Microsatellite Instability and Immune Response: From Microenvironment Features to Therapeutic Actionability—Lessons from Colorectal Cancer
Abstract
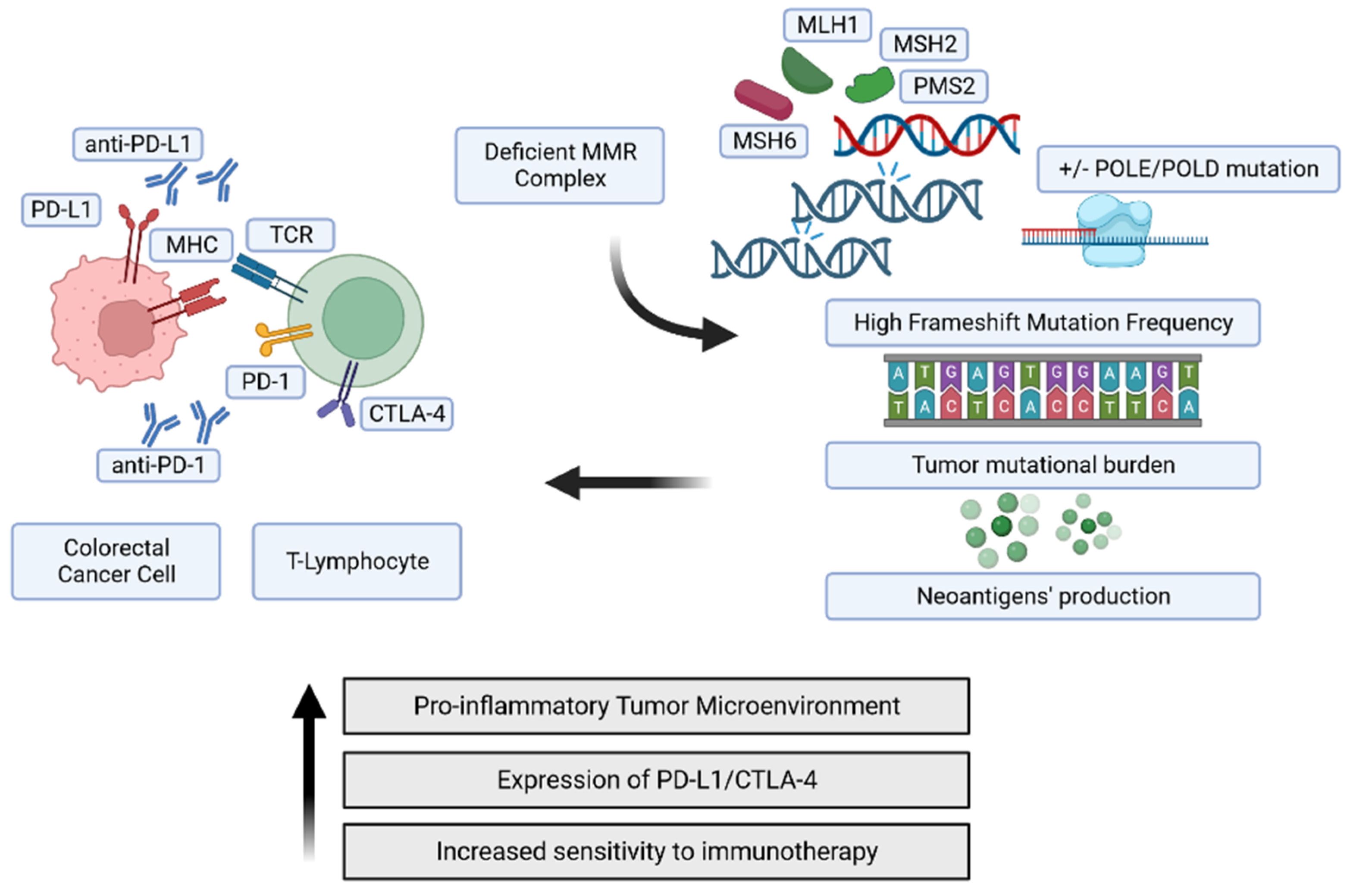
:1. Introduction
2. The Genomic Landscape of MMR-Deficient Cancers
MSI, a Distinguishable Genetic Attractor of the Adaptive Immune Response
3. Prognostic and Predictive Value of MMR defects
3.1. Direct Prognostic Implications
| References | MSS | MSI | ||||
|---|---|---|---|---|---|---|
| HR (95% C.I) | PFS (95% C.I) | OS (95% C.I) | HR (95% C.I) | PFS (95% C.I) | OS (95% C.I) | |
| Malesci et al. [87] | NA | NA | NA | 0.30 (0.16–0.54), p < 0.001, for DSS | NA | NA |
| Popat et al. [88] | NA | NA | NA | 0.65 (0.59–0.71), pooled, for OS 0.67 (0.53–0.83), pooled, for PFS | NA | NA |
| Sankila et al. [89] | NA | NA | NA | 0.64 (0.56–0.72), for OS in Lynch patients | NA | NA |
| Klingbiel et al. [92] | 0.16 (0.04–0.64), p = 0.01, for OS in stage II | NA | NA | 0.26 (0.10–0.65), p = 0.004, for OS in stage II | NA | NA |
| Venderbosch et al. [94] | 1.34 (1.10–1.64), for PFS 1.94 (1.57–2.40), for OS | 7.6 (7.3–8.0) | 16.8 (16.3–17.5) | 1.33 (1.12–1.57), for PFS 1.35 (1.13–1.61), for OS | 6.2 (5.9–7.0) | 13.6 (12.4–15.6) |
| Taieb et al. [95] | NA | NA | NA | 0.82 (0.69–0.98), p = 0.029, for OS after recurrence | 2.2 (1.9–2.7) | NA |
3.2. Predictive Implications for Standard Adjuvant Treatment
4. Actionability of Immune Responses as a Therapeutic Target in CRC according to MS Status
4.1. Immune Checkpoint Targeting
4.2. An Evolving Therapeutic Scenario
5. Immunotherapy and MSI Colorectal Cancer: On-Stage and Incoming Broadcasting
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, R.; Guo, F.; Heisser, T.; Hackl, M.; Ihle, P.; De Schutter, H.; Van Damme, N.; Valerianova, Z.; Atanasov, T.; Májek, O.; et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: An international population-based study. Lancet Oncol. 2021, 22, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.; Dey, N.; Grady, W.M. Emerging Tests for Noninvasive Colorectal Cancer Screening. Clin. Gastroenterol. Hepatol. 2023, 21, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Goel, A. Microsatellite Instability in Colorectal Cancer. Gastroenterology 2010, 138, 2073–2087.e3. [Google Scholar] [CrossRef]
- Pino, M.S.; Chung, D.C. The Chromosomal Instability Pathway in Colon Cancer. Gastroenterology 2010, 138, 2059–2072. [Google Scholar] [CrossRef]
- Dal Buono, A.; Gaiani, F.; Poliani, L.; Correale, C.; Laghi, L. Defects in MMR Genes as a Seminal Example of Personalized Medicine: From Diagnosis to Therapy. J. Pers. Med. 2021, 11, 1333. [Google Scholar] [CrossRef]
- Ionov, Y.; Peinado, M.A.; Malkhosyan, S.; Shibata, D.; Perucho, M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993, 363, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Thibodeau, S.N.; Bren, G.; Schaid, D. Microsatellite Instability in Cancer of the Proximal Colon. Science 1993, 260, 816–819. [Google Scholar] [CrossRef] [PubMed]
- Picard, E.; Verschoor, C.P.; Ma, G.W.; Pawelec, G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front. Immunol. 2020, 11, 369. [Google Scholar] [CrossRef]
- Lynch, H.T.; Snyder, C.L.; Shaw, T.G.; Heinen, C.D.; Hitchins, M.P. Milestones of Lynch syndrome: 1895–2015. Nat. Rev. Cancer 2015, 15, 181–194. [Google Scholar] [CrossRef]
- Sinicrope, F.A. Lynch Syndrome–Associated Colorectal Cancer. N. Engl. J. Med. 2018, 379, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Guarinos, C.; Castillejo, A.; Barberá, V.M.; Pérez-Carbonell, L.; Sánchez-Heras, A.B.; Segura, Á.; Guillén-Ponce, C.; Martínez-Cantó, A.; Castillejo, M.I.; Egoavil, C.M.; et al. EPCAM Germ Line Deletions as Causes of Lynch Syndrome in Spanish Patients. J. Mol. Diagn. 2010, 12, 765–770. [Google Scholar] [CrossRef]
- Tutlewska, K.; Lubinski, J.; Kurzawski, G. Germline deletions in the EPCAM gene as a cause of Lynch syndrome—Literature review. Hered. Cancer Clin. Pract. 2013, 11, 9. [Google Scholar] [CrossRef] [PubMed]
- Pathak, S.J.; Mueller, J.L.; Okamoto, K.; Das, B.; Hertecant, J.; Greenhalgh, L.; Cole, T.; Pinsk, V.; Yerushalmi, B.; Gurkan, O.E.; et al. EPCAM mutation update: Variants associated with congenital tufting enteropathy and Lynch syndrome. Hum. Mutat. 2019, 40, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Møller, P.; Seppälä, T.T.; Bernstein, I.; Holinski-Feder, E.; Sala, P.; Gareth Evans, D.; Lindblom, A.; Macrae, F.; Blanco, I.; Sijmons, R.H.; et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: A report from the Prospective Lynch Syndrome Database. Gut 2018, 67, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppälä, T.T.; ten Broeke, S.W.; Plazzer, J.P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef]
