y.; Zhu, Q.; Zhou, M.; Yang, W.; Shi, H.; Shan, Y.; Zhang, Q.; Yu, F. Restoration of miRNA-148a in pancreatic cancer reduces invasion and metastasis by inhibiting the Wnt/β-catenin signaling pathway via downregulating maternally expressed gene-3. Exp. Med. 2019, 17, 639–648. [Google Scholar] [CrossRef]
- Li, A.; Omura, N.; Hong, S.M.; Vincent, A.; Walter, K.; Griffith, M.; Borges, M.; Goggins, M. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010, 70, 5226–5237. [Google Scholar] [CrossRef]
- Ma, Y.; Chai, N.; Jiang, Q.; Chang, Z.; Chai, Y.; Li, X.; Sun, H.; Hou, J.; Linghu, E. DNA methyltransferase mediates the hypermethylation of the microRNA 34a promoter and enhances the resistance of patient-derived pancreatic cancer cells to molecular targeting agents. Pharm. Res. 2020, 160, 105071. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, L.; Zhang, J.; Chen, H.; Fan, J.; Wang, K.; Luo, J.; Chen, Z.; Meng, Z.; Liu, L. Methylation-mediated silencing of the miR-124 genes facilitates pancreatic cancer progression and metastasis by targeting Rac1. Oncogene 2014, 33, 514–524. [Google Scholar] [CrossRef]
- Lim, B.; Kim, H.J.; Heo, H.; Huh, N.; Baek, S.J.; Kim, J.H.; Bae, D.H.; Seo, E.H.; Lee, S.I.; Song, K.S.; et al. Epigenetic silencing of miR-1271 enhances MEK1 and TEAD4 expression in gastric cancer. Cancer Med. 2018, 7, 3411–3424. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Li, B.; Zhang, Z.; Luo, H.; Wang, Y.; Lu, Z.; Wu, X. Epigenetic silencing of miRNA-9 is correlated with promoter-proximal CpG island hypermethylation in gastric cancer in vitro and in vivo. Int. J. Oncol. 2014, 45, 2576–2586. [Google Scholar] [CrossRef]
- Shao, L.; Chen, Z.; Peng, D.; Soutto, M.; Zhu, S.; Bates, A.; Zhang, S.; El-Rifai, W. Methylation of the HOXA10 Promoter Directs miR-196b-5p-Dependent Cell Proliferation and Invasion of Gastric Cancer Cells. Mol. Cancer Res. 2018, 16, 696–706. [Google Scholar] [CrossRef]
- Xin, L.; Liu, L.; Liu, C.; Zhou, L.Q.; Zhou, Q.; Yuan, Y.W.; Li, S.H.; Zhang, H.T. DNA-methylation-mediated silencing of miR-7-5p promotes gastric cancer stem cell invasion via increasing Smo and Hes1. J. Cell Physiol. 2020, 235, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Song, P.; Su, R.; Yang, G.; Dong, L.; Luo, M.; Wang, B.; Gong, B.; Liu, C.; Song, W.; et al. DNA Methylation mediated down-regulating of MicroRNA-33b and its role in gastric cancer. Sci. Rep. 2016, 6, 18824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zou, Y.; Dai, D.Q. Downregulation of microRNA-27b-3p via aberrant DNA methylation contributes to malignant behavior of gastric cancer cells by targeting GSPT1. Biomed. Pharm. 2019, 119, 109417. [Google Scholar] [CrossRef]
- Zhang, J.K.; Li, Y.S.; Zhang, C.D.; Dai, D.Q. Up-regulation of CRKL by microRNA-335 methylation is associated with poor prognosis in gastric cancer. Cancer Cell Int. 2017, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, D.; Zhang, G.; Xiong, J.; Jie, Z.; Cheng, H.; Cao, Y.; Jiang, M.; Lin, L.; Le, Z.; et al. Methylation-associated silencing of MicroRNA-335 contributes tumor cell invasion and migration by interacting with RASA1 in gastric cancer. Am. J. Cancer Res. 2014, 4, 648–662. [Google Scholar]
- Eun, J.W.; Kim, H.S.; Shen, Q.; Yang, H.D.; Kim, S.Y.; Yoon, J.H.; Park, W.S.; Lee, J.Y.; Nam, S.W. MicroRNA-495-3p functions as a tumor suppressor by regulating multiple epigenetic modifiers in gastric carcinogenesis. J. Pathol. 2018, 244, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Song, Y.; Bi, N.; Shen, J.; Liu, W.; Fan, J.; Sun, G.; Tong, T.; He, J.; Shi, Y.; et al. DNA methylation-mediated repression of miR-886-3p predicts poor outcome of human small cell lung cancer. Cancer Res. 2013, 73, 3326–3335. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Toyooka, S.; Soh, J.; Kubo, T.; Yamamoto, H.; Maki, Y.; Muraoka, T.; Shien, K.; Furukawa, M.; Ueno, T.; et al. Frequent methylation and oncogenic role of microRNA-34b/c in small-cell lung cancer. Lung Cancer 2012, 76, 32–38. [Google Scholar] [CrossRef]
- Cui, R.; Meng, W.; Sun, H.L.; Kim, T.; Ye, Z.; Fassan, M.; Jeon, Y.J.; Li, B.; Vicentini, C.; Peng, Y.; et al. MicroRNA-224 promotes tumor progression in nonsmall cell lung cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E4288–E4297. [Google Scholar] [CrossRef]
- Brueckner, B.; Stresemann, C.; Kuner, R.; Mund, C.; Musch, T.; Meister, M.; Sultmann, H.; Lyko, F. The human let-7a-3 locus contains an epigenetically regulated microRNA gene with oncogenic function. Cancer Res. 2007, 67, 1419–1423. [Google Scholar] [CrossRef]
- Lin, C.W.; Chang, Y.L.; Chang, Y.C.; Lin, J.C.; Chen, C.C.; Pan, S.H.; Wu, C.T.; Chen, H.Y.; Yang, S.C.; Hong, T.M.; et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat. Commun. 2013, 4, 1877. [Google Scholar] [CrossRef]
- Tessema, M.; Yingling, C.M.; Picchi, M.A.; Wu, G.; Ryba, T.; Lin, Y.; Bungum, A.O.; Edell, E.S.; Spira, A.; Belinsky, S.A. ANK1 Methylation regulates expression of MicroRNA-486-5p and discriminates lung tumors by histology and smoking status. Cancer Lett. 2017, 410, 191–200. [Google Scholar] [CrossRef]
- Crawford, M.; Brawner, E.; Batte, K.; Yu, L.; Hunter, M.G.; Otterson, G.A.; Nuovo, G.; Marsh, C.B.; Nana-Sinkam, S.P. MicroRNA-126 inhibits invasion in non-small cell lung carcinoma cell lines. Biochem. Biophys. Res. Commun. 2008, 373, 607–612. [Google Scholar] [CrossRef]
- Watanabe, K.; Emoto, N.; Hamano, E.; Sunohara, M.; Kawakami, M.; Kage, H.; Kitano, K.; Nakajima, J.; Goto, A.; Fukayama, M.; et al. Genome structure-based screening identified epigenetically silenced microRNA associated with invasiveness in non-small-cell lung cancer. Int. J. Cancer 2012, 130, 2580–2590. [Google Scholar] [CrossRef]
- Wilting, S.M.; van Boerdonk, R.A.; Henken, F.E.; Meijer, C.J.; Diosdado, B.; Meijer, G.A.; le Sage, C.; Agami, R.; Snijders, P.J.; Steenbergen, R.D. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer. Mol. Cancer 2010, 9, 167. [Google Scholar] [CrossRef]
- Varghese, V.K.; Shukla, V.; Jishnu, P.V.; Kabekkodu, S.P.; Pandey, D.; Sharan, K.; Satyamoorthy, K. Characterizing methylation regulated miRNA in carcinoma of the human uterine cervix. Life Sci. 2019, 232, 116668. [Google Scholar] [CrossRef]
