Genome-Wide Identification of the NPR1-like Gene Family in Solanum tuberosum and Functional Characterization of StNPR1 in Resistance to Ralstonia solanacearum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis of StNPR1
2.2. Plant Growth and Disease Treatment
2.3. qRT-PCR
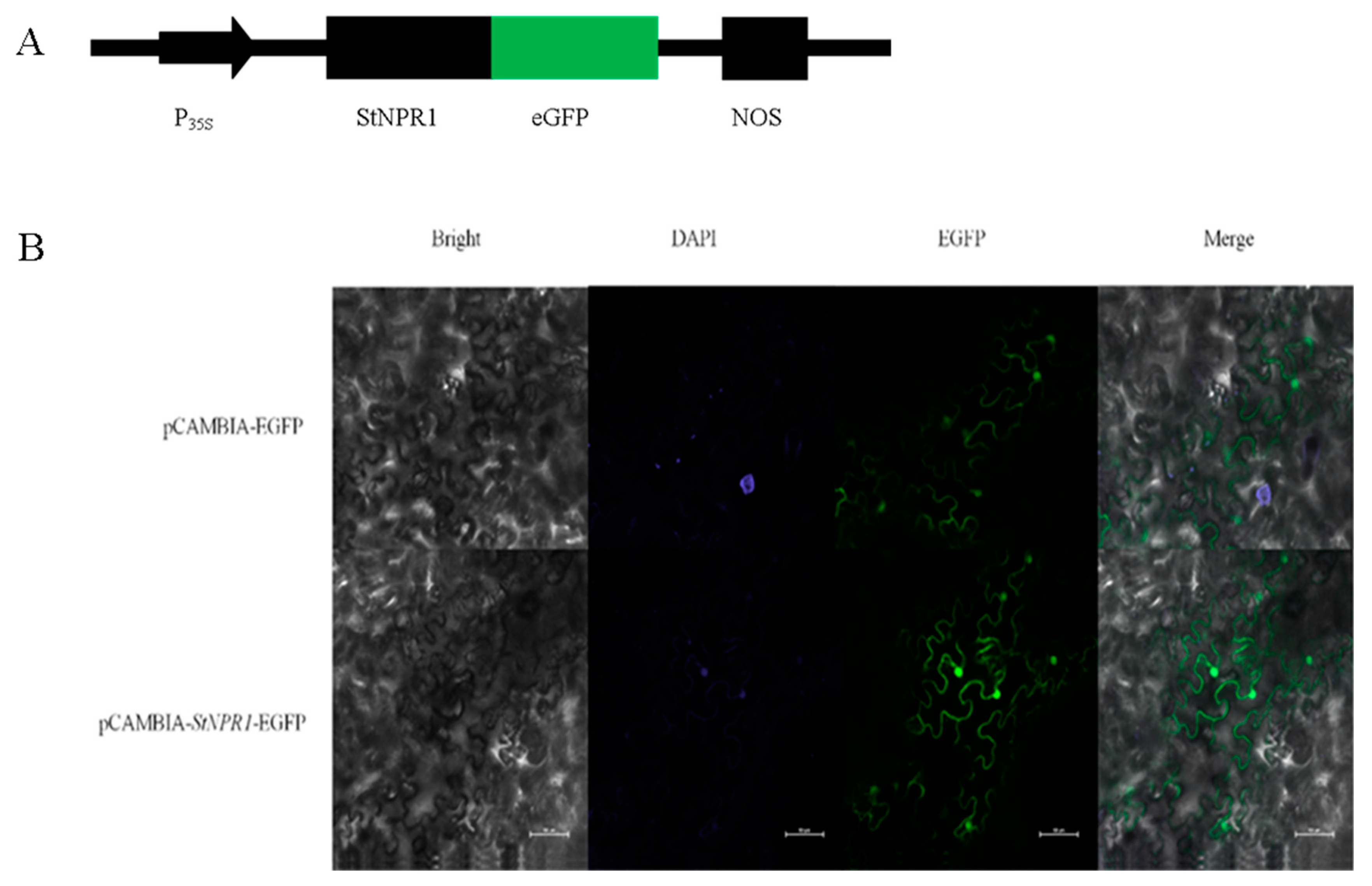
2.4. Subcellular Localization of StNPR1 Protein
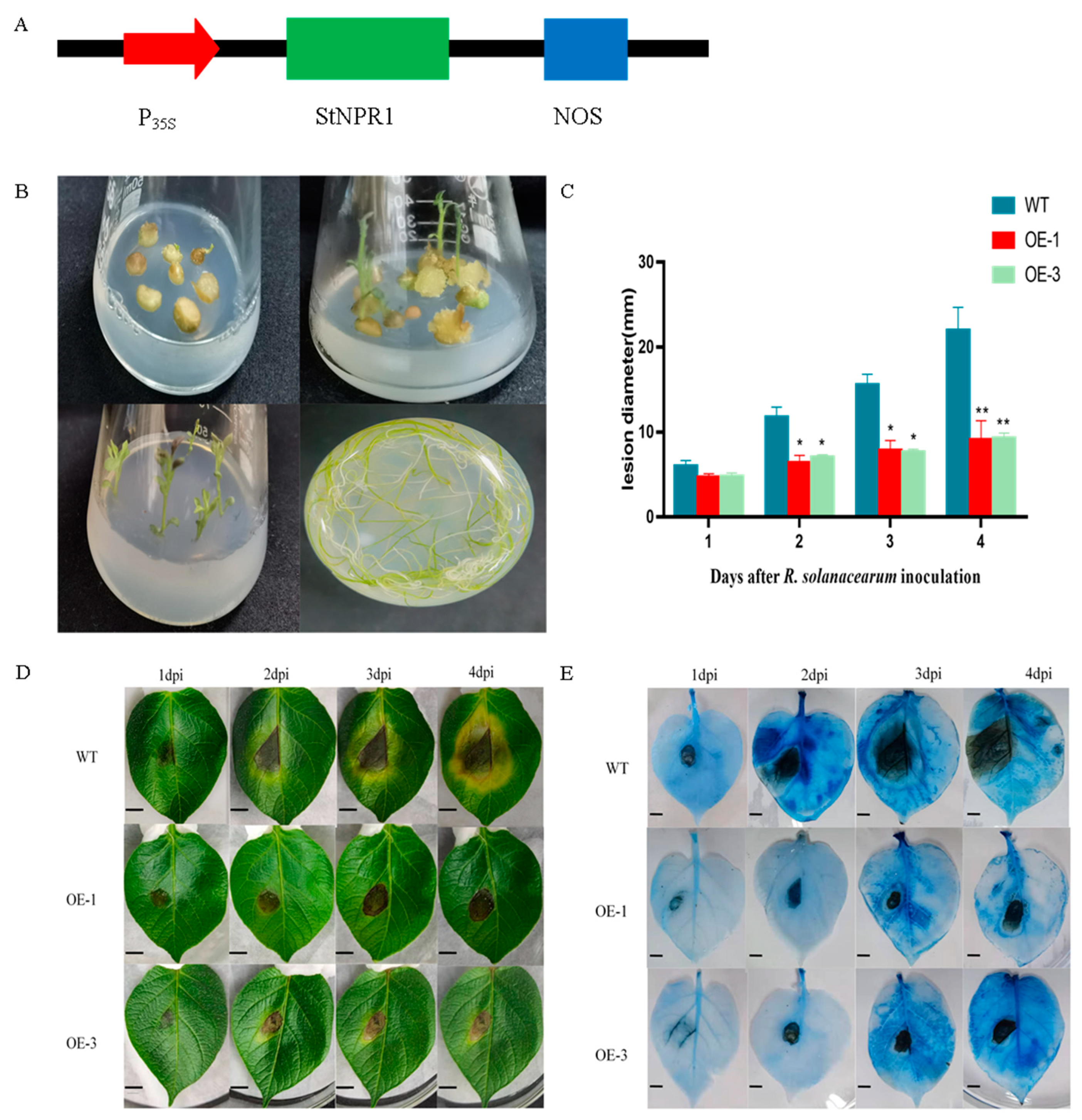
2.5. Overexpression of StNPR1 in Potato
2.6. Analysis of Resistance of StNPR1 Transgenic Plants to R. solanacearum