- Dominguez-Valentin, M.; Haupt, S.; Seppälä, T.T.; Sampson, J.R.; Sunde, L.; Bernstein, I.; Jenkins, M.A.; Engel, C.; Aretz, S.; Nielsen, M.; et al. Mortality by Age, Gene and Gender in Carriers of Pathogenic Mismatch Repair Gene Variants Receiving Surveillance for Early Cancer Diagnosis and Treatment: A Report from the Prospective Lynch Syndrome Database. eClinicalMedicine 2023, 58, 101909. [Google Scholar] [CrossRef]
- Peltomäki, P.; Nyström, M.; Mecklin, J.P.; Seppälä, T.T. Lynch Syndrome Genetics and Clinical Implications. Gastroenterology 2023, 164, 783–799. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar]
- Kloor, M.; von Knebel Doeberitz, M. The Immune Biology of Microsatellite-Unstable Cancer. Trends Cancer 2016, 2, 121–133. [Google Scholar] [CrossRef]
- Ahadova, A.; Seppälä, T.T.; Engel, C.; Gallon, R.; Burn, J.; Holinski-Feder, E.; Steinke-Lange, V.; Möslein, G.; Nielsen, M.; Broeke, S.W.; et al. The “unnatural” history of colorectal cancer in Lynch syndrome: Lessons from colonoscopy surveillance. Int. J. Cancer 2021, 148, 800–811. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef] [PubMed]
- Laghi, L.; Bianchi, P.; Delconte, G.; Celesti, G.; Di Caro, G.; Pedroni, M.; Chiaravalli, A.M.; Jung, B.; Capella, C.; de Leon, M.P.; et al. MSH3 Protein Expression and Nodal Status in MLH1-Deficient Colorectal Cancers. Clin. Cancer Res. 2012, 18, 3142–3153. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Lichtenstern, C.R.; Ngu, R.K.; Shalapour, S.; Karin, M. Immunotherapy, Inflammation and Colorectal Cancer. Cells 2020, 9, 618. [Google Scholar] [CrossRef]
- Mendonça Gorgulho, C.; Krishnamurthy, A.; Lanzi, A.; Galon, J.; Housseau, F.; Kaneno, R.; Lotze, M.T. Gutting it Out: Developing Effective Immunotherapies for Patients with Colorectal Cancer. J. Immunother. 2021, 44, 49–62. [Google Scholar] [CrossRef]
- Boland, C.R.; Thibodeau, S.N.; Hamilton, S.R.; Sidransky, D.; Eshleman, J.R.; Burt, R.W.; Meltzer, S.J.; Rodriguez-Bigas, M.A.; Fodde, R.; Ranzani, G.N.; et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: Development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998, 58, 5248–5257. [Google Scholar]
- Umar, A.; Boland, C.R.; Terdiman, J.P.; Syngal, S.; de la Chapelle, A.; Ruschoff, J.; Fishel, R.; Lindor, N.M.; Burgart, L.J.; Hamelin, R.; et al. Revised Bethesda Guidelines for Hereditary Nonpolyposis Colorectal Cancer (Lynch Syndrome) and Microsatellite Instability. JNCI J. Natl. Cancer Inst. 2004, 96, 261–268. [Google Scholar] [CrossRef]
- Schwitalle, Y.; Kloor, M.; Eiermann, S.; Linnebacher, M.; Kienle, P.; Knaebel, H.P.; Tariverdian, M.; Benner, A.; von Knebel Doeberitz, M. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 2008, 134, 988–997. [Google Scholar] [CrossRef]
- Grady, W.M.; Carethers, J.M. Genomic and Epigenetic Instability in Colorectal Cancer Pathogenesis. Gastroenterology 2008, 135, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Leggett, B.; Whitehall, V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010, 138, 2088–2100. [Google Scholar] [CrossRef] [PubMed]
- Carethers, J.M.; Jung, B.H. Genetics and Genetic Biomarkers in Sporadic Colorectal Cancer. Gastroenterology 2015, 149, 1177–1190.e3. [Google Scholar] [CrossRef] [PubMed]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Kawaguchi, T.; Peng, X.; Qi, Q.; Liu, S.; Yan, L.; Takabe, K. Tumor Infiltrating Lymphocytes and Macrophages Improve Survival in Microsatellite Unstable Colorectal Cancer. Sci. Rep. 2019, 9, 13455. [Google Scholar] [CrossRef]
- Carethers, J.M. Lynch syndrome and Lynch syndrome mimics: The growing complex landscape of hereditary colon cancer. World J. Gastroenterol. 2015, 21, 9253. [Google Scholar] [CrossRef]
- Mensenkamp, A.R.; Vogelaar, I.P.; van Zelst-Stams, W.A.G.; Goossens, M.; Ouchene, H.; Hendriks-Cornelissen, S.J.B.; Kwint, M.P.; Hoogerbrugge, N.; Nagtegaal, I.D.; Ligtenberg, M.J.L. Somatic Mutations in MLH1 and MSH2 Are a Frequent Cause of Mismatch-Repair Deficiency in Lynch Syndrome-Like Tumors. Gastroenterology 2014, 146, 643–646.e8. [Google Scholar] [CrossRef] [PubMed]
- Haraldsdottir, S.; Hampel, H.; Tomsic, J.; Frankel, W.L.; Pearlman, R.; de la Chapelle, A.; Pritchard, C.C. Colon and Endometrial Cancers with Mismatch Repair Deficiency Can Arise from Somatic, Rather than Germline, Mutations. Gastroenterology 2014, 147, 1308–1316.e1. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Soler, M.; Pérez-Carbonell, L.; Guarinos, C.; Zapater, P.; Castillejo, A.; Barberá, V.M.; Juárez, M.; Bessa, X.; Xicola, R.M.; Clofent, J.; et al. Risk of Cancer in Cases of Suspected Lynch Syndrome without Germline Mutation. Gastroenterology 2013, 144, 926–932.e1. [Google Scholar] [CrossRef]
- Kang, S.Y.; Park, C.K.; Chang, D.K.; Kim, J.W.; Son, H.J.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; Kwon, M.; Kim, K.M. Lynch-like syndrome: Characterization and comparison with EPCAM deletion carriers. Int. J. Cancer 2015, 136, 1568–1578. [Google Scholar] [CrossRef]
- Weisenberger, D.J.; Siegmund, K.D.; Campan, M.; Young, J.; Long, T.I.; Faasse, M.A.; Kang, G.H.; Widschwendter, M.; Weener, D.; Buchanan, D.; et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat. Genet. 2006, 38, 787–793. [Google Scholar] [CrossRef]