- Mei, Q.; Li, X.; Zhang, K.; Wu, Z.; Li, X.; Meng, Y.; Guo, M.; Luo, G.; Fu, X.; Han, W. Genetic and Methylation-Induced Loss of miR-181a2/181b2 within chr9q33.3 Facilitates Tumor Growth of Cervical Cancer through the PIK3R3/Akt/FoxO Signaling Pathway. Clin. Cancer Res. 2017, 23, 575–586. [Google Scholar] [CrossRef]
- Tsuruta, T.; Kozaki, K.; Uesugi, A.; Furuta, M.; Hirasawa, A.; Imoto, I.; Susumu, N.; Aoki, D.; Inazawa, J. miR-152 is a tumor suppressor microRNA that is silenced by DNA hypermethylation in endometrial cancer. Cancer Res. 2011, 71, 6450–6462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, J.H.; Shan, T.; Aguilera-Barrantes, I.; Wang, L.S.; Huang, T.H.; Rader, J.S.; Sheng, X.; Huang, Y.W. miR-137 is a tumor suppressor in endometrial cancer and is repressed by DNA hypermethylation. Lab. Investig. 2018, 98, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Liu, J.C.; Deatherage, D.E.; Luo, J.; Mutch, D.G.; Goodfellow, P.J.; Miller, D.S.; Huang, T.H. Epigenetic repression of microRNA-129-2 leads to overexpression of SOX4 oncogene in endometrial cancer. Cancer Res. 2009, 69, 9038–9046. [Google Scholar] [CrossRef]
- Ramalho-Carvalho, J.; Goncalves, C.S.; Graca, I.; Bidarra, D.; Pereira-Silva, E.; Salta, S.; Godinho, M.I.; Gomez, A.; Esteller, M.; Costa, B.M.; et al. A multiplatform approach identifies miR-152-3p as a common epigenetically regulated onco-suppressor in prostate cancer targeting TMEM97. Clin. Epigen. 2018, 10, 40. [Google Scholar] [CrossRef]
- Ramalho-Carvalho, J.; Martins, J.B.; Cekaite, L.; Sveen, A.; Torres-Ferreira, J.; Graca, I.; Costa-Pinheiro, P.; Eilertsen, I.A.; Antunes, L.; Oliveira, J.; et al. Epigenetic disruption of miR-130a promotes prostate cancer by targeting SEC23B and DEPDC1. Cancer Lett. 2017, 385, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Long, X.R.; He, Y.; Huang, C.; Li, J. MicroRNA-148a is silenced by hypermethylation and interacts with DNA methyltransferase 1 in hepatocellular carcinogenesis. Int. J. Oncol. 2014, 44, 1915–1922. [Google Scholar] [CrossRef]
- Yu, Q.; Xiang, L.; Yin, L.; Liu, X.; Yang, D.; Zhou, J. Loss-of-function of miR-142 by hypermethylation promotes TGF-β-mediated tumour growth and metastasis in hepatocellular carcinoma. Cell Prolif. 2017, 50, e12384. [Google Scholar] [CrossRef] [PubMed]
- Rui, T.; Xu, S.; Zhang, X.; Huang, H.; Feng, S.; Zhan, S.; Xie, H.; Zhou, L.; Ling, Q.; Zheng, S. The chromosome 19 microRNA cluster, regulated by promoter hypomethylation, is associated with tumour burden and poor prognosis in patients with hepatocellular carcinoma. J. Cell Physiol. 2020, 235, 6103–6112. [Google Scholar] [CrossRef] [PubMed]
- Kitano, K.; Watanabe, K.; Emoto, N.; Kage, H.; Hamano, E.; Nagase, T.; Sano, A.; Murakawa, T.; Nakajima, J.; Goto, A.; et al. CpG island methylation of microRNAs is associated with tumor size and recurrence of non-small-cell lung cancer. Cancer Sci. 2011, 102, 2126–2131. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Witmer, P.D.; Casey, E.; Valle, D.; Sukumar, S. DNA methylation regulates MicroRNA expression. Cancer Biol. 2007, 6, 1284–1288. [Google Scholar] [CrossRef]
- Fabbri, M.; Calin, G.A. Epigenetics and miRNAs in human cancer. Adv. Genet. 2010, 70, 87–99. [Google Scholar] [CrossRef]
- Lujambio, A.; Calin, G.A.; Villanueva, A.; Ropero, S.; Sanchez-Cespedes, M.; Blanco, D.; Montuenga, L.M.; Rossi, S.; Nicoloso, M.S.; Faller, W.J.; et al. A microRNA DNA methylation signature for human cancer metastasis. Proc. Natl. Acad. Sci. USA 2008, 105, 13556–13561. [Google Scholar] [CrossRef]
- Xia, W.; Chen, Q.; Wang, J.; Mao, Q.; Dong, G.; Shi, R.; Zheng, Y.; Xu, L.; Jiang, F. DNA methylation mediated silencing of microRNA-145 is a potential prognostic marker in patients with lung adenocarcinoma. Sci. Rep. 2015, 5, 16901. [Google Scholar] [CrossRef]
- Hinske, L.C.; Franca, G.S.; Torres, H.A.; Ohara, D.T.; Lopes-Ramos, C.M.; Heyn, J.; Reis, L.F.; Ohno-Machado, L.; Kreth, S.; Galante, P.A. miRIAD-integrating microRNA inter- and intragenic data. Database 2014, 2014, bau099. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.W.; Kao, H.W.; Chen, H.C.; Chen, S.J.; Lin, W.C. Epigenetic control of the expression of a primate-specific microRNA cluster in human cancer cells. Epigenetics 2009, 4, 587–592. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Aure, M.R.; Fleischer, T.; Bjorklund, S.; Ankill, J.; Castro-Mondragon, J.A.; Osbreac; Borresen-Dale, A.L.; Tost, J.; Sahlberg, K.K.; Mathelier, A.; et al. Crosstalk between microRNA expression and DNA methylation drives the hormone-dependent phenotype of breast cancer. Genome Med. 2021, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, N.; Rahimi, K.; Mansouri, K.; Fathi, F.; Menbari, M.N.; Mohammadi, G.; Abdi, M. MiR-646 prevents proliferation and progression of human breast cancer cell lines by suppressing HDAC2 expression. Mol. Cell Probes 2020, 53, 101649. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, R.; Rivenbark, A.G.; Mackler, R.M.; Livasy, C.A.; Coleman, W.B. Dysregulation of microRNA expression drives aberrant DNA hypermethylation in basal-like breast cancer. Int. J. Oncol. 2014, 44, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.J.; Hu, J.Y.; Kuang, X.Y.; Luo, J.M.; Hou, Y.F.; Di, G.H.; Wu, J.; Shen, Z.Z.; Song, H.Y.; Shao, Z.M. MicroRNA-200a promotes anoikis resistance and metastasis by targeting YAP1 in human breast cancer. Clin. Cancer Res. 2013, 19, 1389–1399. [Google Scholar] [CrossRef]
- Becker, L.E.; Takwi, A.A.; Lu, Z.; Li, Y. The role of miR-200a in mammalian epithelial cell transformation. Carcinogenesis 2015, 36, 2–12. [Google Scholar] [CrossRef]
- Yu, S.J.; Yang, L.; Hong, Q.; Kuang, X.Y.; Di, G.H.; Shao, Z.M. MicroRNA-200a confers chemoresistance by antagonizing TP53INP1 and YAP1 in human breast cancer. BMC Cancer 2018, 18, 74. [Google Scholar] [CrossRef]
- Fontana, A.; Barbano, R.; Dama, E.; Pasculli, B.; Rendina, M.; Morritti, M.G.; Melocchi, V.; Castelvetere, M.; Valori, V.M.; Ravaioli, S.; et al. Combined analysis of miR-200 family and its significance for breast cancer. Sci. Rep. 2021, 11, 2980. [Google Scholar] [CrossRef]
- Lee, H.; Shin, C.H.; Kim, H.R.; Choi, K.H.; Kim, H.H. MicroRNA-296-5p Promotes Invasiveness through Downregulation of Nerve Growth Factor Receptor and Caspase-8. Mol. Cells 2017, 40, 254–261. [Google Scholar] [CrossRef]