2.7. Statistical Analysis
3. Results and Analysis
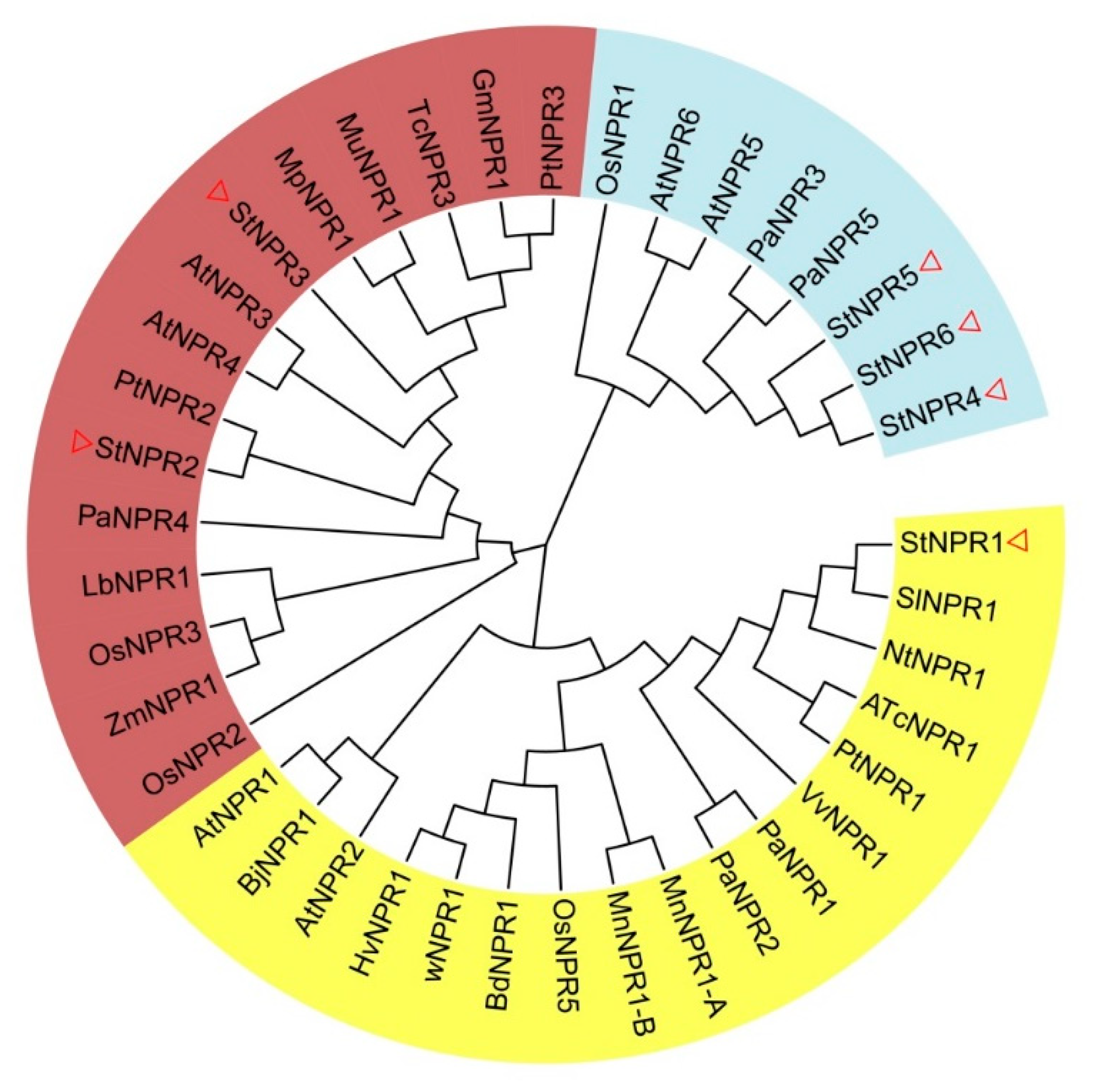
3.1. Identification, Phylogeny, and Characterization of Potato NPR1-like Genes
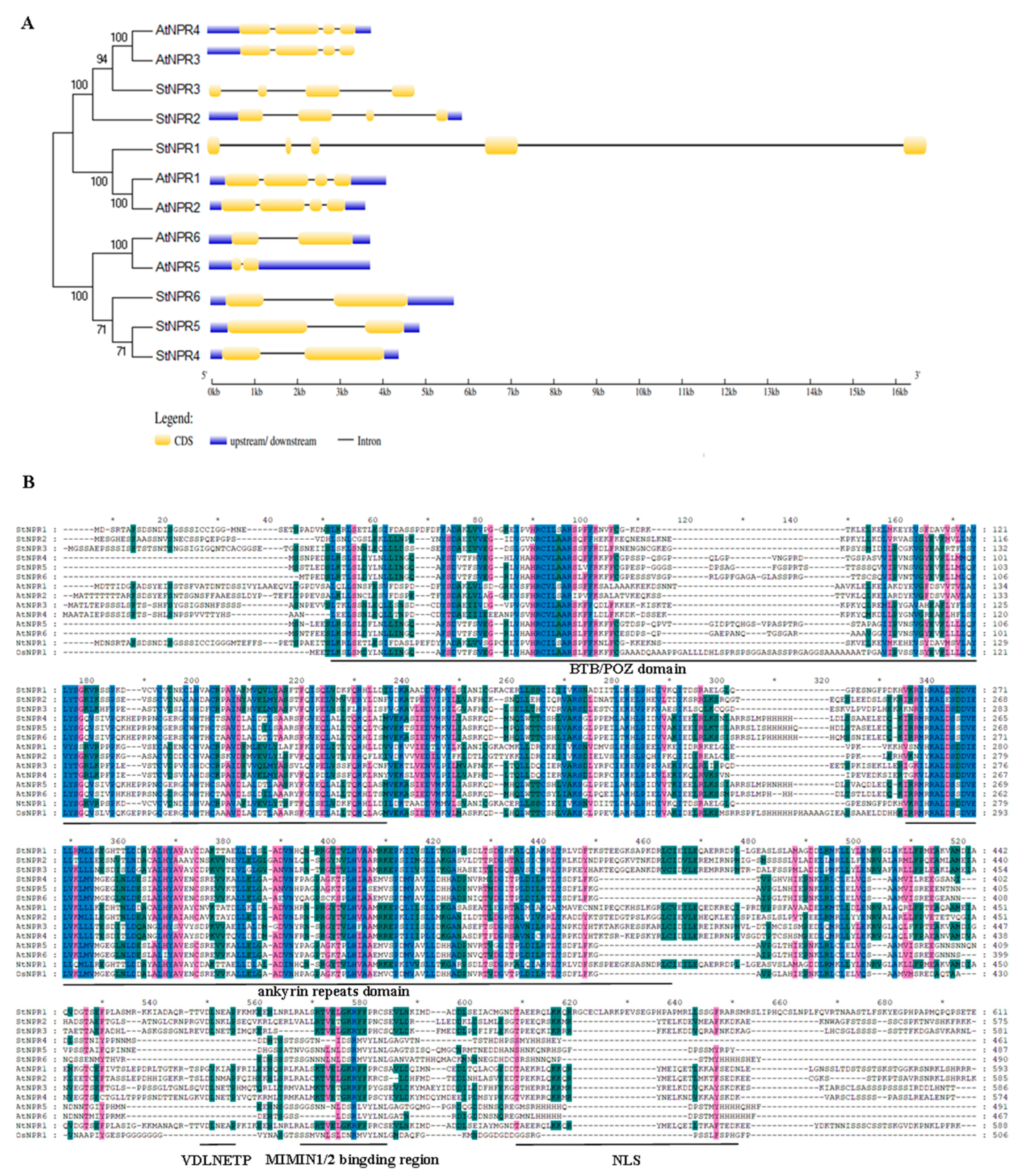
3.2. Sequence and Structural Analysis of StNPR1-like Genes and Proteins
