The Genome of a Pigeonpea Compatible Rhizobial Strain ‘10ap3’ Appears to Lack Common Nodulation Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Rhizobial Strain and DNA Extraction
2.2. Sequencing, DNA Libraries and Cluster Generation
3. Results
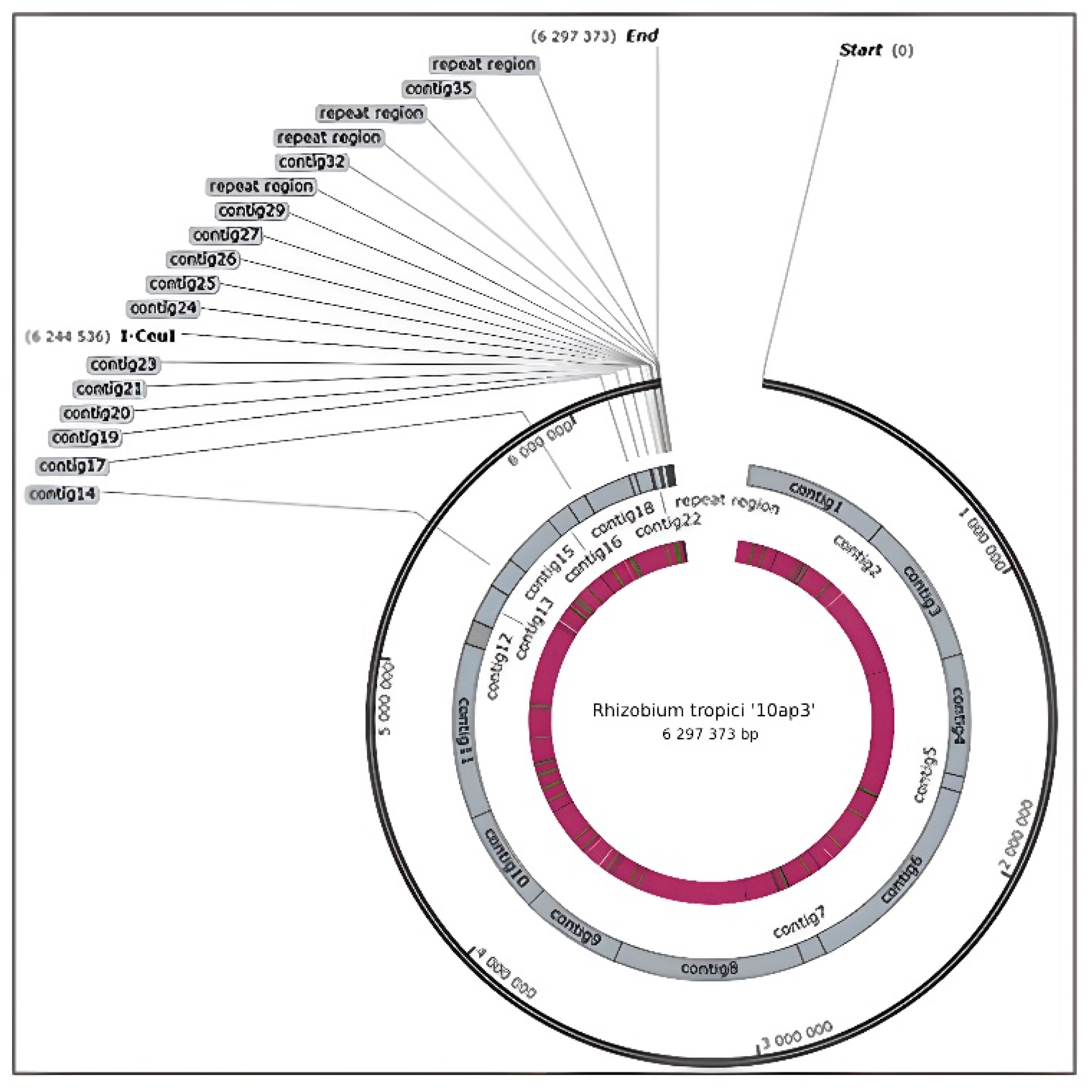
3.1. Genome Sequencing and Size
3.2. Key Genes in the Genome
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amarteifio, J.O.; Munthali, D.C.; Karikari, S.K.; Morake, T.K. The Composition of Pigeonpeas (Cajanus cajan (L. Millsp.) grown in Botswana. Plant Food. Hum. Nutr. 2002, 57, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, J.; Grewal, S.K.; Singh, I.; Kaur, J. Evaluation of Nutritional Quality and Antioxidant Potential of Pigeonpea Genotypes. J. Food Sci. Technol. 2017, 54, 3598–3611. [Google Scholar] [CrossRef] [PubMed]
- Mekonen, T.; Tolera, A.; Nurfeta, A.; Bradford, B.; Mekasha, A. Location and Plant Spacing Affect Biomass Yield and Nutritional Value of Pigeonpea Forage. Agron. J. 2021, 114, 228–247. [Google Scholar] [CrossRef]
- Gwata, E.T.; Siambi, M. Genetic Enhancement of Pigeonpea for High Latitude Areas in Southern Africa. Afric. J. Biotechnol. 2009, 8, 18. [Google Scholar]
- Gwata, E.T. Potential Impact of Edible Tropical Legumes on Crop Productivity in the Small-Holder Sector in Sub-Saharan Africa. J. Food Agric. Environ. 2010, 8, 939–944. [Google Scholar]
- Bopape, F.L.; Beukes, C.W.; Katlego, K.; Hassen, A.I.; Steenkamp, E.T.; Gwata, E.T. Symbiotic Performance and Characterization of Pigeonpea (Cajanus cajan L. Millsp.) Rhizobia Occurring in South African Soils. Agriculture 2023, 13, 30. [Google Scholar] [CrossRef]
- Jaiswal, S.K.; Msimbira, L.A.; Dakora, F.D. Phylogenetically Diverse Group of Native Bacterial Symbionts Isolated from Root Nodules of Groundnut (Arachis hypogaea L.) in South Africa. Syst. Appl. Microbiol. 2007, 40, 215–226. [Google Scholar] [CrossRef]
- Steenkamp, E.T.; Stępkowski, T.; Przymusiak, A.; Botha, W.J.; Law, I.J. Cowpea and Peanut in Southern Africa are Nodulated by Diverse Bradyrhizobium Strains Harboring Nodulation Genes that Belong to the Large Pantropical Clade Common in Africa. Molec. Phylog. Evol. 2008, 48, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Degefu, T.; Wolde-meskel, E.; Adem, M.; Fikre, A.; Amede, T.; Ijiewo, C. Morphophysiological Diversity of Rhizobia Nodulating Pigeonpea (Cajanus cajan L. Millsp.) Growing in Ethiopia. Afric. J. Biotech. 2018, 17, 167–177. [Google Scholar]