- D’Urso, P.I.; D’Urso, O.F.; Storelli, C.; Mallardo, M.; Gianfreda, C.D.; Montinaro, A.; Cimmino, A.; Pietro, C.; Marsigliante, S. miR-155 is up-regulated in primary and secondary glioblastoma and promotes tumour growth by inhibiting GABA receptors. Int. J. Oncol. 2012, 41, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Tynninen, O.; Carpen, O.; Jaaskelainen, J.; Paavonen, T.; Paetau, A. Ezrin expression in tissue microarray of primary and recurrent gliomas. Neuropathol. Appl. Neurobiol. 2004, 30, 472–477. [Google Scholar] [CrossRef]
- Murakami, M.; Ito, H.; Hagiwara, K.; Yoshida, K.; Sobue, S.; Ichihara, M.; Takagi, A.; Kojima, T.; Tanaka, K.; Tamiya-Koizumi, K.; et al. ATRA inhibits ceramide kinase transcription in a human neuroblastoma cell line, SH-SY5Y cells: The role of COUP-TFI. J. Neurochem. 2010, 112, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, M. Modulation of SOX2 and SOX3 gene expression during differentiation of human neuronal precursor cell line NTERA2. Mol. Biol. Rep. 2003, 30, 127–132. [Google Scholar] [CrossRef]
- Baer, C.; Claus, R.; Frenzel, L.P.; Zucknick, M.; Park, Y.J.; Gu, L.; Weichenhan, D.; Fischer, M.; Pallasch, C.P.; Herpel, E.; et al. Extensive promoter DNA hypermethylation and hypomethylation is associated with aberrant microRNA expression in chronic lymphocytic leukemia. Cancer Res. 2012, 72, 3775–3785. [Google Scholar] [CrossRef]
- Carvalho de Oliveira, J.; Mathias, C.; Oliveira, V.C.; Pezuk, J.A.; Brassesco, M.S. The Double Face of miR-708: A Pan-Cancer Player with Dissociative Identity Disorder. Genes 2022, 13, 2375. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, M.; Garzon, R.; Andreeff, M.; Kantarjian, H.M.; Garcia-Manero, G.; Calin, G.A. MicroRNAs and noncoding RNAs in hematological malignancies: Molecular, clinical and therapeutic implications. Leukemia 2008, 22, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Liang, R.; So, C.C.; Jin, D.Y.; Costello, J.F.; Chim, C.S. Epigenetic silencing of MIR203 in multiple myeloma. Br. J. Haematol. 2011, 154, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Yim, R.L.; Kwong, Y.L.; Leung, C.Y.; Hui, P.K.; Cheung, F.; Liang, R.; Jin, D.Y.; Chim, C.S. Epigenetic inactivation of the MIR129-2 in hematological malignancies. J. Hematol. Oncol. 2013, 6, 16. [Google Scholar] [CrossRef]
- Li, Z.; Wong, K.Y.; Chan, G.C.; Chng, W.J.; Chim, C.S. Epigenetic silencing of EVL/miR-342 in multiple myeloma. Transl. Res. 2018, 192, 46–53. [Google Scholar] [CrossRef]
- Yim, R.L.; Kwong, Y.L.; Wong, K.Y.; Chim, C.S. DNA Methylation of Tumor Suppressive miRNAs in Non-Hodgkin’s Lymphomas. Front. Genet. 2012, 3, 233. [Google Scholar] [CrossRef] [PubMed]
- Farazi, P.A.; DePinho, R.A. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Yang, Y. Hepatic Hippo signaling inhibits development of hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 26, 742–750. [Google Scholar] [CrossRef]
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Prim. 2016, 2, 16022. [Google Scholar] [CrossRef]
- Yonemori, K.; Kurahara, H.; Maemura, K.; Natsugoe, S. MicroRNA in pancreatic cancer. J. Hum. Genet. 2017, 62, 33–40. [Google Scholar] [CrossRef]
- Hanoun, N.; Delpu, Y.; Suriawinata, A.A.; Bournet, B.; Bureau, C.; Selves, J.; Tsongalis, G.J.; Dufresne, M.; Buscail, L.; Cordelier, P.; et al. The silencing of microRNA 148a production by DNA hypermethylation is an early event in pancreatic carcinogenesis. Clin. Chem. 2010, 56, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Riascos, Z.V.; Ginesta, M.M.; Fabregat, J.; Serrano, T.; Busquets, J.; Buscail, L.; Cordelier, P.; Capella, G. Expression and Role of MicroRNAs from the miR-200 Family in the Tumor Formation and Metastatic Propensity of Pancreatic Cancer. Mol. Nucleic Acids 2019, 17, 491–503. [Google Scholar] [CrossRef]
- Lujambio, A.; Ropero, S.; Ballestar, E.; Fraga, M.F.; Cerrato, C.; Setien, F.; Casado, S.; Suarez-Gauthier, A.; Sanchez-Cespedes, M.; Git, A.; et al. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007, 67, 1424–1429. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, P.; Li, Y.; Ye, F.; Wang, F.; Wan, X.; Cheng, X.; Lu, W.; Xie, X. miR-375 is upregulated in acquired paclitaxel resistance in cervical cancer. Br. J. Cancer 2013, 109, 92–99. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Gravina, G.L.; Ranieri, G.; Muzi, P.; Marampon, F.; Mancini, A.; Di Pasquale, B.; Di Clemente, L.; Dolo, V.; D’Alessandro, A.M.; Festuccia, C. Increased levels of DNA methyltransferases are associated with the tumorigenic capacity of prostate cancer cells. Oncol. Rep. 2013, 29, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Porkka, K.P.; Pfeiffer, M.J.; Waltering, K.K.; Vessella, R.L.; Tammela, T.L.; Visakorpi, T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007, 67, 6130–6135. [Google Scholar] [CrossRef] [PubMed]
- Yaqinuddin, A.; Qureshi, S.A.; Qazi, R.; Abbas, F. Down-regulation of DNMT3b in PC3 cells effects locus-specific DNA methylation, and represses cellular growth and migration. Cancer Cell Int. 2008, 8, 13. [Google Scholar] [CrossRef]
- Fuse, M.; Nohata, N.; Kojima, S.; Sakamoto, S.; Chiyomaru, T.; Kawakami, K.; Enokida, H.; Nakagawa, M.; Naya, Y.; Ichikawa, T.; et al. Restoration of miR-145 expression suppresses cell proliferation, migration and invasion in prostate cancer by targeting FSCN1. Int. J. Oncol. 2011, 38, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Goel, A. Non-coding RNAs as liquid biopsy biomarkers in cancer. Br. J. Cancer 2022, 126, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Saviana, M.; Romano, G.; Le, P.; Acunzo, M.; Nana-Sinkam, P. Extracellular Vesicles in Lung Cancer Metastasis and Their Clinical Applications. Cancers 2021, 13, 5633. [Google Scholar] [CrossRef]
- Romano, G.; Saviana, M.; Le, P.; Li, H.; Micalo, L.; Nigita, G.; Acunzo, M.; Nana-Sinkam, P. Non-Coding RNA Editing in Cancer Pathogenesis. Cancers 2020, 12, 1845. [Google Scholar] [CrossRef]
- Uzuner, E.; Ulu, G.T.; Gurler, S.B.; Baran, Y. The Role of MiRNA in Cancer: Pathogenesis, Diagnosis, and Treatment. Methods Mol. Biol. 2022, 2257, 375–422. [Google Scholar] [CrossRef]
- Fuertes, T.; Ramiro, A.R.; de Yebenes, V.G. miRNA-Based Therapies in B Cell Non-Hodgkin Lymphoma. Trends Immunol. 2020, 41, 932–947. [Google Scholar] [CrossRef]
