Direct and Secondary Transfer of Touch DNA on a Credit Card: Evidence Evaluation Given Activity Level Propositions and Application of Bayesian Networks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Circumstances
2.2. Phase I—Shedder Status
2.3. Phase II—Direct Transfer
2.4. Phase III—Secondary Transfer
2.5. Phase IV—Laboratory Processing and Statistical Analysis
3. Results
3.1. Phase I—Shedder Status
3.2. Phases II and III—Direct and Secondary Transfer
3.3. Background DNA
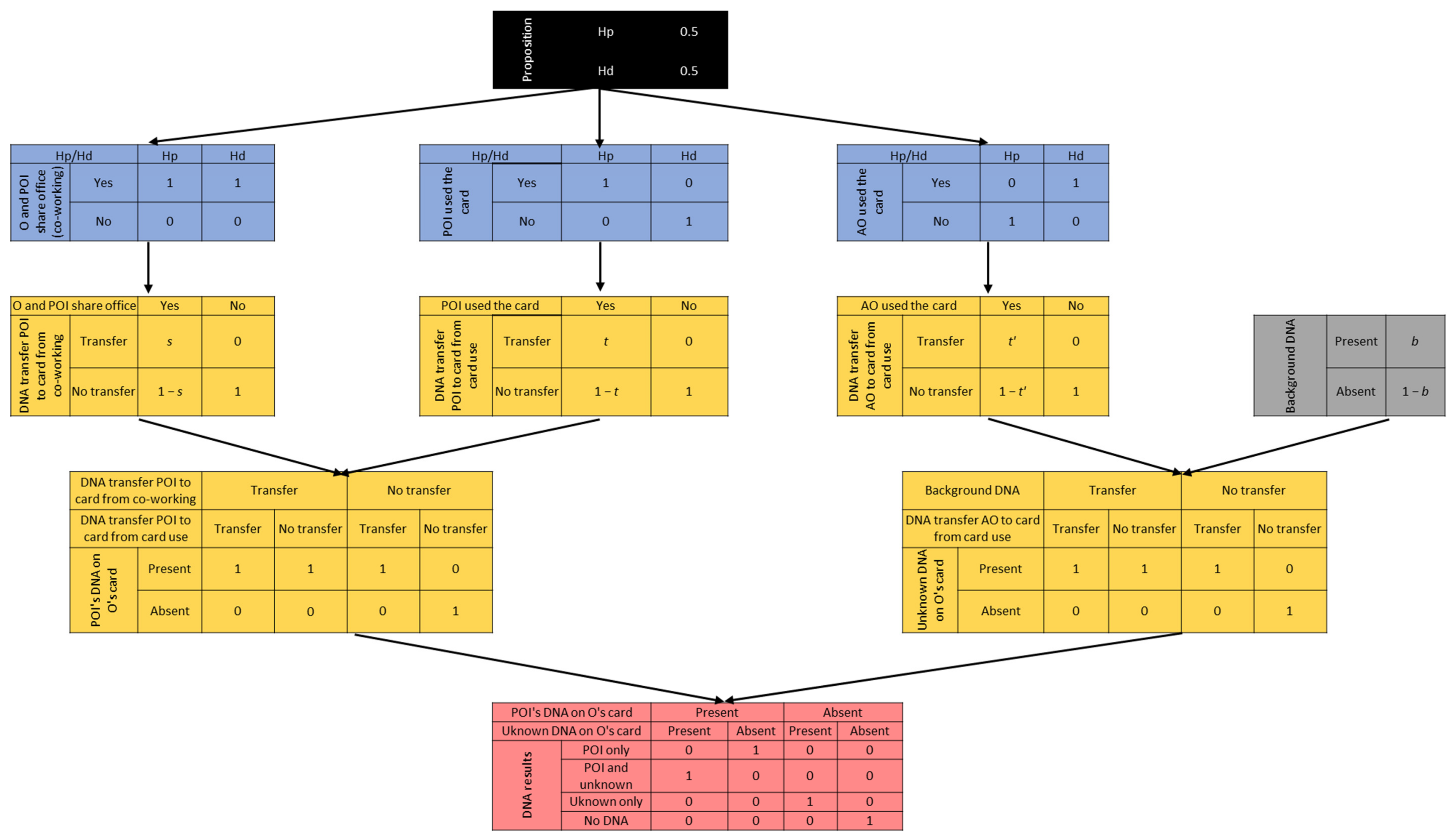
3.4. Constructing a Bayesian Network
- O and POI share the office workspace;
- O often left her credit card on POI and other co-workers’ desks;
- If POI did not use the card, someone else did (evidence given by the bank withdrawal).
- POI and O share the workspace;
- POI used the card (without gloves);
- An unknown alternative offender (AO) used the card (without gloves).
- POI’s DNA is on the card;
- Unknown DNA is on the card.
- t = the probability that a DNA amount, leading to a reportable major contribution from POI, will be transferred from POI and recovered on the card, given that POI used the card.
- t’ = the probability that a DNA amount, leading to a reportable major contribution from an unknown, alternative offender (AO), will be transferred from UNK and recovered on the card, given that UNK used the card.
- s = the probability that a DNA amount, leading to a reportable major contribution from POI, will be transferred and recovered on O’s card, given that O and POI share the same workspace, and O left the card on POI’s desk.
- b = probability of observing background DNA on the card.
- 1st Level: probability of log10LRϕ > 6 = 9/11 = 0.818;
- 2nd Level: probability of POI being a major contributor (ng) = 7/9 = 0.778.