- Oldroyd, G.E.; Murray, J.D.; Poole, P.S.; Downie, J.A. The Rules of Engagement in the Legume-Rhizobial Symbiosis. Ann. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Zhu, H. Genetic and Molecular Mechanism Underlying Symbiotic Specificity in Legume-Rhizobium Interactions. Front. Plant Sci. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Remigi, P.; Zhu, J.; Young, J.P.W.; Masson-Boivin, C. Symbiosis Within Symbiosis: Evolving Nitrogen-Fixing Legume Symbionts. Trends Microbiol. 2016, 24, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Broughton, W.J.; Zhang, F.; Perret, X.; Staehelin, C. Signal Exchanged Between Legumes and Rhizobium: Agricultural Uses and Perspectives. Plant Soil 2003, 252, 129–137. [Google Scholar] [CrossRef]
- Laranjo, M.; Alexandre, A.; Oliveira, S. Legume growth-promoting rhizobia: An Overview on the Mesorhizobium Genus. Microbiol. Res. 2014, 169, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Lindstrom, K.; Mousavi, S.A. Effectiveness of Nitrogen Fixation in Rhizobia. Microb. Biotechnol. 2019, 13, 1314–1335. [Google Scholar] [CrossRef]
- Perret, X.; Staehelin, C.; Broughton, W.J. Molecular Basis of Symbiotic Promiscuity. Microbiol. Molec. Biol. Rev. 2000, 64, 180–201. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Host-Secreted Antimicrobial Peptide Enforces Symbiotic Selectivity in Medicago truncatula. Proc. Natl. Acad. Sci. USA 2017, 114, 6854–6859. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Q.; Fedorova, E.; Liu, J.; Qin, Q.; Zheng, Q. Microsymbiont Discrimination Mediated by a Host-Secreted Peptide in Medicago truncatula. Proc. Natl. Acad. Sci. USA 2017, 114, 6848–6853. [Google Scholar] [CrossRef]
- Kosugi, S.; Momozawa, Y.; Liu, X.; Terao, C.; Kubo, M.; Kamatani, Y. Comprehensive Evaluation of Structural Variation Detection Algorithms for Whole Genome Sequencing. Gen. Biol. 2019, 20, 1–18. [Google Scholar] [CrossRef]
- Bopape, F.L.; Gwata, E.T.; Hassen, A.I.; Zhou, M.M. Symbiotic Efficiency of Pigeonpea (Cajanus cajan) with Different Sources of Nitrogen. Plant Genet. Resour. 2021, 19, 1–4. [Google Scholar] [CrossRef]
- Gautam, S.S.; Rajendra, K.C.; Leong, K.W.C.; Mac Aogáin, M.; O’Toole, R.F. Step-by Step Beginner’s Protocol for Whole Genome Sequencing of Human Bacterial Pathogens. J. Biol. Meth. 2019, 6, 1–9. [Google Scholar] [CrossRef]
- Tatusova, T.; DiCuccio, M.; Badretdin, A.; Chetvernin, V.; Nawrocki, E.P.; Zaslavsky, L.; Lomsadze, A.; Pruitt, K.D.; Borodovsky, M.; Ostell, J. NCBI Prokaryotic Genome Annotation Pipeline. Nucleic Acids Res. 2016, 44, 6614–6624. [Google Scholar] [CrossRef]
- Kwong, J.C.; McCallum, N.; Sintchenko, V.; Howden, B.P. Whole Genome Sequencing in Clinical and Public Health Microbiology. Pathology 2015, 47, 199–210. [Google Scholar] [CrossRef]