- Pottoo, F.H.; Javed, M.N.; Rahman, J.U.; Abu-Izneid, T.; Khan, F.A. Targeted delivery of miRNA based therapeuticals in the clinical management of Glioblastoma Multiforme. Semin. Cancer Biol. 2021, 69, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.M.; Salvatore, M.; Incoronato, M. miRNA-Based Therapeutics in Breast Cancer: A Systematic Review. Front. Oncol. 2021, 11, 668464. [Google Scholar] [CrossRef] [PubMed]
- De Rubis, G.; Rajeev Krishnan, S.; Bebawy, M. Liquid Biopsies in Cancer Diagnosis, Monitoring, and Prognosis. Trends Pharm. Sci. 2019, 40, 172–186. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, D.; Wu, L.; He, M.; Zhou, X.; Zhang, L.; Zhang, H.; Wang, W.; Zhu, J.; Cheng, W.; et al. Six Serum-Based miRNAs as Potential Diagnostic Biomarkers for Gastric Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 188–196. [Google Scholar] [CrossRef]
- Aiso, T.; Ohtsuka, K.; Ueda, M.; Karita, S.; Yokoyama, T.; Takata, S.; Matsuki, N.; Kondo, H.; Takizawa, H.; Okada, A.A.; et al. Serum levels of candidate microRNA diagnostic markers differ among the stages of non-small-cell lung cancer. Oncol. Lett. 2018, 16, 6643–6651. [Google Scholar] [CrossRef]
- Sudo, K.; Kato, K.; Matsuzaki, J.; Takizawa, S.; Aoki, Y.; Shoji, H.; Iwasa, S.; Honma, Y.; Takashima, A.; Sakamoto, H.; et al. Identification of serum microRNAs predicting the response of esophageal squamous-cell carcinoma to nivolumab. Jpn. J. Clin. Oncol. 2020, 50, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Kong, H.; Hou, Y.; Ge, D.; Huang, W.; Ou, J.; Yang, D.; Zhang, L.; Wu, G.; Song, Y.; et al. Two plasma microRNA panels for diagnosis and subtype discrimination of lung cancer. Lung Cancer 2018, 123, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Lu, Z.; Wang, T.; Huang, Z.; Zhu, W.; Miao, Y. Plasma miRNAs in diagnosis and prognosis of pancreatic cancer: A miRNA expression analysis. Gene 2018, 673, 181–193. [Google Scholar] [CrossRef]
- Herreros-Villanueva, M.; Duran-Sanchon, S.; Martin, A.C.; Perez-Palacios, R.; Vila-Navarro, E.; Marcuello, M.; Diaz-Centeno, M.; Cubiella, J.; Diez, M.S.; Bujanda, L.; et al. Plasma MicroRNA Signature Validation for Early Detection of Colorectal Cancer. Clin. Transl. Gastroenterol. 2019, 10, e00003. [Google Scholar] [CrossRef]
- Okuda, Y.; Shimura, T.; Iwasaki, H.; Fukusada, S.; Nishigaki, R.; Kitagawa, M.; Katano, T.; Okamoto, Y.; Yamada, T.; Horike, S.I.; et al. Urinary microRNA biomarkers for detecting the presence of esophageal cancer. Sci. Rep. 2021, 11, 8508. [Google Scholar] [CrossRef]
- Aftab, M.; Poojary, S.S.; Seshan, V.; Kumar, S.; Agarwal, P.; Tandon, S.; Zutshi, V.; Das, B.C. Urine miRNA signature as a potential non-invasive diagnostic and prognostic biomarker in cervical cancer. Sci. Rep. 2021, 11, 10323. [Google Scholar] [CrossRef]
- Braicu, C.; Buiga, R.; Cojocneanu, R.; Buse, M.; Raduly, L.; Pop, L.A.; Chira, S.; Budisan, L.; Jurj, A.; Ciocan, C.; et al. Connecting the dots between different networks: miRNAs associated with bladder cancer risk and progression. J. Exp. Clin. Cancer Res. 2019, 38, 433. [Google Scholar] [CrossRef]
- Homami, A.; Ghazi, F. MicroRNAs as biomarkers associated with bladder cancer. Med. J. Islam Repub. Iran 2016, 30, 475. [Google Scholar]
- Lin, H.; Shi, X.; Li, H.; Hui, J.; Liu, R.; Chen, Z.; Lu, Y.; Tan, W. Urinary Exosomal miRNAs as biomarkers of bladder Cancer and experimental verification of mechanism of miR-93-5p in bladder Cancer. BMC Cancer 2021, 21, 1293. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Shimura, T.; Kitagawa, M.; Yamada, T.; Nishigaki, R.; Fukusada, S.; Okuda, Y.; Katano, T.; Horike, S.I.; Kataoka, H. A Novel Urinary miRNA Biomarker for Early Detection of Colorectal Cancer. Cancers 2022, 14, 461. [Google Scholar] [CrossRef]
- Koh, Y.; Bustos, M.A.; Moon, J.; Gross, R.; Ramos, R.I.; Ryu, S.; Choe, J.; Lin, S.Y.; Allen, W.M.; Krasne, D.L.; et al. Urine Cell-Free MicroRNAs in Localized Prostate Cancer Patients. Cancers 2022, 14, 2388. [Google Scholar] [CrossRef] [PubMed]
- Kopkova, A.; Sana, J.; Machackova, T.; Vecera, M.; Radova, L.; Trachtova, K.; Vybihal, V.; Smrcka, M.; Kazda, T.; Slaby, O.; et al. Cerebrospinal Fluid MicroRNA Signatures as Diagnostic Biomarkers in Brain Tumors. Cancers 2019, 11, 1546. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Raimondo, M.; Guha, S.; Chen, J.; Diao, L.; Dong, X.; Wallace, M.B.; Killary, A.M.; Frazier, M.L.; Woodward, T.A.; et al. Circulating microRNAs in Pancreatic Juice as Candidate Biomarkers of Pancreatic Cancer. J. Cancer 2014, 5, 696–705. [Google Scholar] [CrossRef]
- Su, Y.; Fang, H.; Jiang, F. Integrating DNA methylation and microRNA biomarkers in sputum for lung cancer detection. Clin. Epigen. 2016, 8, 109. [Google Scholar] [CrossRef]
- Shojaee, S.; Romano, G.; Sanchez, T.M.; Yermakhanova, G.; Saviana, M.; Le, P.; Nigita, G.; Calore, F.; Guthrie, R.; Hess, K.; et al. Extracellular Vesicle MicroRNA in Malignant Pleural Effusion. Genes 2022, 13, 2159. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef]
- Rodriguez-Paredes, M.; Esteller, M. Cancer epigenetics reaches mainstream oncology. Nat. Med. 2011, 17, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Laird, P.W. Cancer epigenetics. Hum. Mol. Genet. 2005, 14, R65–R76. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Power, B.E.; Molloy, P.L. DNA hypomethylation and human diseases. Biochim. Biophys. Acta 2007, 1775, 138–162. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.D.; Timp, W.; Bravo, H.C.; Sabunciyan, S.; Langmead, B.; McDonald, O.G.; Wen, B.; Wu, H.; Liu, Y.; Diep, D.; et al. Increased methylation variation in epigenetic domains across cancer types. Nat. Genet. 2011, 43, 768–775. [Google Scholar] [CrossRef]
- Brennan, K.; Flanagan, J.M. Is there a link between genome-wide hypomethylation in blood and cancer risk? Cancer Prev. Res. 2012, 5, 1345–1357. [Google Scholar] [CrossRef]
- Joyce, B.T.; Gao, T.; Liu, L.; Zheng, Y.; Liu, S.; Zhang, W.; Penedo, F.; Dai, Q.; Schwartz, J.; Baccarelli, A.A.; et al. Longitudinal Study of DNA Methylation of Inflammatory Genes and Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1531–1538. [Google Scholar] [CrossRef]