- 1st Level: probability of log10LRϕ > 6 = 4/11 = 0.364;
- 2nd Level: probability of POI being a major contributor (ng) = 2/4 = 0.5.

| DNA Analysis Outcome | Formulae Pr(E|Hp)/Pr(E|Hd) | LRα |
|---|---|---|
| POI only | (1−((1−s) (1−t))) (1−b)/s(1−t’) (1−b) | 10.6003 |
| POI and unknown | (1−((1−s) (1−t)))b/s(1−((1−t’) (1−b))) | 3.507 |
| Unknown only | (1−s) (1−t)b/(1−((1−t’) (1−b))) (1−s) | 0.331 |
| No DNA | (1−s) (1−t) (1−b)/(1−s) (1−t’) (1−b) | 1 |
4. Discussion
4.1. Phase I–Shedder Status
4.2. Phase II—Direct and Secondary Transfer
4.3. Background DNA
4.4. General Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynch, M. Science, Truth, and Forensic Cultures: The Exceptional Legal Status of DNA Evidence. Stud. Hist. Philos. Sci. Part C Stud. Hist. Philos. Biol. Biomed. Sci. 2013, 44, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Wickenheiser, R.A. Trace DNA: A Review, Discussion of Theory, and Application of the Transfer of Trace Quantities of DNA through Skin Contact. J. Forensic Sci. 2002, 47, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Burrill, J.; Daniel, B.; Frascione, N. A Review of Trace “Touch DNA” Deposits: Variability Factors and an Exploration of Cellular Composition. Forensic Sci. Int. Genet. 2019, 39, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, A.; Murray, C.; Whitaker, J.; Tully, G.; Gill, P. The Propensity of Individuals to Deposit DNA and Secondary Transfer of Low Level DNA from Individuals to Inert Surfaces. Forensic Sci. Int. 2002, 129, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Quinones, I.; Daniel, B. Cell Free DNA as a Component of Forensic Evidence Recovered from Touched Surfaces. Forensic Sci. Int. Genet. 2012, 6, 26–30. [Google Scholar] [CrossRef]
- van Oorschot, R.A.H.; Szkuta, B.; Meakin, G.E.; Kokshoorn, B.; Goray, M. DNA Transfer in Forensic Science: A Review. Forensic Sci. Int. Genet. 2019, 38, 140–166. [Google Scholar] [CrossRef]
- Miller, M.; Philpott, M.K.; Olsen, A.; Tootham, M.; Yadavalli, V.K.; Ehrhardt, C.J. Technical Note: Survey of Extracellular and Cell-Pellet-Associated DNA from ‘Touch’/Trace Samples. Forensic Sci. Int. 2021, 318, 110557. [Google Scholar] [CrossRef]
- Johannessen, H.; Gill, P.; Roseth, A.; Fonneløp, A.E. Determination of Shedder Status: A Comparison of Two Methods Involving Cell Counting in Fingerprints and the DNA Analysis of Handheld Tubes. Forensic Sci. Int. Genet. 2021, 53, 102541. [Google Scholar] [CrossRef]
- Jansson, L.; Swensson, M.; Gifvars, E.; Hedell, R.; Forsberg, C.; Ansell, R.; Hedman, J. Individual Shedder Status and the Origin of Touch DNA. Forensic Sci. Int. Genet. 2022, 56, 102626. [Google Scholar] [CrossRef]
- Ladd, C.; Adamowicz, M.S.; Bourke, M.T.; Scherczinger, C.A.; Lee, H.C. A Systematic Analysis of Secondary DNA Transfer. J. Forensic Sci. 1999, 44, 1270–1272. [Google Scholar] [CrossRef]
- Kamphausen, T.; Schadendorf, D.; von Wurmb-Schwark, N.; Bajanowski, T.; Poetsch, M. Good Shedder or Bad Shedder—The Influence of Skin Diseases on Forensic DNA Analysis from Epithelial Abrasions. Int. J. Leg. Med. 2012, 126, 179–183. [Google Scholar] [CrossRef]
- Phipps, M.; Petricevic, S. The Tendency of Individuals to Transfer DNA to Handled Items. Forensic Sci. Int. 2007, 168, 162–168. [Google Scholar] [CrossRef]
- Zoppis, S.; Muciaccia, B.; D’Alessio, A.; Ziparo, E.; Vecchiotti, C.; Filippini, A. DNA Fingerprinting Secondary Transfer from Different Skin Areas: Morphological and Genetic Studies. Forensic Sci. Int. Genet. 2014, 11, 137–143. [Google Scholar] [CrossRef]
- Goray, M.; Fowler, S.; Szkuta, B.; van Oorschot, R.A.H. Shedder Status—An Analysis of Self and Non-Self DNA in Multiple Handprints Deposited by the Same Individuals over Time. Forensic Sci. Int. Genet. 2016, 23, 190–196. [Google Scholar] [CrossRef]
- Tobias, S.H.A.; Jacques, G.S.; Morgan, R.M.; Meakin, G.E. The Effect of Pressure on DNA Deposition by Touch. Forensic Sci. Int. Genet. Suppl. Ser. 2017, 6, e12–e14. [Google Scholar] [CrossRef] [Green Version]
- Gosch, A.; Courts, C. On DNA Transfer: The Lack and Difficulty of Systematic Research and How to Do It Better. Forensic Sci. Int. Genet. 2019, 40, 24–36. [Google Scholar] [CrossRef]
- Poetsch, M.; Bajanowski, T.; Kamphausen, T. Influence of an Individual’s Age on the Amount and Interpretability of DNA Left on Touched Items. Int. J. Leg. Med. 2013, 127, 1093–1096. [Google Scholar] [CrossRef]
- van Oorschot, R.A.H.; Glavich, G.; Mitchell, R.J. Persistence of DNA Deposited by the Original User on Objects after Subsequent Use by a Second Person. Forensic Sci. Int. Genet. 2014, 8, 219–225. [Google Scholar] [CrossRef]