- Pauline, C.N.; Kirkness, E.F. Whole Genome Sequencing. Meth. Molec. Biol. 2010, 628, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Ormeño-Orrillo, E.; Menna, P.; Almeida, L.G.P.; Ollero, F.J.; Nicolás, M.F.; Rodrigues, E.P.; Nakatani, A.S.; Batista, J.S.S.; Chueire, L.M.O.; Souza, R.C.; et al. Genomic Basis of Broad Host Range and Environmental Adaptability of Rhizobium Tropici CIAT 899 and Rhizobium Sp. PRF 81 Which are Used in Inoculants for Common Bean (Phaseolus vulgaris L.). BMC Genom. 2012, 13, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Garcίa, P.; Jimѐnez-Guerrero, I.; Jacott, C.N.; López-Baena, J.; Ollero, F.J.; Del Cerro, P.; Pѐrez-Montaño, F. The Rhizobium Tropici CIAT 899 NodD2 Protein Promotes Symbiosis and Extends Rhizobia Nodulation Range by Constitutive Nodulation Factor Synthesis. J. Exp. Bot. 2022, 73, 6931–6941. [Google Scholar] [CrossRef] [PubMed]
- Giraud, E.; Moulin, L.; Vallenet, D.; Barbe, V.; Cytryn, E.; Avarre, J.-C.; Jaubert, M.; Simon, D.; Cartieaux, F.; Prin, Y.; et al. Legumes Symbioses: Absence of Nod Genes in Photosynthetic Bradyrhizobia. Science 2007, 316, 1307–1312. [Google Scholar] [CrossRef]
- Okazaki, S.; Hattori, Y.; Saeki, K. The Mesorhizobium loti purB Gene is Involved in Infection Thread Formation and Nodule Development in Lotus japonicas. J. Bacteriol. 2007, 189, 8347–8352. [Google Scholar] [CrossRef]
- Inomura, K.; Deutsch, C.; Masuda, T.; Prášil, O.; Follows, M.J. Quantitative Models of Nitrogen-Fixing Organisms. Comput. Struct. Biotechnol. J. 2020, 18, 3905–3924. [Google Scholar] [CrossRef]
- Xie, B.; Chen, D.; Cheng, G. Effect of the PurL Gene Expression Level on the Competitive Nodulation Ability of Sinorhizobium fredii. Curr. Microbiol. 2009, 59, 193–198. [Google Scholar] [CrossRef]
- Young, J.P.W.; Crossman, L.C.; Johnston, A.W.; Thomson, N.R.; Ghazoui, Z.F.; Hull, K.H.; Wexler, M.; Curson, A.R.J.; Todd, J.D.; Poole, P.S.; et al. The Genome of Rhizobium leguminosarum has Recognizable Core and Accessory Components. Genom. Biol. 2006, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Reeve, W.; O’Hara, G.; Chain, P.; Ardley, J.; Bräu, L.; Nandesena, K.; Tiwari, R.; Malfatti, S.; Kiss, H.; Lapidus, A.; et al. Complete Genome Sequence of Rhizobium leguminosarum bv trifolii strain WSM2304, an Effective Microsymbiont of the South American Clover Trifolium polymorphum. Stand. Genom. Sci. 2010, 2, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, Y.; Hemissi, I.; Salem, I.B.; Mejri, S.; Saidi, M.; Belhadj, O. Potential of Rhizobia in Improving Nitrogen Fixation and Yields of Legumes. Symbiosis 2018, 107, 107–117. [Google Scholar]
- Atieno, M.; Lesueur, D. Opportunities for Improved Legume Inoculants: Enhanced Stress Tolerance of Rhizobia and Benefits to Agroecosystems. Symbiosis 2019, 77, 191–205. [Google Scholar] [CrossRef]
- Sindhu, S.; Dahiya, A.; Gera, R.; Sindhu, S.S. Mitigation of Abiotic Stress in Legume-Nodulating Rhizobia for Sustainable Crop Production. Agric. Res. 2020, 9, 444–459. [Google Scholar] [CrossRef]