- Koestler, D.C.; Marsit, C.J.; Christensen, B.C.; Accomando, W.; Langevin, S.M.; Houseman, E.A.; Nelson, H.H.; Karagas, M.R.; Wiencke, J.K.; Kelsey, K.T. Peripheral blood immune cell methylation profiles are associated with nonhematopoietic cancers. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1293–1302. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jin, H. Methylated DNA and microRNA in body fluids as biomarkers for cancer detection. Int. J. Mol. Sci. 2013, 14, 10307–10331. [Google Scholar] [CrossRef]
- Joyce, B.T.; Zheng, Y.; Zhang, Z.; Liu, L.; Kocherginsky, M.; Murphy, R.; Achenbach, C.J.; Musa, J.; Wehbe, F.; Just, A.; et al. miRNA-Processing Gene Methylation and Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2018, 27, 550–557. [Google Scholar] [CrossRef]
- Roman-Gomez, J.; Agirre, X.; Jimenez-Velasco, A.; Arqueros, V.; Vilas-Zornoza, A.; Rodriguez-Otero, P.; Martin-Subero, I.; Garate, L.; Cordeu, L.; San Jose-Eneriz, E.; et al. Epigenetic regulation of microRNAs in acute lymphoblastic leukemia. J. Clin. Oncol. 2009, 27, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Heller, G.; Altenberger, C.; Steiner, I.; Topakian, T.; Ziegler, B.; Tomasich, E.; Lang, G.; End-Pfutzenreuter, A.; Zehetmayer, S.; Dome, B.; et al. DNA methylation of microRNA-coding genes in non-small-cell lung cancer patients. J. Pathol. 2018, 245, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yi, X.M.; Zhang, Z.Y.; Ge, J.P.; Zhou, W.Q. miR-129 predicts prognosis and inhibits cell growth in human prostate carcinoma. Mol. Med. Rep. 2016, 14, 5025–5032. [Google Scholar] [CrossRef]
- Liu, Z.; Dou, C.; Yao, B.; Xu, M.; Ding, L.; Wang, Y.; Jia, Y.; Li, Q.; Zhang, H.; Tu, K.; et al. Methylation-mediated repression of microRNA-129-2 suppresses cell aggressiveness by inhibiting high mobility group box 1 in human hepatocellular carcinoma. Oncotarget 2016, 7, 36909–36923. [Google Scholar] [CrossRef]
- Zhao, F.; Vesprini, D.; Liu, R.S.C.; Olkhov-Mitsel, E.; Klotz, L.H.; Loblaw, A.; Liu, S.K.; Bapat, B. Combining urinary DNA methylation and cell-free microRNA biomarkers for improved monitoring of prostate cancer patients on active surveillance. Urol. Oncol. 2019, 37, 297.e9–297.e17. [Google Scholar] [CrossRef] [PubMed]
- Torres-Ferreira, J.; Ramalho-Carvalho, J.; Gomez, A.; Menezes, F.D.; Freitas, R.; Oliveira, J.; Antunes, L.; Bento, M.J.; Esteller, M.; Henrique, R.; et al. MiR-193b promoter methylation accurately detects prostate cancer in urine sediments and miR-34b/c or miR-129-2 promoter methylation define subsets of clinically aggressive tumors. Mol. Cancer 2017, 16, 26. [Google Scholar] [CrossRef]
- Rogeri, C.D.; Silveira, H.C.S.; Causin, R.L.; Villa, L.L.; Stein, M.D.; de Carvalho, A.C.; Arantes, L.; Scapulatempo-Neto, C.; Possati-Resende, J.C.; Antoniazzi, M.; et al. Methylation of the hsa-miR-124, SOX1, TERT, and LMX1A genes as biomarkers for precursor lesions in cervical cancer. Gynecol. Oncol. 2018, 150, 545–551. [Google Scholar] [CrossRef]
- De Strooper, L.M.A.; Berkhof, J.; Steenbergen, R.D.M.; Lissenberg-Witte, B.I.; Snijders, P.J.F.; Meijer, C.; Heideman, D.A.M. Cervical cancer risk in HPV-positive women after a negative FAM19A4/mir124-2 methylation test: A post hoc analysis in the POBASCAM trial with 14 year follow-up. Int. J. Cancer 2018, 143, 1541–1548. [Google Scholar] [CrossRef]
- Datta, J.; Kutay, H.; Nasser, M.W.; Nuovo, G.J.; Wang, B.; Majumder, S.; Liu, C.G.; Volinia, S.; Croce, C.M.; Schmittgen, T.D.; et al. Methylation mediated silencing of MicroRNA-1 gene and its role in hepatocellular carcinogenesis. Cancer Res. 2008, 68, 5049–5058. [Google Scholar] [CrossRef]
- Yuan, R.; Wang, G.; Xu, Z.; Zhao, H.; Chen, H.; Han, Y.; Wang, B.; Zhou, J.; Hu, H.; Guo, Z.; et al. Up-regulated Circulating miR-106a by DNA Methylation Promised a Potential Diagnostic and Prognostic Marker for Gastric Cancer. Anticancer Agents Med. Chem. 2016, 16, 1093–1100. [Google Scholar] [CrossRef]
- Juergens, R.A.; Wrangle, J.; Vendetti, F.P.; Murphy, S.C.; Zhao, M.; Coleman, B.; Sebree, R.; Rodgers, K.; Hooker, C.M.; Franco, N.; et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 2011, 1, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Liu, X.; Zeng, Y.; Liu, J.; Wu, F. DNA methyltransferase inhibitors combination therapy for the treatment of solid tumor: Mechanism and clinical application. Clin. Epigenet. 2021, 13, 166. [Google Scholar] [CrossRef] [PubMed]
| Cancer | miRNA | Effect on DNA Methylation | Functional Consequence | Ref |
|---|---|---|---|---|
| Brain | miR-152 | DNMT1 | Negatively affects cell invasiveness. miR-152 is upregulated when treating neuroblastoma with ATRA and can be a possible marker for treatment effectiveness. | [63] |
| miR-148a | DNMT1 | miR-148a directly targets DNMT1 in IDHMT gliomas, thereby altering the DNA methylation status of other miRNAs. | [47] | |
| miR-152-3p | DNMT1 | miR-152-3p is proposed to target DNMT1, which targets NF2. DNMT1 methylates and downregulates miR-152-3p. Overexpression of both NF2 and miR-152-3p induces apoptosis. | [64] | |
| Breast | miR-646 | Targets TET1 | It affects DNA demethylation and leads to a downregulation of IRX1, which normally suppresses HIST2H2BE. This promotes malignancy and tumorigenesis in breast cancer’s invasive ductal carcinoma subtype. | [65] |
| miR-200b | Targets DNMT3A | A potential feedback loop exists where miR-200b targets DNMT3A yet is hypermethylated through MYC-recruited DNMT3A. | [66] | |
| Lung | miR-708-5p | Regulates DNMT3a | Upregulation of miR-708-5p is associated with decreased DNMT3a protein levels, leading to elevated levels of CDH1, a metastasis suppressor. | [67] |
| miR-29b | Regulates DNMT1, 3a, and 3b | High miR-29b levels lead to reduced levels of DNMTs 1, 3a, and 3b. Subsequently, PTEN’s promoter is hypomethylated and re-expressed, resulting in tumor growth delay. | [68] | |
| miR-101 | Regulates DNMT3a | Overexpression of miR-101 is associated with reduced DNMT3a levels and global DNA methylation and, ultimately, the re-expression of CDH1, a tumor suppressor. | [69] | |
| miR-185-3p | Regulates MeCP2 | Reduced MeCP2-WT luciferase activity was reported after ectopic miR-185-3p expression, suggesting that miR-185-3p negatively regulates MeCP2, a methylation-related protein, in lung cancer. | [70] | |