- Tahara, T.; Yamamoto, E.; Madireddi, P.; Suzuki, H.; Maruyama, R.; Chung, W.; Garriga, J.; Jelinek, J.; Yamano, H.-O.; Sugai, T.; et al. Colorectal Carcinomas with CpG Island Methylator Phenotype 1 Frequently Contain Mutations in Chromatin Regulators. Gastroenterology 2014, 146, 530–538.e5. [Google Scholar] [CrossRef]
- Kim, H.; Jen, J.; Vogelstein, B.; Hamilton, S.R. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am. J. Pathol. 1994, 145, 148–156. [Google Scholar] [PubMed]
- Guidoboni, M.; Gafà, R.; Viel, A.; Doglioni, C.; Russo, A.; Santini, A.; Del Tin, L.; Macrì, E.; Lanza, G.; Boiocchi, M.; et al. Microsatellite instability and high content of activated cytotoxic lymphocytes identify colon cancer patients with a favorable prognosis. Am. J. Pathol. 2001, 159, 297–304. [Google Scholar] [CrossRef]
- Germano, G.; Amirouchene-Angelozzi, N.; Rospo, G.; Bardelli, A. The Clinical Impact of the Genomic Landscape of Mismatch Repair-Deficient Cancers. Cancer Discov. 2018, 8, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Dolcetti, R.; Viel, A.; Doglioni, C.; Russo, A.; Guidoboni, M.; Capozzi, E.; Vecchiato, N.; Macrì, E.; Fornasarig, M.; Boiocchi, M. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am. J. Pathol. 1999, 154, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Bortolomeazzi, M.; Montorsi, L.; Temelkovski, D.; Keddar, M.R.; Acha-Sagredo, A.; Pitcher, M.J.; Basso, G.; Laghi, L.; Rodriguez-Justo, M.; Spencer, J.; et al. A SIMPLI (Single-cell Identification from MultiPLexed Images) approach for spatially-resolved tissue phenotyping at single-cell resolution. Nat. Commun. 2022, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Di Caro, G.; Celesti, G.; Grizzi, F.; Bianchi, P.; Basso, G.; Marchesi, F.; Mantovani, A.; Malesci, A.; Laghi, L. Combined low-FOXP3+ and -CD3+tumor infiltrating lymphocytes: A signature of stage II MSS colorectal cancer at high-risk of recurrence. J. Immunother. Cancer. 2013, 1 (Suppl. 1), 49. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Jass, J.R.; Love, S.B.; Northover, J.M. A new prognostic classification of rectal cancer. Lancet 1987, 1, 1303–1306. [Google Scholar] [CrossRef]
- Gaiani, F.; Marchesi, F.; Negri, F.; Greco, L.; Malesci, A.; de’Angelis, G.L.; Laghi, L. Heterogeneity of Colorectal Cancer Progression: Molecular Gas and Brakes. Int. J. Mol. Sci. 2021, 22, 5246. [Google Scholar] [CrossRef]
- Laghi, L.; Bianchi, P.; Miranda, E.; Balladore, E.; Pacetti, V.; Grizzi, F.; Allavena, P.; Torri, V.; Repici, A.; Santoro, A.; et al. CD3+ cells at the invasive margin of deeply invading (pT3-T4) colorectal cancer and risk of post-surgical metastasis: A longitudinal study. Lancet Oncol. 2009, 10, 877–884. [Google Scholar] [CrossRef]
- Cavalleri, T.; Bianchi, P.; Basso, G.; Celesti, G.; Grizzi, F.; Bossi, P.; Greco, L.; Pitrone, C.; Valtorta, E.; Mauri, G.; et al. Combined Low Densities of FoxP3+ and CD3+ Tumor-Infiltrating Lymphocytes Identify Stage II Colorectal Cancer at High Risk of Progression. Cancer Immunol. Res. 2019, 7, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International validation of the consensus Immunoscore for the classification of colon cancer: A prognostic and accuracy study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef] [PubMed]
- Pagès, F.; Kirilovsky, A.; Mlecnik, B.; Asslaber, M.; Tosolini, M.; Bindea, G.; Lagorce, C.; Wind, P.; Marliot, F.; Bruneval, P.; et al. In Situ Cytotoxic and Memory T Cells Predict Outcome in Patients With Early-Stage Colorectal Cancer. J. Clin. Oncol. 2009, 27, 5944–5951. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bifulco, C.; Bindea, G.; Marliot, F.; Lugli, A.; Lee, J.J.; Zlobec, I.; Rau, T.T.; Berger, M.D.; Nagtegaal, I.D.; et al. Multicenter International Society for Immunotherapy of Cancer Study of the Consensus Immunoscore for the Prediction of Survival and Response to Chemotherapy in Stage III Colon Cancer. J. Clin. Oncol. 2020, 38, 3638–3651. [Google Scholar] [CrossRef]
- Pagès, F.; André, T.; Taieb, J.; Vernerey, D.; Henriques, J.; Borg, C.; Marliot, F.; Ben Jannet, R.; Louvet, C.; Mineur, L.; et al. Prognostic and predictive value of the Immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1276. [Google Scholar] [CrossRef]
- Gubin, M.M.; Schreiber, R.D. CANCER. The odds of immunotherapy success. Science 2015, 350, 158–159. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Freeman-Mills, L.; Rayner, E.; Glaire, M.; Briggs, S.; Vermeulen, L.; Fessler, E.; Medema, J.P.; Boot, A.; Morreau, H.; et al. Somatic POLE proofreading domain mutation, immune response, and prognosis in colorectal cancer: A retrospective, pooled biomarker study. Lancet Gastroenterol. Hepatol. 2016, 1, 207–216. [Google Scholar] [CrossRef]