- Brear, E.M.; Day, D.A.; Smith, P.M.C. Iron: An Essential Micronutrient for the Legume-Rhizobium Symbiosis. Front. Plant Sci. 2013, 4, 1–15. [Google Scholar] [CrossRef]
- Geetha, S.J.; Joshi, S.J. Engineering Rhizobial Bio-Inoculants: A Strategy to Improve Iron Nutrition. Sci. World J. 2013, 2013, 1–15. [Google Scholar]
- Delepelaire, P. Bacterial ABC Transporters of Iron Containing Compounds. Res. Microbiol. 2019, 170, 345–357. [Google Scholar] [CrossRef]
- Chhibber, S.; Nag, D.; Bansal, S. Inhibiting Biofilm Formation by Klebsiella pneumoniae B5055 Using an Iron Antagonizing Molecule and a Bacteriophage. BMC Microbiol. 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Simões, L.C.; Simões, M.; Vieira, M.J. Biofilm Interactions Between Distinct Bacterial Genera Isolated from Drinking Water. Appl. Environ. Microbiol. 2007, 73, 6192–6200. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in Environmental Research: Roles and Applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.C.; Maheshwari, D.K. Role of PGPR in Integrated Nutrient Management of Oil Seed Crops. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–15. [Google Scholar]
- Harrow, J.; Nagy, A.; Reymond, A.; Alioto, T.; Patty, L.; Antonarakis, S.E.; Guigo, R. Identifying Protein-coding Genes in Genomic Sequences. Genom. Biol. 2009, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]



| Genome Feature | Rhizobial Strain ‘10ap3’ |
|---|---|
| Genome size (bp) | 6,297,373 |
| G + C content (%) | 60 |
| Ribosomal RNA operons | 3 |
| Transfer RNAs | 45 |
| Protein coding genes/sequences | 5961 |
| Assigned functionality (%) | 5833 |
| Genes (total) (%) | 6013 |
| CDSs (total) (%) | 5961 |
| Genes (coding) (%) | 5833 |
| CDSs (with protein) (%) | 5833 |
| Number of plasmids | 0 |
| ncRNAs | 4 |
| Pseudogenes | 128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bopape, F.L.; Hassen, A.I.; Chiulele, R.M.; Shonhai, A.; Gwata, E.T. The Genome of a Pigeonpea Compatible Rhizobial Strain ‘10ap3’ Appears to Lack Common Nodulation Genes. Genes 2023, 14, 1084. https://doi.org/10.3390/genes14051084
Bopape FL, Hassen AI, Chiulele RM, Shonhai A, Gwata ET. The Genome of a Pigeonpea Compatible Rhizobial Strain ‘10ap3’ Appears to Lack Common Nodulation Genes. Genes. 2023; 14(5):1084. https://doi.org/10.3390/genes14051084
Chicago/Turabian StyleBopape, Francina L., Ahmed Idris Hassen, Rogerio M. Chiulele, Addmore Shonhai, and Eastonce T. Gwata. 2023. "The Genome of a Pigeonpea Compatible Rhizobial Strain ‘10ap3’ Appears to Lack Common Nodulation Genes" Genes 14, no. 5: 1084. https://doi.org/10.3390/genes14051084
APA StyleBopape, F. L., Hassen, A. I., Chiulele, R. M., Shonhai, A., & Gwata, E. T. (2023). The Genome of a Pigeonpea Compatible Rhizobial Strain ‘10ap3’ Appears to Lack Common Nodulation Genes. Genes, 14(5), 1084. https://doi.org/10.3390/genes14051084