- Lehmann, V.; Mitchell, R.; Ballantyne, K.; van Oorschot, R. Following the Transfer of DNA: How Does the Presence of Background DNA Affect the Transfer and Detection of a Target Source of DNA? Forensic Sci. Int. Genet. 2015, 19, 68–75. [Google Scholar] [CrossRef]
- Samie, L.; Hicks, T.; Castella, V.; Taroni, F. Stabbing Simulations and DNA Transfer. Forensic Sci. Int. Genet. 2016, 22, 73–80. [Google Scholar] [CrossRef]
- Oldoni, F.; Castella, V.; Hall, D. Shedding Light on the Relative DNA Contribution of Two Persons Handling the Same Object. Forensic Sci. Int. Genet. 2016, 24, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Fonneløp, A.E.; Ramse, M.; Egeland, T.; Gill, P. The Implications of Shedder Status and Background DNA on Direct and Secondary Transfer in an Attack Scenario. Forensic Sci. Int. Genet. 2017, 29, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Meakin, G.E.; Butcher, E.V.; van Oorschot, R.A.H.; Morgan, R.M. Trace DNA Evidence Dynamics: An Investigation into the Deposition and Persistence of Directly- and Indirectly-Transferred DNA on Regularly-Used Knives. Forensic Sci. Int. Genet. 2017, 29, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, K.; Arsenault, H.; Taylor, C.; Volgin, L.; Millman, J. Transfer and Persistence of DNA on Items Routinely Encountered in Forensic Casework Following Habitual and Short-Duration One-Time Use. Forensic Sci. Int. Genet. 2022, 60, 102737. [Google Scholar] [CrossRef] [PubMed]
- Goray, M.; Mitchell, J.R.; van Oorschot, R.A.H. Evaluation of Multiple Transfer of DNA Using Mock Case Scenarios. Leg. Med. 2012, 14, 40–46. [Google Scholar] [CrossRef]
- Taroni, F.; Biedermann, A.; Vuille, J.; Morling, N. Whose DNA Is This? How Relevant a Question? (A Note for Forensic Scientists). Forensic Sci. Int. Genet. 2013, 7, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Champod, C. DNA Transfer: Informed Judgment or Mere Guesswork? Front. Genet. 2013, 4, 300. [Google Scholar] [CrossRef] [Green Version]
- Goray, M.; van Oorschot, R.A.H. The Complexities of DNA Transfer during a Social Setting. Leg. Med. 2015, 17, 82–91. [Google Scholar] [CrossRef]
- Fonneløp, A.E.; Egeland, T.; Gill, P. Secondary and Subsequent DNA Transfer during Criminal Investigation. Forensic Sci. Int. Genet. 2015, 17, 155–162. [Google Scholar] [CrossRef]
- Cale, C.M.; Earll, M.E.; Latham, K.E.; Bush, G.L. Could Secondary DNA Transfer Falsely Place Someone at the Scene of a Crime? J. Forensic Sci. 2016, 61, 196–203. [Google Scholar] [CrossRef]
- Taylor, D.; Biedermann, A.; Samie, L.; Pun, K.-M.; Hicks, T.; Champod, C. Helping to Distinguish Primary from Secondary Transfer Events for Trace DNA. Forensic Sci. Int. Genet. 2017, 28, 155–177. [Google Scholar] [CrossRef] [Green Version]
- Cook, R.; Evett, I.W.; Jackson, G.; Jones, P.J.; Lambert, J.A. A Hierarchy of Propositions: Deciding Which Level to Address in Casework. Sci. Justice 1998, 38, 231–239. [Google Scholar] [CrossRef]
- Evett, I.W.; Jackson, G.; Lambert, J.A. More on the Hierarchy of Propositions: Exploring the Distinction between Explanations and Propositions. Sci. Justice 2000, 40, 3–10. [Google Scholar] [CrossRef]
- Gill, P.; Hicks, T.; Butler, J.M.; Connolly, E.; Gusmão, L.; Kokshoorn, B.; Morling, N.; van Oorschot, R.A.H.; Parson, W.; Prinz, M.; et al. DNA Commission of the International Society for Forensic Genetics: Assessing the Value of Forensic Biological Evidence—Guidelines Highlighting the Importance of Propositions. Part I: Evaluation of DNA profiling comparisons given (sub-) source propositions. Forensic Sci. Int. Genet. 2018, 36, 189–202. [Google Scholar] [CrossRef] [Green Version]
- Taylor, D.; Kokshoorn, B.; Biedermann, A. Evaluation of Forensic Genetics Findings given Activity Level Propositions: A Review. Forensic Sci. Int. Genet. 2018, 36, 34–49. [Google Scholar] [CrossRef] [Green Version]
- Gill, P.; Hicks, T.; Butler, J.M.; Connolly, E.; Gusmão, L.; Kokshoorn, B.; Morling, N.; van Oorschot, R.A.H.; Parson, W.; Prinz, M.; et al. DNA Commission of the International Society for Forensic Genetics: Assessing the Value of Forensic Biological Evidence—Guidelines Highlighting the Importance of Propositions. Part II: Evaluation of Biological Traces Considering Activity Level Propositions. Forensic Sci. Int. Genet. 2020, 44, 102186. [Google Scholar] [CrossRef] [Green Version]
- Willis, S.; European Network of Forensic Science Institutes. ENFSI Guideline for Evaluative Reporting in Forensic Science—Strengthening the Evaluation of Forensic Results across Europe (STEOFRAE). 2015. Available online: https://enfsi.eu/wp-content/uploads/2016/09/m1_guideline.pdf (accessed on 12 April 2023).