- Mo, S.; Ma, X.; Li, Y.; Zhang, L.; Hou, T.; Han-Zhang, H.; Qian, J.; Cai, S.; Huang, D.; Peng, J. Somatic POLE exonuclease domain mutations elicit enhanced intratumoral immune responses in stage II colorectal cancer. J. Immunother. Cancer 2020, 8, e000881. [Google Scholar] [CrossRef]
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019, 76, 22–32. [Google Scholar] [CrossRef]
- Bupathi, M.; Wu, C. Biomarkers for immune therapy in colorectal cancer: Mismatch-repair deficiency and others. J. Gastrointest. Oncol. 2016, 7, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C. Tim-3: An Emerging Target in the Cancer Immunotherapy Landscape. Cancer Immunol. Res. 2014, 2, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Pennock, G.K.; Chow, L.Q.M. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. Oncologist 2015, 20, 812–822. [Google Scholar] [CrossRef]
- Toh, J.W.T.; de Souza, P.; Lim, S.H.; Singh, P.; Chua, W.; Ng, W.; Spring, K.J. The Potential Value of Immunotherapy in Colorectal Cancers: Review of the Evidence for Programmed Death-1 Inhibitor Therapy. Clin. Color. Cancer 2016, 15, 285–291. [Google Scholar] [CrossRef]
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 2016, 29, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Wolf, Y.; Anderson, A.C.; Kuchroo, V.K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Cereda, M.; Gambardella, G.; Benedetti, L.; Iannelli, F.; Patel, D.; Basso, G.; Guerra, R.F.; Mourikis, T.P.; Puccio, I.; Sinha, S.; et al. Patients with genetically heterogeneous synchronous colorectal cancer carry rare damaging germline mutations in immune-related genes. Nat. Commun. 2016, 7, 12072. [Google Scholar] [CrossRef] [PubMed]
- Maby, P.; Tougeron, D.; Hamieh, M.; Mlecnik, B.; Kora, H.; Bindea, G.; Angell, H.K.; Fredriksen, T.; Elie, N.; Fauquembergue, E.; et al. Correlation between Density of CD8+ T-cell Infiltrate in Microsatellite Unstable Colorectal Cancers and Frameshift Mutations: A Rationale for Personalized Immunotherapy. Cancer Res. 2015, 75, 3446–3455. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Maby, P.; Angelova, M.; Tougeron, D.; Church, S.E.; Lafontaine, L.; Fischer, M.; Fredriksen, T.; et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival than Microsatellite Instability. Immunity 2016, 44, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.R.; Ryan, C.; Nolan, B.; Tosetto, M.; Geraghty, R.; Winter, D.C.; O’Connell, P.R.; Hyland, J.M.; Doherty, G.A.; Sheahan, K.; et al. Enrichment of Inflammatory IL-17 and TNF-α Secreting CD4(+) T Cells within Colorectal Tumors despite the Presence of Elevated CD39(+) T Regulatory Cells and Increased Expression of the Immune Checkpoint Molecule, PD-1. Front. Oncol. 2016, 6, 50. [Google Scholar] [CrossRef]
- Liu, S.S.; Yang, Y.Z.; Jiang, C.; Quan, Q.; Xie, Q.K.; Wang, X.P.; He, W.Z.; Rong, Y.M.; Chen, P.; Yang, Q.; et al. Comparison of immunological characteristics between paired mismatch repair-proficient and -deficient colorectal cancer patients. J. Transl. Med. 2018, 16, 195. [Google Scholar] [CrossRef]
- Bohaumilitzky, L.; von Knebel Doeberitz, M.; Kloor, M.; Ahadova, A. Implications of Hereditary Origin on the Immune Phenotype of Mismatch Repair-Deficient Cancers: Systematic Literature Review. J. Clin. Med. 2020, 9, 1741. [Google Scholar] [CrossRef]
- Janikovits, J.; Müller, M.; Krzykalla, J.; Körner, S.; Echterdiek, F.; Lahrmann, B.; Grabe, N.; Schneider, M.; Benner, A.; von Doeberitz, M.; et al. High numbers of PDCD1 (PD-1)-positive T cells and B2M mutations in microsatellite-unstable colorectal cancer. Oncoimmunology 2018, 7, e1390640. [Google Scholar] [CrossRef]
- Shia, J.; Ellis, N.A.; Paty, P.B.; Nash, G.M.; Qin, J.; Offit, K.; Zhang, X.M.; Markowitz, A.J.; Nafa, K.; Guillem, J.G.; et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am. J. Surg. Pathol. 2003, 27, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Koornstra, J.J.; de Jong, S.; Boersma-van Eck, W.; Zwart, N.; Hollema, H.; de Vries, E.G.E.; Kleibeuker, J.H. Fas ligand expression in lynch syndrome-associated colorectal tumours. Pathol. Oncol. Res. 2009, 15, 399–406. [Google Scholar] [CrossRef]
- Takemoto, N.; Konishi, F.; Yamashita, K.; Kojima, M.; Furukawa, T.; Miyakura, Y.; Shitoh, K.; Nagai, H. The correlation of microsatellite instability and tumor-infiltrating lymphocytes in hereditary non-polyposis colorectal cancer (HNPCC) and sporadic colorectal cancers: The significance of different types of lymphocyte infiltration. Jpn. J. Clin. Oncol. 2004, 34, 90–98. [Google Scholar] [CrossRef]
- Ozcan, M.; Janikovits, J.; von Knebel Doeberitz, M.; Kloor, M. Complex pattern of immune evasion in MSI colorectal cancer. Oncoimmunology 2018, 7, e1445453. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, D.C.; Kaklamanis, L.; Hampson, R.; Bodmer, W.F.; Karran, P. Selection for β 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr. Biol. 1996, 6, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Ballhausen, A.; Przybilla, M.J.; Jendrusch, M.; Haupt, S.; Pfaffendorf, E.; Seidler, F.; Witt, J.; Hernandez Sanchez, A.; Urban, K.; Draxlbauer, M.; et al. The shared frameshift mutation landscape of microsatellite-unstable cancers suggests immunoediting during tumor evolution. Nat. Commun. 2020, 11, 4740. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, G.H. Molecular and prognostic heterogeneity of microsatellite-unstable colorectal cancer. World J. Gastroenterol. 2014, 20, 4230–4243. [Google Scholar] [CrossRef] [PubMed]