| miR-200 family | Regulates MBD2 | A positive correlation between MBD2 and the miR-200 family in lung adenocarcinoma clinical samples has been reported and suggests miR-200 as a potential target for modulating MBD2, a protein involved in the methylation process. | [71] | |
| Ovarian | miR-30a/c-5p | DNMT1 | Directly targets DNMT1 and negatively regulates cisplatin resistance and EMT. | [72] |
| miR-145 | DNMT3A | Indirectly inhibits DNMT3A by targeting c-myc. | [73] | |
| Prostate | miR-34b | DNMT1 and DNMT3b | Reduces DNMT1 and DNMT3b, decreases cell proliferation, inhibits EMT, and induces apoptosis in PC3 and LNCaP cells. | [74] |
| miR-145 | DNMT3b | Targets DNMT3b. Its overexpression sensitizes PC3 cells to irradiation. | [75] | |
| Leukemia | miR-29b | DNMT1, DNMT3A, DNMT3B | Directly targets DNMT3A and DNMT3B and indirectly downregulates DNMT1 by targeting its transactivator SP1. | [76] |
| Hepatocellular | miR-29c-3p | DNMT-3B | Leads to the methylation of LATS1, which inactivates the Hippo signaling pathway. | [77] |
| Cancer | miRNA | Mechanism | Functional Consequence | Ref |
|---|---|---|---|---|
| Brain | miR-340 | Demethylated with ATRA treatment | Targets SOX2 transcription factor, which is responsible for maintaining stem cells undifferentiated. | [78] |
| miR-29b | Hypermethylation | Overexpression of DCST1-AS1 induces the methylation and subsequent downregulation of miR-29b, which normally inhibits cell proliferation. | [79] | |
| miR-155 | Hypermethylation | Targets FAM133A, a negative regulator of cell invasion/migration, by regulating MMP14. | [80] | |
| miR-204-5p | Hypermethylation | Targets Ezrin and inhibits invasion/migration. | [81] | |
| miR-148a | Hypermethylated | DNMT1 hypermethylates miR-148a, which itself directly targets DNMT1. The suppression of miR-148a is linked to DNA methylation changes. | [47] | |
| miR-296-5p | Hypermethylation | Inhibits stem cell self-renewal by targeting HMGA1, a chromatin remodeling protein that regulates the stem cell transcription factor SOX2. | [82] | |
| miR-338-5p | Hypermethylation | Targets the protooncogene EST-1, which is associated with proliferation and invasion of cancer cells. | [83] | |
| miR-204 | Hypermethylation | Targets SOX4 and EphB2 and reduces cell invasion and tumorigenesis. It is downregulated in glioblastoma by hypermethylation. | [84] | |
| miR-20a | Demethylated | DNMT1 methylates and downregulates miR-20a, which normally targets LRIG1; this leads to chemosensitivity. | [85] | |
| Leukemia | miR-124a | Hypermethylation | miR-124a is hypermethylated and downregulated in ALL, leading to cell growth through the CDK6-Rb oncogenic pathway. | [86] |
| miR-708 | Hypermethylation | miR-708 targets IKKβ and regulates the NF-κB pathway. | [87] | |
| miR-181a/b, miR-107, miR-424 | Hypermethylation | Target 3′UTR of the oncogene PLAG1. | [88] | |
| miR-146a | Hypermethylation | Represses NF-KB signaling. | [89] | |
| Myeloma | miR-1258 | Hypermethylation | Targets PDL1. | [90] |
| miR-375 | Hypermethylation | Represses the expression of PDPK1, IGF1R, and JAK2 in HMCLs. | [91] | |
| Breast | miR-9 family | Increased H3K27me3 and H3K9me2 along with hypermethylation of the miR-9-3 promoter CpG island | Results in a downregulation of miR-9-3, which is involved in p53-related apoptotic pathways. | [92] |
| Hypermethylation | Function was not assessed, but there is a statistical significance in miR-9-1 methylation status in breast carcinoma vs benign tumors. | [93] | ||
| Mechanical compression induces DNMT3A-mediated hypermethylation of promoter | Results in a downregulation of miR-9 and an upregulation of its targets (LAMC2, ITGA6, EIF4E), leading to the production of vascular endothelial growth factors. | [94] | ||
| miR-10b* (Note: * refers to an old miRNA nomenclature) | Hypermethylation of two CpG islands upstream of miR-10b/10b* locus | Results in a downregulation of miR-10b*, which was demonstrated to inhibit cell proliferation in vitro and tumor growth in vivo. The targets of miR-10b * include BUB1, PLK1, and CCNA2. | [95] | |
| miR-34c | Hypermethylation | Reduces miR-34c in breast tumor-initiating cells, which normally targets Notch4, reducing migratory ability and EMT. The hypermethylation of the miR-34c promoter prevents the transcription factor Sp1 from binding to its regulatory element. | [96] | |
| miR-124-2 | Hypomethylation | Overexpresses miR-124-2, particularly in young women with breast cancer, and is associated with poor survival in patients. | [97] | |
| miR-125b | Hypermethylation | Reduces miR-125b, which normally targets ETS1, promoting cell cycle arrest and suppression of proliferation and tumorigenesis. | [98] | |
| miR-133a-3p | Hypermethylation | Reduces tumor suppressor miR-133a-3p, leading to an increase in its target MAML1, thereby promoting metastasis, proliferation, invasion, and stemness. A feedback loop exists where MAML1 upregulates DNMT3A, leading to hypermethylation of the promoter of miR-133a-3p. | [99] | |
| miR-195 | Hypermethylation of select upstream CpG islands to promoter | Leads to a downregulation of miR-195 and upregulation of its targets Raf-1 and Ccnd1. miR-195 normally functions to inhibit colony formation and invasion. | [100] | |
| miR-196a-2 | Hypermethylation of CpG island upstream of the miR-196a-2 precursor | The effect of CpG island hypermethylation on mature miR-196a-2 levels was not confirmed, but the increased methylation of this site is correlated with increased breast cancer risk. | [101] | |
| miR-200 family members | Hypermethylation | Reduces miR-200c and miR-141 levels, thereby leading to stem-like/mesenchymal phenotype. | [102] | |
| Hypermethylation of two CpG cites denoted “P1” and “P2” | Reduces miR-200b. P1 hypermethylation is associated with metastatic lymph node samples, while P2 hypermethylation is associated with estrogen or progesterone receptor loss, and its hypomethylation is associated with HER2 and androgen receptor expression. | [103] | ||
| MYC recruits DNMT3A and induces hypermethylation of CpG island to promoter | Reduces miR-200b in triple negative breast cancer, leading to EMT. miR-200b was demonstrated to target DNMT3A, suggesting a regulatory feedback loop. | [66] | ||
| miR-205 | Hypermethylation of CpG sites in the promoter region since DMNT recruitment is no longer inhibited by Mel-18 | Leads to a downregulation of miR-205 and increased level of its targets ZEB1 and ZEB2, which promote epithelial-to-mesenchymal transition. | [104] | |