- De March, I.; Taroni, F. Bayesian Networks and Dissonant Items of Evidence: A Case Study. Forensic Sci. Int. Genet. 2020, 44, 102172. [Google Scholar] [CrossRef] [Green Version]
- Perepechina, I.O. Considering DNA Transfer Issues in a Retrospective Analysis of Forensic Examinations. Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 853–855. [Google Scholar] [CrossRef]
- Meakin, G.; Jamieson, A. DNA Transfer: Review and Implications for Casework. Forensic Sci. Int. Genet. 2013, 7, 434–443. [Google Scholar] [CrossRef]
- van den Berge, M.; Ozcanhan, G.; Zijlstra, S.; Lindenbergh, A.; Sijen, T. Prevalence of Human Cell Material: DNA and RNA Profiling of Public and Private Objects and after Activity Scenarios. Forensic Sci. Int. Genet. 2016, 21, 81–89. [Google Scholar] [CrossRef]
- Szkuta, B.; Ballantyne, K.N.; van Oorschot, R.A.H. Transfer and Persistence of DNA on the Hands and the Influence of Activities Performed. Forensic Sci. Int. Genet. 2017, 28, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Szkuta, B.; Ballantyne, K.N.; Kokshoorn, B.; van Oorschot, R.A.H. Transfer and Persistence of Non-Self DNA on Hands over Time: Using Empirical Data to Evaluate DNA Evidence given Activity Level Propositions. Forensic Sci. Int. Genet. 2018, 33, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.; Hicks, T.; Champod, C. Using Sensitivity Analyses in Bayesian Networks to Highlight the Impact of Data Paucity and Direct Future Analyses: A Contribution to the Debate on Measuring and Reporting the Precision of Likelihood Ratios. Sci. Justice 2016, 56, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.; Biedermann, A.; Hicks, T.; Champod, C. A Template for Constructing Bayesian Networks in Forensic Biology Cases When Considering Activity Level Propositions. Forensic Sci. Int. Genet. 2018, 33, 136–146. [Google Scholar] [CrossRef] [Green Version]
- Schaapveld, T.E.M.; Opperman, S.L.; Harbison, S. Bayesian Networks for the Interpretation of Biological Evidence. WIREs Forensic Sci. 2019, 1, e1325. [Google Scholar] [CrossRef]
- Fenton, N.E.; Neil, M. Risk Assessment and Decision Analysis with Bayesian Networks; Taylor & Francis: Boca Raton, FL, USA, 2012; ISBN 978-1-4398-0910-5. [Google Scholar]
- Taroni, F. Bayesian Networks for Probabilistic Inference and Decision Analysis in Forensic Science, 2nd ed.; Wiley: Chichester, UK; Hoboken, NJ, USA, 2014; ISBN 978-1-118-91474-8. [Google Scholar]
- Gill, P.; Bleka, Ø.; Roseth, A.; Fonneløp, A.E. An L R Framework Incorporating Sensitivity Analysis to Model Multiple Direct and Secondary Transfer Events on Skin Surface. Forensic Sci. Int. Genet. 2021, 53, 102509. [Google Scholar] [CrossRef]
- Johannessen, H.; Gill, P.; Shanthan, G.; Fonneløp, A.E. Transfer, Persistence and Recovery of DNA and MRNA Vaginal Mucosa Markers after Intimate and Social Contact with Bayesian Network Analysis for Activity Level Reporting. Forensic Sci. Int. Genet. 2022, 60, 102750. [Google Scholar] [CrossRef]
- Mayuoni-Kirshenbaum, L.; Waiskopf, O.; Finkelstein, N.; Pasternak, Z. How Did the DNA of a Suspect Get to the Crime Scene? A Practical Study in DNA Transfer during Lock-Picking. Aust. J. Forensic Sci. 2022, 54, 15–25. [Google Scholar] [CrossRef]
- Hansson, O.; Finnebraaten, M.; Heitmann, I.K.; Ramse, M.; Bouzga, M. Trace DNA Collection—Performance of Minitape and Three Different Swabs. Forensic Sci. Int. Genet. Suppl. Ser. 2009, 2, 189–190. [Google Scholar] [CrossRef]
- Verdon, T.J.; Mitchell, R.J.; van Oorschot, R.A.H. Evaluation of Tapelifting as a Collection Method for Touch DNA. Forensic Sci. Int. Genet. 2014, 8, 179–186. [Google Scholar] [CrossRef]
- Plaza, D.T.; Mealy, J.L.; Lane, J.N.; Parsons, M.N.; Bathrick, A.S.; Slack, D.P. Nondestructive Biological Evidence Collection with Alternative Swabs and Adhesive Lifters. J. Forensic Sci. 2016, 61, 485–488. [Google Scholar] [CrossRef]
- Forsberg, C.; Jansson, L.; Ansell, R.; Hedman, J. High-Throughput DNA Extraction of Forensic Adhesive Tapes. Forensic Sci. Int. Genet. 2016, 24, 158–163. [Google Scholar] [CrossRef] [Green Version]
- Pääbo, S. Amplifying Ancient DNA. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., Eds.; Academic Press, Inc.: San Diego, CA, USA, 1990. [Google Scholar]
- Stoop, B.; Defaux, P.M.; Utz, S.; Zieger, M. Touch DNA Sampling with SceneSafe FastTM Minitapes. Leg. Med. 2017, 29, 68–71. [Google Scholar] [CrossRef]
- Ewing, M.M.; Thompson, J.M.; McLaren, R.S.; Purpero, V.M.; Thomas, K.J.; Dobrowski, P.A.; DeGroot, G.A.; Romsos, E.L.; Storts, D.R. Human DNA Quantification and Sample Quality Assessment: Developmental Validation of the PowerQuant¨r) System. Forensic Sci. Int. Genet. 2016, 23, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Promega. PowerQuant® System—Technical Manual. 2022. Available online: https://ita.promega.com/-/media/files/resources/protocols/technical-manuals/tmd/powerquant-system-technical-manual.pdf?rev=eed77410b69d43a2b0ea03fc78c7b69c&sc_lang=en (accessed on 12 April 2023).