- Pfuderer, P.L.; Ballhausen, A.; Seidler, F.; Stark, H.J.; Grabe, N.; Frayling, I.M.; Ager, A.; von Knebel Doeberitz, M.; Kloor, M.; Ahadova, A. High endothelial venules are associated with microsatellite instability, hereditary background and immune evasion in colorectal cancer. Br. J. Cancer. 2019, 121, 395–404. [Google Scholar] [CrossRef]
- Echterdiek, F.; Janikovits, J.; Staffa, L.; Müller, M.; Lahrmann, B.; Frühschütz, M.; Hartog, B.; Nelius, N.; Benner, A.; Tariverdian, M.; et al. Low density of FOXP3-positive T cells in normal colonic mucosa is related to the presence of beta2-microglobulin mutations in Lynch syndrome-associated colorectal cancer. Oncoimmunology 2016, 5, e1075692. [Google Scholar] [CrossRef]
- Surmann, E.M.; Voigt, A.Y.; Michel, S.; Bauer, K.; Reuschenbach, M.; Ferrone, S.; von Knebel Doeberitz, M.; Kloor, M. Association of high CD4-positive T cell infiltration with mutations in HLA class II-regulatory genes in microsatellite-unstable colorectal cancer. Cancer Immunol. Immunother. 2015, 64, 357–366. [Google Scholar] [CrossRef]
- Malesci, A.; Laghi, L.; Bianchi, P.; Delconte, G.; Randolph, A.; Torri, V.; Carnaghi, C.; Doci, R.; Rosati, R.; Montorsi, M.; et al. Reduced Likelihood of Metastases in Patients with Microsatellite-Unstable Colorectal Cancer. Clin. Cancer Res. 2007, 13, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic Review of Microsatellite Instability and Colorectal Cancer Prognosis. J. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef]
- Sankila, R.; Aaltonen, L.; Jarvinen, H.; Mecklin, J. Better survival rates in patients with MLH1-associated hereditary colorectal cancer. Gastroenterology 1996, 110, 682–687. [Google Scholar] [CrossRef]
- Sargent, D.J.; Shi, Q.; Yothers, G.; Tejpar, S.; Bertagnolli, M.M.; Thibodeau, S.N.; Andre, T.; Labianca, R.; Gallinger, S.; Hamilton, S.R.; et al. Prognostic impact of deficient mismatch repair (dMMR) in 7803 stage II/III colon cancer (CC) patients (pts): A pooled individual pt data analysis of 17 adjuvant trials in the ACCENT database. J. Clin. Oncol. 2014, 32 (Suppl. 15), 3507. [Google Scholar] [CrossRef]
- Laghi, L.; Malesci, A. Microsatellite instability and therapeutic consequences in colorectal cancer. Dig. Dis. 2012, 30, 304–309. [Google Scholar] [CrossRef]
- Klingbiel, D.; Saridaki, Z.; Roth, A.D.; Bosman, F.T.; Delorenzi, M.; Tejpar, S. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: Results of the PETACC-3 trial. Ann. Oncol. 2015, 26, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, F.; Zhou, X.; Ma, Y.; Fu, W. Is microsatellite instability-high really a favorable prognostic factor for advanced colorectal cancer? A meta-analysis. World J. Surg. Oncol. 2019, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Venderbosch, S.; Nagtegaal, I.D.; Maughan, T.S.; Smith, C.G.; Cheadle, J.P.; Fisher, D.; Kaplan, R.; Quirke, P.; Seymour, M.T.; Richman, S.D.; et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014, 20, 5322–5330. [Google Scholar] [CrossRef]
- Taieb, J.; Shi, Q.; Pederson, L.; Alberts, S.; Wolmark, N.; Van Cutsem, E.; de Gramont, A.; Kerr, R.; Grothey, A.; Lonardi, S.; et al. Prognosis of microsatellite instability and/or mismatch repair deficiency stage III colon cancer patients after disease recurrence following adjuvant treatment: Results of an ACCENT pooled analysis of seven studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Samowitz, W.S.; Curtin, K.; Wolff, R.K.; Tripp, S.R.; Caan, B.J.; Slattery, M.L. Microsatellite instability and survival in rectal cancer. Cancer Causes Control 2009, 20, 1763–1768. [Google Scholar] [CrossRef]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Shah, M.A.; Renfro, L.A.; Allegra, C.J.; André, T.; de Gramont, A.; Schmoll, H.J.; Haller, D.G.; Alberts, S.R.; Yothers, G.; Sargent, D.J. Impact of Patient Factors on Recurrence Risk and Time Dependency of Oxaliplatin Benefit in Patients with Colon Cancer: Analysis from Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database. J. Clin. Oncol. 2016, 34, 843–853. [Google Scholar] [CrossRef]
- Hochster, H.S.; Sargent, D.J. One good DNA-damage deserves another: Oxaliplatin in MSI-high colon cancer. J. Natl. Cancer Inst. 2016, 108, djw011. [Google Scholar] [CrossRef]
- Renfro, L.A.; Grothey, A.; Xue, Y.; Saltz, L.B.; André, T.; Twelves, C.; Labianca, R.; Allegra, C.J.; Alberts, S.R.; Loprinzi, C.L.; et al. ACCENT-based web calculators to predict recurrence and overall survival in stage III colon cancer. J. Natl. Cancer Inst. 2014, 106, dju333. [Google Scholar] [CrossRef]