| miR-216a | Limonin mediates hypomethylation of CpG island in its promoter | Increases levels of miR-216a, which targets WNT3A, inactivating the Wnt/β-catenin signaling cascade and attenuating stemness and adriamycin resistance. | [105] | |
| miR-335 | Genetic copy loss at the miR-335 locus along with hypermethylation of CpG island in the promoter of miR-335/Mest | Reduces levels of the tumor suppressor miR-335, which has a role in suppressing tumor reinitiation, invasion, and metastasis. | [106] | |
| miR-362-3p | Hypermethylation of CLCN5 promoter | Leads to a reduction in tumor suppressive miR-362-3p, which targets p130Cas, a regulator of receptor tyrosine kinase signaling, and normally suppresses cell viability, migration, invasiveness, and tumor growth. | [107] | |
| miR-375 | Hypomethylation | Contributes to an upregulation of miR-375, which targets RASD1 and leads to enhanced ERα signaling and cell proliferation. | [108] | |
| miR-497 | DNMT-mediated methylation of CpG islands to promoter | Leads to a downregulation of miR-497 and upregulation of its targets Raf-1, Ccnd1, GPRC5A, and MUC1. miR-497 normally inhibits colony formation, invasion, and malignancy and promotes apoptosis. The repression of miR-497 is linked to chemotherapy resistance and metastases. | [100,109,110] | |
| miR-663 | Hypomethylation of CpG sites | Leads to an upregulation of miR-663, which targets HSPG2 in multidrug-resistant breast cancer cell lines and leads to chemoresistance. | [111] | |
| miR-892b | Hypermethylation | Leads to a reduction in miR-892b, which normally suppresses several components of the NFκB cascade, including TRAF, TAB3, and TAK1, and decreases tumor growth, metastasis, and angiogenesis in breast cancer cells. | [112] | |
| Colorectal | miR-342 | Methylation of the EVL/miR-342 locus | Resulted in a downregulation of miR-342, potentially inducing anti-apoptotic pathways. | [113] |
| miR-34b/c | Hypermethylation of neighboring CpG island | Resulted in the epigenetic silencing of the tumor suppressors miR-34b/c, whose functions include suppressing colony formation. | [114,115] | |
| miR-34a | Increased methylation of CpG islands in the promoter and transcribed region | Is elevated in primary tumors with liver metastases. When combined with elevated c-Met and β-catenin expression, it has potential prognostic value for distant metastasis. | [116] | |
| miR-1247 | Hypermethylation of promoter regions | Leads to decreased levels of miR-1247 in hypermethylated CRC cell lines and tissue specimens, leading to an upregulation of its target MYCBP2. Introduction of miR-1247 impairs cell viability, induces apoptosis, and inhibits cell motility in vitro while reducing tumor mass and size in vivo. | [117] | |
| miR-484 | Hypermethylation of CpG on the island promoter | Is observed in CRC with microsatellite instability, leading to lower levels of miR-484, which functions as a tumor suppressor and targets CD137L, arresting IL-8 production. | [118] | |
| miR-126 | Methylation of its host gene EGFL7 | Leads to the silencing of miR-126, which targets VEGF and acts as a tumor suppressor by inhibiting cell growth, invasion, migration, and angiogenesis. | [119] | |
| Esophageal | miR-652-5p | Hypermethylation | Reduces the expression of exosomal miR-652-5p, which targets PARG and VEGF, suppressing cell growth and metastasis in vitro and in vivo. | [120] |
| miR-10b-3p | Hypomethylation of CpG islands upstream to the miR-10-3p gene | Increases the expression of miR-10b-3p, which targets FOXO3, inducing cell growth and metastasis in vitro and in vivo. | [121] | |
| miR-128 | Hypermethylation | In response to zinc deficiency, there are increased levels of DNMT1 and DNMT3B. The methylation of miR-128 leads to an upregulation of its target: the pro-inflammatory COX-2. | [122] | |
| miR-126-3p | Hypermethylation of its host gene EGFL7 | Resulted in a downregulation of miR-126-3p, which suppresses proliferation and migration. It targets ADAM9 and subsequently reduces the downstream signaling of the EGFR-AKT pathway. | [123] | |
| miR-216a | Hypermethylation | Resulted in a downregulation of miR-216a, which targets HMGB3 and decreases cell survival through the Wnt/β-catenin pathway. | [124] | |
| miR-124-3p | Hypermethylation of miR-124 loci | Reduces levels of miR-124, which inhibits proliferation, migration, and invasion by targeting EZH2. | [125] | |
| miR-149 | Hypermethylation | Leads to low expression of miR-149, which targets RNF2, impacting the Wnt/β-catenin pathway and suppressing growth and metastases. | [126] | |
| miR-217 | Cigarette smoke condensate induces DNMT2b-dependent hypermethylation of the miR-217 genomic locus. | Leads to reduced levels of miR-217, which targets KLK7 and decreases proliferation and invasion. | [127] | |
| Pancreatic | miR-192 | Hypermethylation | Low levels of miR-192 promote EMT, while its overexpression inhibits cell migration and invasion. Specifically, miR-192 affects the expression of SERPINE1. | [128] |
| miR-615-5p | Hypermethylation | miR-615-5p reduces cell proliferation, migration, invasion, and tumor growth in vivo. It directly targets IGF2, which is responsible for the cancerous phenotype. Rescue of IGF2 expression impairs the tumor suppressive activity of miR-615-5p. | [129] | |
| miR-142-3p | Hypermethylation | DNMT1 is upregulated with p53 mutant pancreatic ductal adenocarcinoma and methylates miR-142-3p in a p53 mutation-dependent manner. The overexpression of miR-142-3p inhibits cell invasion in vitro. | [130] | |
| miR-148a | Hypermethylation | miR-148a is methylated in pancreatic cancer. Restoration of miR-148 downregulates the Wnt/β-catenin pathway and inhibits mesenchymal-to-epithelial transition. | [131] | |
| miR-200a/b | Hypomethylation | miR-200 is primarily hypomethylated in pancreatic cancers, which contributes to its upregulation. | [132] | |
| miR-34a | Hypermethylation | miR-34a is methylated by DNMT1, leading to the activation of the Notch pathway, which promotes drug resistance. | [133] | |
| miR-124 family (124-1/2/3) | Hypermethylation | miR-124 inhibits cell proliferation and metastasis by targeting Rac1, a pro-tumor enhancer that activates the MKK4-JNK-c-Jun pathway. | [134] | |