- McLaren, R.S.; Bourdeau-Heller, J.; Patel, J.; Thompson, J.M.; Pagram, J.; Loake, T.; Beesley, D.; Pirttimaa, M.; Hill, C.R.; Duewer, D.L.; et al. Developmental Validation of the PowerPlex® ESI 16/17 Fast and PowerPlex® ESX 16/17 Fast Systems. Forensic Sci. Int. Genet. 2014, 13, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Promega. PowerPlex® ESX 17 Fast System for Use on the Applied Biosystems® Genetic Analyzers. 2021. Available online: https://ita.promega.com/-/media/files/resources/protocols/technical-manuals/tmd/powerplex-esx-17-fast-system-protocol.pdf?rev=a09a557843694c8da2d264b7722c102b&la=en (accessed on 12 April 2023).
- Goray, M.; Mitchell, R.J.; Oorschot, R.A.H. van Investigation of Secondary DNA Transfer of Skin Cells under Controlled Test Conditions. Leg. Med. 2010, 12, 117–120. [Google Scholar] [CrossRef]
- Goray, M.; Eken, E.; Mitchell, R.J.; van Oorschot, R.A.H. Secondary DNA Transfer of Biological Substances under Varying Test Conditions. Forensic Sci. Int. Genet. 2010, 4, 62–67. [Google Scholar] [CrossRef]
- Pfeifer, C.M.; Wiegand, P. Persistence of Touch DNA on Burglary-Related Tools. Int. J. Leg. Med. 2017, 131, 941–953. [Google Scholar] [CrossRef]
- Fonneløp, A.E.; Faria, S.; Shanthan, G.; Gill, P. Who Packed the Drugs? Application of Bayesian Networks to Address Questions of DNA Transfer, Persistence, and Recovery from Plastic Bags and Tape. Genes 2021, 13, 18. [Google Scholar] [CrossRef]
- Calafell, F. The Probability Distribution of the Number of Loci Indicating Exclusion in a Core Set of STR Markers. Int. J. Leg. Med. 2000, 114, 61–65. [Google Scholar] [CrossRef]
- Clayton, T.M.; Whitaker, J.P.; Sparkes, R.; Gill, P. Analysis and Interpretation of Mixed Forensic Stains Using DNA STR Profiling. Forensic Sci. Int. 1998, 91, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Bleka, Ø.; Storvik, G.; Gill, P. EuroForMix: An Open Source Software Based on a Continuous Model to Evaluate STR DNA Profiles from a Mixture of Contributors with Artefacts. Forensic Sci. Int. Genet. 2016, 21, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kokshoorn, B.; Aarts, L.H.J.; Ansell, R.; Connolly, E.; Drotz, W.; Kloosterman, A.D.; McKenna, L.G.; Szkuta, B.; van Oorschot, R.A.H. Sharing Data on DNA Transfer, Persistence, Prevalence and Recovery: Arguments for Harmonization and Standardization. Forensic Sci. Int. Genet. 2018, 37, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Gill, P.; Bleka, Ø.; Fonneløp, A.E. RFU Derived LRs for Activity Level Assignments Using Bayesian Networks. Forensic Sci. Int. Genet. 2022, 56, 102608. [Google Scholar] [CrossRef] [PubMed]
- van Oorschot, R.A.H.; Jones, M.K. DNA Fingerprints from Fingerprints. Nature 1997, 387, 767. [Google Scholar] [CrossRef]
- Buckingham, A.K.; Harvey, M.L.; van Oorschot, R.A.H. The Origin of Unknown Source DNA from Touched Objects. Forensic Sci. Int. Genet. 2016, 25, 26–33. [Google Scholar] [CrossRef]
- Nordgaard, A.; Rasmusson, B. The Likelihood Ratio as Value of Evidence--More than a Question of Numbers. Law Probab. Risk 2012, 11, 303–315. [Google Scholar] [CrossRef]
- Ge.F.I. Ge.F.I. Recommendations for Personal Identification Analysis by Forensic Laboratories. 2018. Available online: https://www.gefi-isfg.org/temp/GeFI%20guidelines-ONLINEdef.pdf (accessed on 12 April 2023).