- Kim, J.E.; Hong, Y.S.; Kim, H.J.; Kim, K.P.; Lee, J.L.; Park, S.J.; Lim, S.B.; Park, I.J.; Kim, C.W.; Yoon, Y.S.; et al. Defective Mismatch Repair Status was not Associated with DFS and OS in Stage II Colon Cancer Treated with Adjuvant Chemotherapy. Ann. Surg. Oncol. 2015, 22 (Suppl. 3), 630–637. [Google Scholar] [CrossRef] [PubMed]
- Ribic, C.M.; Sargent, D.J.; Moore, M.J.; Thibodeau, S.N.; French, A.J.; Goldberg, R.M.; Hamilton, S.R.; Laurent-Puig, P.; Gryfe, R.; Shepherd, L.E.; et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N. Engl. J. Med. 2003, 349, 247–257. [Google Scholar] [CrossRef]
- Sargent, D.J.; Marsoni, S.; Monges, G.; Thibodeau, S.N.; Labianca, R.; Hamilton, S.R.; French, A.J.; Kabat, B.; Foster, N.R.; Torri, V.; et al. Defective Mismatch Repair As a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J. Clin. Oncol. 2010, 28, 3219–3226. [Google Scholar] [CrossRef]
- Hutchins, G.; Southward, K.; Handley, K.; Magill, L.; Beaumont, C.; Stahlschmidt, J.; Richman, S.; Chambers, P.; Seymour, M.; Kerr, D.; et al. Value of Mismatch Repair, KRAS, and BRAF Mutations in Predicting Recurrence and Benefits From Chemotherapy in Colorectal Cancer. J. Clin. Oncol. 2011, 29, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, M.; Cabiddu, M.; Pezzica, E.; Corti, D.; Turati, L.; Costanzo, A.; Varricchio, A.; Ghidini, A.; Barni, S.; et al. Microsatellite Instability and Survival in Stage II Colorectal Cancer: A Systematic Review and Meta-analysis. Anticancer Res. 2019, 39, 6431–6441. [Google Scholar] [CrossRef]
- Romiti, A.; Rulli, E.; Pilozzi, E.; Gerardi, C.; Roberto, M.; Legramandi, L.; Falcone, R.; Pacchetti, I.; Marchetti, P.; Floriani, I. Exploring the Prognostic Role of Microsatellite Instability in Patients with Stage II Colorectal Cancer: A Systematic Review and Meta-Analysis. Clin. Color. Cancer 2017, 16, e55–e59. [Google Scholar] [CrossRef]
- André, T.; de Gramont, A.; Vernerey, D.; Chibaudel, B.; Bonnetain, F.; Tijeras-Raballand, A.; Scriva, A.; Hickish, T.; Tabernero, J.; Van Laethem, J.L.; et al. Adjuvant Fluorouracil, Leucovorin, and Oxaliplatin in Stage II to III Colon Cancer: Updated 10-Year Survival and Outcomes According to BRAF Mutation and Mismatch Repair Status of the MOSAIC Study. J. Clin. Oncol. 2015, 33, 4176–4187. [Google Scholar] [CrossRef]
- Tougeron, D.; Mouillet, G.; Trouilloud, I.; Lecomte, T.; Coriat, R.; Aparicio, T.; Des Guetz, G.; Lécaille, C.; Artru, P.; Sickersen, G.; et al. Efficacy of Adjuvant Chemotherapy in Colon Cancer with Microsatellite Instability: A Large Multicenter AGEO Study. J. Natl. Cancer Inst. 2016, 108, djv438. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.; Taieb, J.; Fiskum, J.; Yothers, G.; Goldberg, R.; Yoshino, T.; Alberts, S.; Allegra, C.; de Gramont, A.; Seitz, J.F.; et al. Microsatellite Instability in Patients with Stage III Colon Cancer Receiving Fluoropyrimidine with or without Oxaliplatin: An ACCENT Pooled Analysis of 12 Adjuvant Trials. J. Clin. Oncol. 2021, 39, 642–651. [Google Scholar] [CrossRef]
- Tu, L.; Guan, R.; Yang, H.; Zhou, Y.; Hong, W.; Ma, L.; Zhao, G.; Yu, M. Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. Int. J. Cancer 2020, 147, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Shih, K.; Arkenau, H.T.; Infante, J.R. Clinical impact of checkpoint inhibitors as novel cancer therapies. Drugs 2014, 74, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.Y.; Otsuji, M.; Gorski, K.; Huang, X.; Slansky, J.E.; Pai, S.I.; Shalabi, A.; Shin, T.; Pardoll, D.M.; Tsuchiya, H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001, 193, 839–846. [Google Scholar] [CrossRef]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef]
- Passardi, A.; Canale, M.; Valgiusti, M.; Ulivi, P. Immune Checkpoints as a Target for Colorectal Cancer Treatment. Int. J. Mol. Sci. 2017, 18, 1324. [Google Scholar] [CrossRef] [PubMed]
- Francisco, L.M.; Sage, P.T.; Sharpe, A.H. The PD-1 pathway in tolerance and autoimmunity. Immunol. Rev. 2010, 236, 219–242. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Lonardi, S.; Wong, M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.; Van Cutsem, E.; McDermott, R.S.; Hill, A.G.; et al. Nivolumab + ipilimumab combination in patients with DNA mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) metastatic colorectal cancer (mCRC): First report of the full cohort from CheckMate-142. J. Clin. Oncol. 2018, 36 (Suppl. 4), 553. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Lipson, E.J.; Sharfman, W.H.; Drake, C.G.; Wollner, I.; Taube, J.M.; Anders, R.A.; Xu, H.; Yao, S.; Pons, A.; Chen, L.; et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 2013, 19, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Oncology (Cancer)/Hematologic Malignancies Approval Notifications. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/hematologyoncology-cancer-approvals-safety-notifications (accessed on 2 February 2023).