| Gastric | miR-1271 | Hypermethylation | Leads to lowered levels of miR-1271, which targets TEAD4, potentially leading to an enrichment of the YAP signature and represses MAP2K1 (MEK1), thereby downregulating the ERK/MAPK pathway. | [135] |
| miR-9 | Hypermethylation of promoter-proximal CpG island | Results in a downregulation of miR-9, which is associated with the clinicopathological features of tumor size and lymph node metastasis. | [136] | |
| miR-196-5p | Hypomethylation of HOXA10 promoter | Is associated with increased levels of HOXA10 and miR-196-5p, thereby enhancing proliferation and invasion. TFF1 reconstitution represses HOXA10 and miR-196-5p by inducing methylation of HOXA10. | [137] | |
| miR-7-5p | Hypermethylation | Partially mediates the lower expression of miR-7-5p in stem cells. When cultured with methionine-depleted medium, there is less methylation of the promoter and greater expression of miR-7-5p, which regulates colony formation and cell invasion by targeting Smo and Hes1. | [138] | |
| miR-33b | Hypermethylation | Downregulates miR-33b, which suppresses proliferation, migration, and invasion, possibly by regulating c-Myc. | [139] | |
| miR-27b-3p | Hypermethylation | Leads to decreased miR-27b-3p, which targets GSTP1 and inhibits proliferation, migration, and invasion. | [140] | |
| miR-335 | Hypermethylation | Leads to decreased miR-335, which targets CRKL and represses proliferation and migration while inducing apoptosis and cell cycle arrest at G0/G1 phase. Another potential target of miR-335 is RASA1, which has reported roles in cell invasion and metastasis. | [141,142] | |
| miR-495-3p | Hypermethylation | Downregulates miR-495-3p, which regulates ten oncogenic epigenetic modifiers of HDAC2, KDM1A, KDM2B, KDM5B, CREBBP, EP300, MYST3, SMYD3, DNMT1, and MTA1. | [143] | |
| Lung | miR-886-3p | Hypermethylation | Loss of miR-886-3p and consequently reduced levels of PLK1 and TGF-β1, thereby inhibiting cell invasion, migration, and proliferation. | [144] |
| miR-34b/c | Hypermethylation | Results in lower levels of miR-34b/c. Functional analysis of miR-34b/c revealed that ectopic expression of the miR-34 family suppressed cell proliferation, invasion, and migration in SCLC cell lines. | [145] | |
| miR-224 | Hypomethylation | miR-224 promoter hypomethylation status was linked to high levels of miR-224, promoting cell proliferation and migration by targeting TNFAIP1 and SMAD4, genes known for their respective proapoptotic and anti-migratory functions in lung cancer. | [146] | |
| Let-7a-3 | Hypomethylation | Re-expression of Let-7a-3 following DAC treatment revealed the involvement of DNA hypomethylation in the regulation of let-7a-3 in lung cancer. Ectopic expression of let-7a-3 was associated with anchorage-independent cell growth. | [147] | |
| miR-135b | Hypomethylation | Upregulation of miR-135b was observed in highly invasive CLI-5 lung cancer cells. miR-135b promotes cell invasion, tumor growth, and metastasis by targeting LZTS1 and some players of the Hippo signaling pathway. | [148] | |
| miR-486-5p | Hypermethylation of ANK1 promoter | No functional studies were performed, but an inverse correlation between the hypermethylated ANK1 promoter and intronic miR-486-5p expression levels in NSCLC cell lines was reported. | [149] | |
| miR-126 | Hypermethylation of EGFL7 promoter | Reduces miR-126 levels, which impedes cell invasion in NSCLC by targeting Crk, a key regulator of cell growth, motility, differentiation, and adhesion. | [150,151] | |
| Cervical | miR-124 | Hypermethylation | Reduces levels of mature miR-124, which has tumor suppressor activity in cervical cancer. | [152] |
| miR-375 and miR-196a-1 | Hypermethylation | Downregulation of miR-375 and miR-196a-1 inhibits the proliferation of SiHa cells. | [153] | |
| miR-181a2/181b2 | Hypermethylation | Targets the PIK3R3/Akt/FoxO signaling, and its reduction is associated with poor prognosis and advanced-stage cervical cancer. | [154] | |
| Ovarian | miR-30a/c-5p | Hypermethylation | Inhibits cisplatin resistance and EMT by targeting Snail. | [72] |
| miR-133b | Hypermethylation | Targets PKM2, inhibiting the Warburg effect. | [73] | |
| Endometrial | miR-152 | Hypermethylation | Targets E2F3, MET, and Rictor. | [155] |
| miR-137 | Hypermethylation | Targets EZH2 and LSD1 and inhibits tumor growth. | [156] | |
| miR-129-2 | Hypermethylation | Targets the oncogene SOX4. | [157] | |
| Prostate | miR-152-3p | Hypermethylation | Suppresses cell viability and invasion potential. | [158] |
| miR-130a | Hypermethylation | Inhibits cell viability, increased apoptosis, and reduced invasive potential of prostate cancer cell lines. | [159] | |
| miR-34b | Hypermethylation | Decreases cell proliferation, inhibits EMT, and induces apoptosis in PC3 and LNCaP cells. | [74] | |
| miR-145 | Hypermethylation | Sensitizes PC3 cells to irradiation. | [75] | |
| Hepatocellular | miR-148a | Hypermethylation | Reduces cell proliferation and cell cycle progression. | [160] |
| miR-142 | Hypermethylation | Targets TGF-β, reducing cell viability, proliferation, and angiogenesis. | [161] | |
| C19MC | Hypomethylation | Observed in high T-stage HCC tumors with high invasive ability. | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saviana, M.; Le, P.; Micalo, L.; Del Valle-Morales, D.; Romano, G.; Acunzo, M.; Li, H.; Nana-Sinkam, P. Crosstalk between miRNAs and DNA Methylation in Cancer. Genes 2023, 14, 1075. https://doi.org/10.3390/genes14051075
Saviana M, Le P, Micalo L, Del Valle-Morales D, Romano G, Acunzo M, Li H, Nana-Sinkam P. Crosstalk between miRNAs and DNA Methylation in Cancer. Genes. 2023; 14(5):1075. https://doi.org/10.3390/genes14051075
Chicago/Turabian StyleSaviana, Michela, Patricia Le, Lavender Micalo, Daniel Del Valle-Morales, Giulia Romano, Mario Acunzo, Howard Li, and Patrick Nana-Sinkam. 2023. "Crosstalk between miRNAs and DNA Methylation in Cancer" Genes 14, no. 5: 1075. https://doi.org/10.3390/genes14051075
APA StyleSaviana, M., Le, P., Micalo, L., Del Valle-Morales, D., Romano, G., Acunzo, M., Li, H., & Nana-Sinkam, P. (2023). Crosstalk between miRNAs and DNA Methylation in Cancer. Genes, 14(5), 1075. https://doi.org/10.3390/genes14051075