- Hugin Expert A/S. Hugin Expert Software v 9.3. Denmark. Available online: https://www.hugin.com/ (accessed on 12 April 2023).
- Kirgiz, I.A.; Calloway, C. Increased Recovery of Touch DNA Evidence Using FTA Paper Compared to Conventional Collection Methods. J. Forensic Leg. Med. 2017, 47, 9–15. [Google Scholar] [CrossRef]
- Farmen, R.K.; Jaghø, R.; Cortez, P.; Frøyland, E.S. Assessment of Individual Shedder Status and Implication for Secondary DNA Transfer. Forensic Sci. Int. Genet. Suppl. Ser. 2008, 1, 415–417. [Google Scholar] [CrossRef]
- Tan, J.; Lee, J.Y.; Lee, L.Y.C.; Aw, Z.Q.; Chew, M.H.; Ishak, N.I.B.; Lee, Y.S.; Mugni, M.A.; Syn, C.K.C. Shedder Status: Does It Really Exist? Forensic Sci. Int. Genet. Suppl. Ser. 2019, 7, 360–362. [Google Scholar] [CrossRef]
- Oleiwi, A.A.; Morris, M.R.; Schmerer, W.M.; Sutton, R. The Relative DNA-Shedding Propensity of the Palm and Finger Surfaces. Sci. Justice 2015, 55, 329–334. [Google Scholar] [CrossRef]
- Kanokwongnuwut, P.; Martin, B.; Kirkbride, K.P.; Linacre, A. Shedding Light on Shedders. Forensic Sci. Int. Genet. 2018, 36, 20–25. [Google Scholar] [CrossRef] [Green Version]
- Thornbury, D.; Goray, M.; van Oorschot, R.A.H. Indirect DNA Transfer without Contact from Dried Biological Materials on Various Surfaces. Forensic Sci. Int. Genet. 2021, 51, 102457. [Google Scholar] [CrossRef]
- Hefetz, I.; Einot, N.; Faerman, M.; Horowitz, M.; Almog, J. Touch DNA: The Effect of the Deposition Pressure on the Quality of Latent Fingermarks and STR Profiles. Forensic Sci. Int. Genet. 2019, 38, 105–112. [Google Scholar] [CrossRef]
- Noël, S.; Lagacé, K.; Rogic, A.; Granger, D.; Bourgoin, S.; Jolicoeur, C.; Séguin, D. DNA Transfer during Laundering May Yield Complete Genetic Profiles. Forensic Sci. Int. Genet. 2016, 23, 240–247. [Google Scholar] [CrossRef]
- Breathnach, M.; Williams, L.; McKenna, L.; Moore, E. Probability of Detection of DNA Deposited by Habitual Wearer and/or the Second Individual Who Touched the Garment. Forensic Sci. Int. Genet. 2016, 20, 53–60. [Google Scholar] [CrossRef]
- Walsh, P.S.; Erlich, H.A.; Higuchi, R. Preferential PCR Amplification of Alleles: Mechanisms and Solutions. Genome Res. 1992, 1, 241–250. [Google Scholar] [CrossRef]
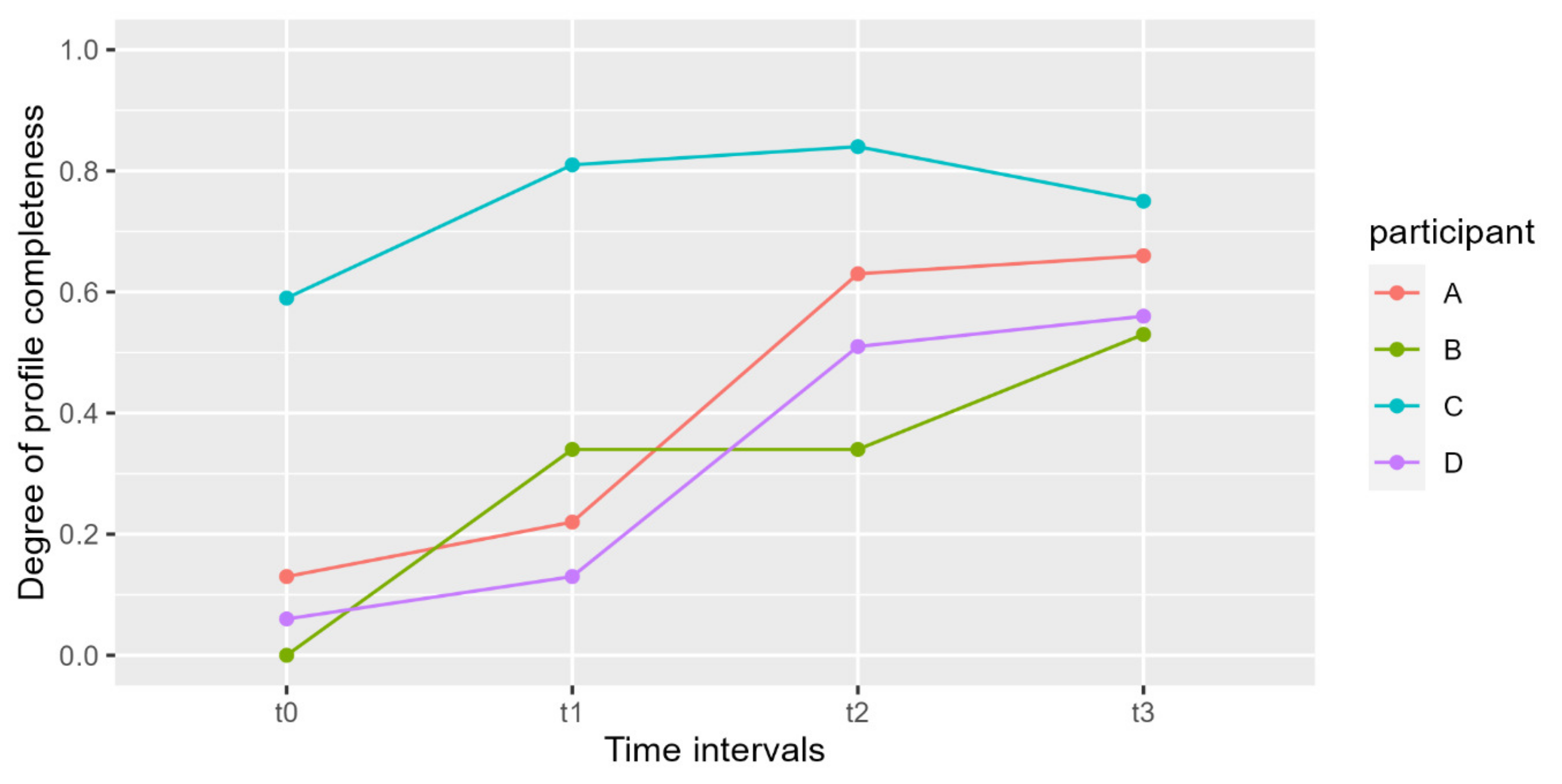

| Participant | Time Point | Concentration (ng/μL) | DNA Quantity (ng in 30 μL) | Profile Completeness | Shedder Category | |||
|---|---|---|---|---|---|---|---|---|
| Values | Average | Values | Average | Values | Average | |||
| A | t0 | 0.0011 | >0.0029 | 0.033 | >0.086 | 0.13 | 0.41 | Intermediate |
| t1 | 0.0032 | 0.096 | 0.22 | |||||
| t2 | 0.004 | 0.120 | 0.63 | |||||