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Neoadjuvant Immune Checkpoint Inhibition and Novel IO Combinations in Early-Stage Colon Cancer (NICHE). Available online: https://clinicaltrials.gov/ct2/show/NCT03026140 (accessed on 2 February 2023).
- Hu, H.; Kang, L.; Zhang, J.; Wu, Z.; Wang, H.; Huang, M.; Lan, P.; Wu, X.; Wang, C.; Cao, W.; et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): A single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 2022, 7, 38–48. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Ma, R.; Qu, X.; Che, X.; Yang, B.; Li, C.; Hou, K.; Guo, T.; Xiao, J.; Liu, Y. Comparative Analysis and in vitro Experiments of Signatures and Prognostic Value of Immune Checkpoint Genes in Colorectal Cancer. OncoTargets Ther. 2021, 14, 3517–3534. [Google Scholar] [CrossRef] [PubMed]
- Naboush, A.; Roman, C.A.J.; Shapira, I. Immune checkpoint inhibitors in malignancies with mismatch repair deficiency: A review of the state of the current knowledge. J. Investig. Med. 2017, 65, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Krummel, M.F.; Allison, J.P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 1996, 183, 2533–2540. [Google Scholar] [CrossRef] [PubMed]
- Héninger, E.; Krueger, T.E.G.; Lang, J.M. Augmenting antitumor immune responses with epigenetic modifying agents. Front. Immunol. 2015, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Sun, H.; Liu, Y.; Xu, J.; Huang, H.; Fu, J.; Zhang, D.; Tian, T.; Zhao, Y.; et al. Inhibitory immune checkpoints PDCD-1 and LAG-3 hypermethylation may reduce the risk of colorectal cancer. Mol. Med. 2021, 27, 114. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Yothers, G.; Hong, T.S.; Russell, M.M.; You, Y.N.; Parker, W.; Jacobs, S.A.; Colangelo, L.H.; Lucas, P.C.; Gollub, M.J.; et al. Use of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: Initial Results from the Pembrolizumab Arm of a Phase 2 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 1225–1230. [Google Scholar] [CrossRef]
- Tarpgaard, L.S.; Andersen, P.V.; Øgaard, N.; Demuth, C.; Andersen, C.L.; Pfeiffer, P. Complete pathological and serological response to immunotherapy in a patient with MMR-deficient early rectal cancer. Ann. Oncol. 2021, 32, 805–806. [Google Scholar] [CrossRef] [PubMed]
- Study of Pembrolizumab (MK-3475) vs. Standard Therapy in Participants with Microsatellite Instability-High (MSI-H) or Mismatch Repair Deficient (dMMR) Stage IV Colorectal Carcinoma (MK-3475-177/KEYNOTE-177). Available online: https://clinicaltrials.gov/ct2/show/NCT02563002 (accessed on 2 February 2023).
- An Investigational Immuno-Therapy Study of Nivolumab, and Nivolumab in Combination with Other Anti-Cancer Drugs, in Colon Cancer That Has Come Back or Has Spread (CheckMate142). Available online: https://clinicaltrials.gov/ct2/show/NCT02060188 (accessed on 2 February 2023).
- Combination Chemotherapy, Bevacizumab, and/or Atezolizumab in Treating Patients with Deficient DNA Mismatch Repair Metastatic Colorectal Cancer, the COMMIT Study. Available online: https://clinicaltrials.gov/ct2/show/NCT02997228 (accessed on 2 February 2023).
- Overman, M.J.; Yothers, G.; Jacobs, S.A.; Sanoff, H.K.; Cohen, D.J.; Guthrie, K.A.; Henry, N.L.; Ganz, P.A.; Kopetz, S.; Lucas, P.C.; et al. NRG-GI004/SWOG-S1610: Colorectal Cancer Metastatic dMMR Immuno-Therapy (COMMIT) Study—A randomized phase III study of atezolizumab (atezo) monotherapy versus mFOLFOX6/bevacizumab/atezo in the first-line treatment of patients (pts) with deficient DNA mismatch repair (dMMR) or microsatellite instability high (MSI-H) metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2021, 39 (Suppl. 3), TPS158. [Google Scholar]
- Neoadjuvant Treatment in Rectal Cancer with Radiotherapy Followed by Atezolizumab and Bevacizumab (TARZAN) (TARZAN). Available online: https://clinicaltrials.gov/ct2/show/NCT04017455 (accessed on 2 February 2023).
- Regorafenib and Pembrolizumab in Treating Participants with Advanced or Metastatic Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03657641 (accessed on 2 February 2023).
- Efficacy and Safety of Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) in Previously Treated Participants with Select Solid Tumors (MK-7902-005/E7080-G000-224/LEAP-005). Available online: https://clinicaltrials.gov/ct2/show/NCT03797326 (accessed on 2 February 2023).
- Cadonilimab for PD-1/PD-L1 Blockade-Refractory, MSI-H/dMMR, Advanced Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT05426005 (accessed on 2 February 2023).