| t3 | 0.0032 | 0.096 | 0.66 | |||||
| B | t0 | 0.0006 | 0.0018 | 0.018 | 0.053 | 0 | 0.30 | Poor |
| t1 | 0.0016 | 0.048 | 0.34 | |||||
| t2 | 0.0021 | 0.063 | 0.34 | |||||
| t3 | 0.0028 | 0.084 | 0.53 | |||||
| C | t0 | 0.0094 | 0.0191 | 0.282 | 0.572 | 0.59 | 0.75 | High |
| t1 | 0.0176 | 0.528 | 0.81 | |||||
| t2 | 0.0223 | 0.669 | 0.84 | |||||
| t3 | 0.0270 | 0.810 | 0.75 | |||||
| D | t0 | 0.0005 | 0.0033 | 0.015 | 0.097 | 0.06 | 0.32 | Intermediate |
| t1 | 0.0016 | 0.048 | 0.13 | |||||
| t2 | 0.0038 | 0.114 | 0.51 | |||||
| t3 | 0.0070 | 0.210 | 0.56 | |||||
| Transfer Mechanism | Sample Code | Concentration (ng/μL) | DNA Quantity (ng in 30 μL) | Shared Alleles | Profile Completeness (Unique Alleles) | Profile Completeness (All Alleles) | |||
|---|---|---|---|---|---|---|---|---|---|
| O with POI | POI with O | O | POI | O | POI | ||||
| Direct transfer | D1 | 0.012 | 0.345 | 5 | 5 | 0.63 | 0.78 | 0.69 | 0.81 |
| D2 | 0.071 | 2.13 | 5 | 5 | 0.67 | 0.96 | 0.72 | 0.97 | |
| D3 | 0.061 | 1.836 | 10 | 8 | 0.77 | 1 | 0.84 | 1 | |
| D4 | 0.034 | 1.026 | 8 | 10 | 0.89 | 0.93 | 0.91 | 0.94 | |
| D5 | 0.065 | 1.944 | 5 | 5 | 0.96 | 0.96 | 0.97 | 0.97 | |
| D6 | 0.074 | 2.226 | 10 | 8 | 1 | 1 | 1 | 1 | |
| D7 | 0.023 | 0.687 | 8 | 8 | 0.54 | 0.63 | 0.63 | 0.69 | |
| D8 | 0.031 | 0.942 | 8 | 8 | 0.58 | 0.58 | 0.59 | 0.63 | |
| D9 | 0.078 | 2.343 | 5 | 5 | 0.85 | 1 | 0.88 | 1 | |
| D10 | 0.056 | 1.692 | 5 | 5 | 0.63 | 1 | 0.69 | 1 | |
| D11 | 0.069 | 2.07 | 5 | 5 | 0.93 | 1 | 0.94 | 1 | |
| Secondary transfer | S1 | 0.013 | 0.39 | 5 | 5 | 0.96 | 0.24 | 0.88 | 0.31 |
| S2 | 0.025 | 0.753 | 5 | 5 | 0.84 | 0.64 | 0.81 | 0.72 | |
| S3 | 0.018 | 0.537 | 10 | 8 | 0.77 | 0.63 | 0.81 | 0.69 | |
| S4 | 0.026 | 0.783 | 8 | 10 | 0.92 | 0.77 | 0.94 | 0.84 | |
| S5 | 0.036 | 1.071 | 8 | 8 | 0.82 | 0.96 | 0.84 | 0.97 | |
| S6 | 0.057 | 1.701 | 10 | 8 | 1 | 0.68 | 1 | 0.78 | |
| S7 | 0.006 | 0.171 | 8 | 8 | 0.33 | 0.44 | 0.38 | 0.47 | |
| S8 | 0.023 | 0.675 | 5 | 5 | 0.74 | 0.96 | 0.75 | 0.97 | |
| S9 | 0.016 | 0.468 | 5 | 5 | 0.93 | 0.93 | 0.91 | 0.88 | |
| S10 | 0.017 | 0.504 | 5 | 5 | 0.85 | 0.78 | 0.91 | 0.78 | |
| S11 | 0.006 | 0.174 | 5 | 5 | 0.22 | 0.41 | 0.31 | 0.5 | |
| Transfer Mechanism | Sample Code | Number of Contributors | log10LRϕ | Mixture Proportion | Contribution in ng | ||||
|---|---|---|---|---|---|---|---|---|---|
| O | POI | Unknown | O | POI | Unknown | ||||
| Direct transfer | D1 | 3 | 12.36 | 0.49 | 0.35 | 0.16 | 0.170 | 0.119 | 0.057 |
| D2 | 3 | 14.29 | 0.19 | 0.57 | 0.24 | 0.405 | 1.214 | 0.511 | |
| D3 | 3 | 21.13 | 0.72 | 0.16 | 0.12 | 1.322 | 0.294 | 0.220 | |
| D4 | 3 | 12.84 | 0.33 | 0.49 | 0.19 | 0.334 | 0.502 | 0.190 | |
| D5 | 3 | 14.88 | 0.37 | 0.48 | 0.15 | 0.719 | 0.933 | 0.292 | |
| D6 | 3 | 15 | 0.40 | 0.45 | 0.15 | 0.890 | 0.979 | 0.334 | |
| D7 | 3 | 5.08 | 0.40 | 0.36 | 0.24 | 0.247 | 0.275 | 0.165 | |
| D8 | 3 | 2.76 | 0.39 | 0.29 | 0.32 | 0.363 | 0.269 | 0.309 | |
| D9 | 3 | 22.58 | 0.14 | 0.77 | 0.09 | 0.328 | 1.804 | 0.209 | |
| D10 | 3 | 20.71 | 0.09 | 0.73 | 0.18 | 0.154 | 1.235 | 0.305 | |
| D11 | 3 | 22.63 | 0.16 | 0.81 | 0.03 | 0.331 | 1.677 | 0.056 | |
| Secondary transfer | S1 | 2 | 1.30 | 0.12 | 0.88 | / | 0.046 | 0.344 | / |
| S2 | 3 | 5.55 | 0.48 | 0.32 | 0.20 | 0.361 | 0.241 | 0.151 | |
| S3 | 3 | 1.06 | 0.27 | 0.24 | 0.49 | 0.145 | 0.129 | 0.263 | |
| S4 | 3 | −0.17 | 0.57 | 0.14 | 0.29 | 0.446 | 0.110 | 0.227 | |
| S5 | 3 | 10.14 | 0.27 | 0.46 | 0.27 | 0.289 | 0.493 | 0.289 | |
| S6 | 3 | 7.03 | 0.78 | 0.14 | 0.08 | 1.327 | 0.238 | 0.136 | |
| S7 | 2 | 6.48 | 0.38 | 0.62 | / | 0.066 | 0.106 | / | |
| S8 | 3 | 7.24 | 0.46 | 0.17 | 0.37 | 0.322 | 0.115 | 0.250 | |
| S9 | 3 | 3.99 | 0.39 | 0.29 | 0.32 | 0.183 | 0.136 | 0.150 | |
| S10 | 3 | 4.45 | 0.45 | 0.30 | 0.25 | 0.227 | 0.151 | 0.126 | |
| S11 | 2 | 5.79 | 0.29 | 0.71 | / | 0.051 | 0.124 | / | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onofri, M.; Altomare, C.; Severini, S.; Tommolini, F.; Lancia, M.; Carlini, L.; Gambelunghe, C.; Carnevali, E. Direct and Secondary Transfer of Touch DNA on a Credit Card: Evidence Evaluation Given Activity Level Propositions and Application of Bayesian Networks. Genes 2023, 14, 996. https://doi.org/10.3390/genes14050996
Onofri M, Altomare C, Severini S, Tommolini F, Lancia M, Carlini L, Gambelunghe C, Carnevali E. Direct and Secondary Transfer of Touch DNA on a Credit Card: Evidence Evaluation Given Activity Level Propositions and Application of Bayesian Networks. Genes. 2023; 14(5):996. https://doi.org/10.3390/genes14050996
Chicago/Turabian StyleOnofri, Martina, Cristina Altomare, Simona Severini, Federica Tommolini, Massimo Lancia, Luigi Carlini, Cristiana Gambelunghe, and Eugenia Carnevali. 2023. "Direct and Secondary Transfer of Touch DNA on a Credit Card: Evidence Evaluation Given Activity Level Propositions and Application of Bayesian Networks" Genes 14, no. 5: 996. https://doi.org/10.3390/genes14050996
APA StyleOnofri, M., Altomare, C., Severini, S., Tommolini, F., Lancia, M., Carlini, L., Gambelunghe, C., & Carnevali, E. (2023). Direct and Secondary Transfer of Touch DNA on a Credit Card: Evidence Evaluation Given Activity Level Propositions and Application of Bayesian Networks. Genes, 14(5), 996. https://doi.org/10.3390/genes14050996