- Ghiringhelli, F.; Apetoh, L. Enhancing the anticancer effects of 5-fluorouracil: Current challenges and future perspectives. Biomed. J. 2015, 38, 111–116. [Google Scholar] [PubMed]
- Orecchioni, S.; Talarico, G.; Labanca, V.; Calleri, A.; Mancuso, P.; Bertolini, F. Vinorelbine, cyclophosphamide and 5-FU effects on the circulating and intratumoural landscape of immune cells improve anti-PD-L1 efficacy in preclinical models of breast cancer and lymphoma. Br. J. Cancer. 2018, 118, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Farhood, B.; Najafi, M.; Mortezaee, K. CD8 + cytotoxic T lymphocytes in cancer immunotherapy: A review. J. Cell. Physiol. 2019, 234, 8509–8521. [Google Scholar] [CrossRef]
- Malesci, A.; Bianchi, P.; Celesti, G.; Basso, G.; Marchesi, F.; Grizzi, F.; Di Caro, G.; Cavalleri, T.; Rimassa, L.; Palmqvist, R.; et al. Tumor-associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. Oncoimmunology 2017, 6, e1342918. [Google Scholar] [CrossRef]
- Cavalleri, T.; Greco, L.; Rubbino, F.; Hamada, T.; Quaranta, M.; Grizzi, F.; Sauta, E.; Craviotto, V.; Bossi, P.; Vetrano, S.; et al. Tumor-associated macrophages and risk of recurrence in stage III colorectal cancer. J. Pathol. Clin. Res. 2022, 8, 307–312. [Google Scholar] [CrossRef]
- Goggi, J.L.; Tan, Y.X.; Hartimath, S.V.; Jieu, B.; Hwang, Y.Y.; Jiang, L.; Boominathan, R.; Cheng, P.; Yuen, T.Y.; Chin, H.X.; et al. Granzyme B PET Imaging of Immune Checkpoint Inhibitor Combinations in Colon Cancer Phenotypes. Mol. Imaging Biol. 2020, 22, 1392–1402. [Google Scholar] [CrossRef] [PubMed]
- Larimer, B.M.; Wehrenberg-Klee, E.; Dubois, F.; Mehta, A.; Kalomeris, T.; Flaherty, K.; Boland, G.; Mahmood, U. Granzyme B PET Imaging as a Predictive Biomarker of Immunotherapy Response. Cancer Res. 2017, 77, 2318–2327. [Google Scholar] [CrossRef]
- Larimer, B.M.; Bloch, E.; Nesti, S.; Austin, E.E.; Wehrenberg-Klee, E.; Boland, G.; Mahmood, U. The Effectiveness of Checkpoint Inhibitor Combinations and Administration Timing Can Be Measured by Granzyme B PET Imaging. Clin. Cancer Res. 2019, 25, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Nivolumab plus low-dose ipilimumab in previously treated patients with microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: 4-year follow-up from CheckMate 142. Ann. Oncol. 2022, 33, 1052–1060. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Pembrolizumab + Poly-ICLC in MRP Colon Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02834052 (accessed on 2 February 2023).
- Olaptesed (NOX-A12) Alone and in Combination With Pembrolizumab in Colorectal and Pancreatic Cancer (Keynote-559). Available online: https://clinicaltrials.gov/ct2/show/NCT03168139 (accessed on 2 February 2023).
- Segal, N.H.; Saro, J.; Melero, I.; Ros, W.; Argiles, G.; Marabelle, A.; Rodriguez Ruiz, M.E.; Albanell, J.; Calvo, E.; Moreno, V.; et al. Phase I studies of the novel carcinoembryonic antigen T-cell bispecific (CEA-CD3 TCB) antibody as a single agent and in combination with atezolizumab: Preliminary efficacy and safety in patients (pts) with metastatic colorectal cancer (mCRC). Ann. Oncol. 2017, 28, v134. [Google Scholar] [CrossRef]
- Study of Favezelimab (MK-4280) as Monotherapy and in Combination With Pembrolizumab (MK-3475) with or without Chemotherapy or Lenvatinib (MK-7902) and Favezelimab/Pembrolizumab (MK-4280A) as Monotherapy in Adults with Advanced Solid Tumors (MK-4280-001). Available online: https://clinicaltrials.gov/ct2/show/NCT02720068 (accessed on 2 February 2023).
- Garralda, E.; Sukari, A.; Lakhani, N.J.; Patnaik, A.; Lou, Y.; Im, S.A.; Golan, T.; Geva, R.; Wermke, M.; de Miguel, M.; et al. A first-in-human study of the anti-LAG-3 antibody favezelimab plus pembrolizumab in previously treated, advanced microsatellite stable colorectal cancer. ESMO Open 2022, 7, 100639. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Villarejo-Campos, P.; Guijo, I.; Hernández-Villafranca, S.; García-Olmo, D.; González-Soares, S.; Guadalajara, H.; Jiménez-Galanes, S.; Qian, C. Update for Advance CAR-T Therapy in Solid Tumors, Clinical Application in Peritoneal Carcinomatosis from Colorectal Cancer and Future Prospects. Front. Immunol. 2022, 13, 841425. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Crisafulli, G.; Sogari, A.; Reilly, N.M.; Arena, S.; Lamba, S.; Bartolini, A.; Amodio, V.; Magrì, A.; Novara, L.; et al. Adaptive mutability of colorectal cancers in response to targeted therapies. Science 2019, 366, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, L.; Rubbino, F.; Dal Buono, A.; Laghi, L. Microsatellite Instability and Immune Response: From Microenvironment Features to Therapeutic Actionability—Lessons from Colorectal Cancer. Genes 2023, 14, 1169. https://doi.org/10.3390/genes14061169
Greco L, Rubbino F, Dal Buono A, Laghi L. Microsatellite Instability and Immune Response: From Microenvironment Features to Therapeutic Actionability—Lessons from Colorectal Cancer. Genes. 2023; 14(6):1169. https://doi.org/10.3390/genes14061169
Chicago/Turabian StyleGreco, Luana, Federica Rubbino, Arianna Dal Buono, and Luigi Laghi. 2023. "Microsatellite Instability and Immune Response: From Microenvironment Features to Therapeutic Actionability—Lessons from Colorectal Cancer" Genes 14, no. 6: 1169. https://doi.org/10.3390/genes14061169