3.3. Expression Analysis of StNPR1-like Genes in Different Tissues and Organs
3.4. Expression Analysis of StNPR1-like Genes under Biotic Stress
3.5. Subcellular Localization of StNPR1 Gene in Tobacco
3.6. Overexpression of the StNPR1 Gene Enhances Potato Resistance to R. solanacearum
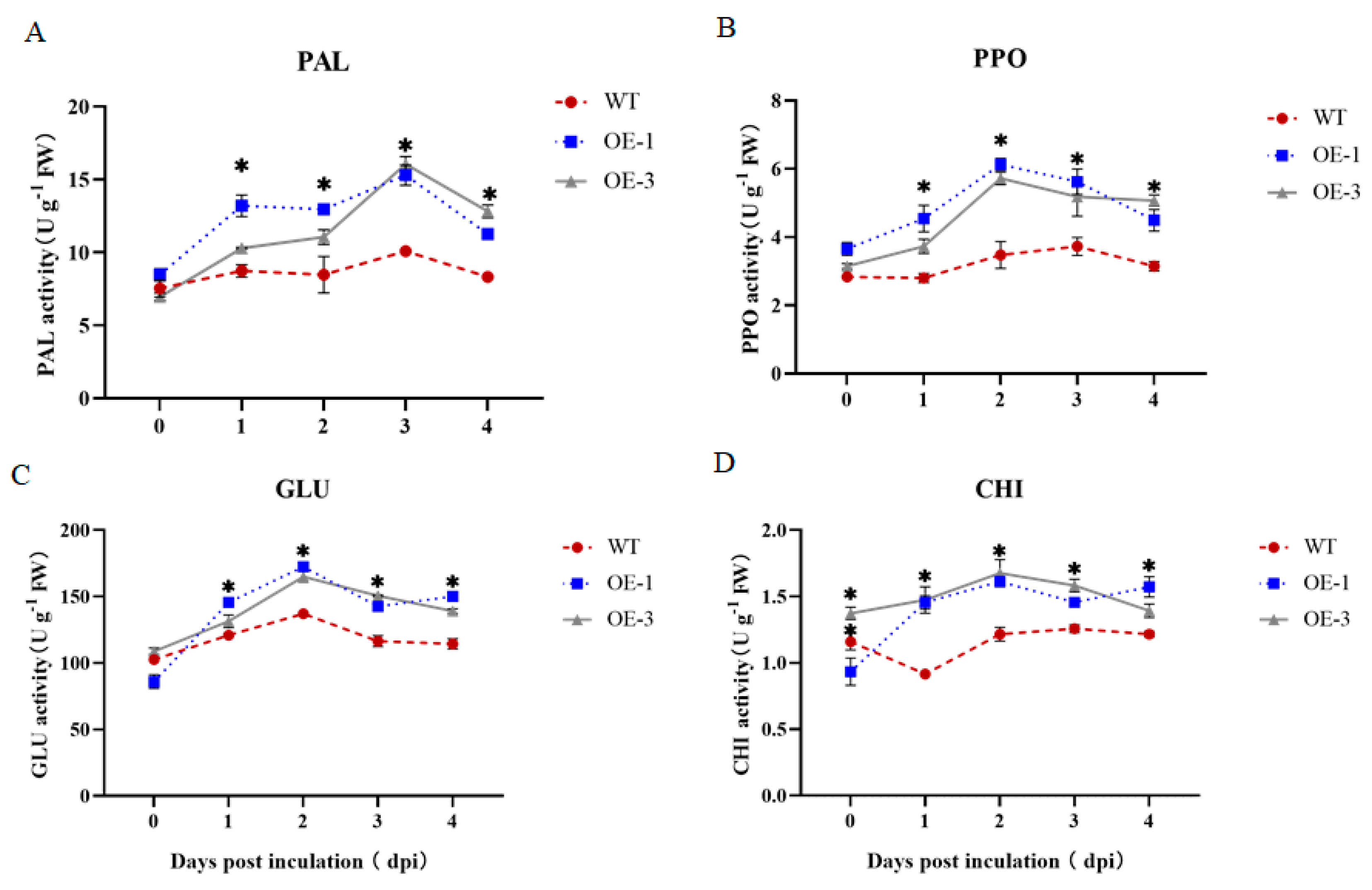
3.7. Effect of StNPR1 Overexpression on Defensive Enzyme Activities
3.8. Effect of Overexpression of StNPR1 on ROS Content and Antioxidant Enzyme Activity
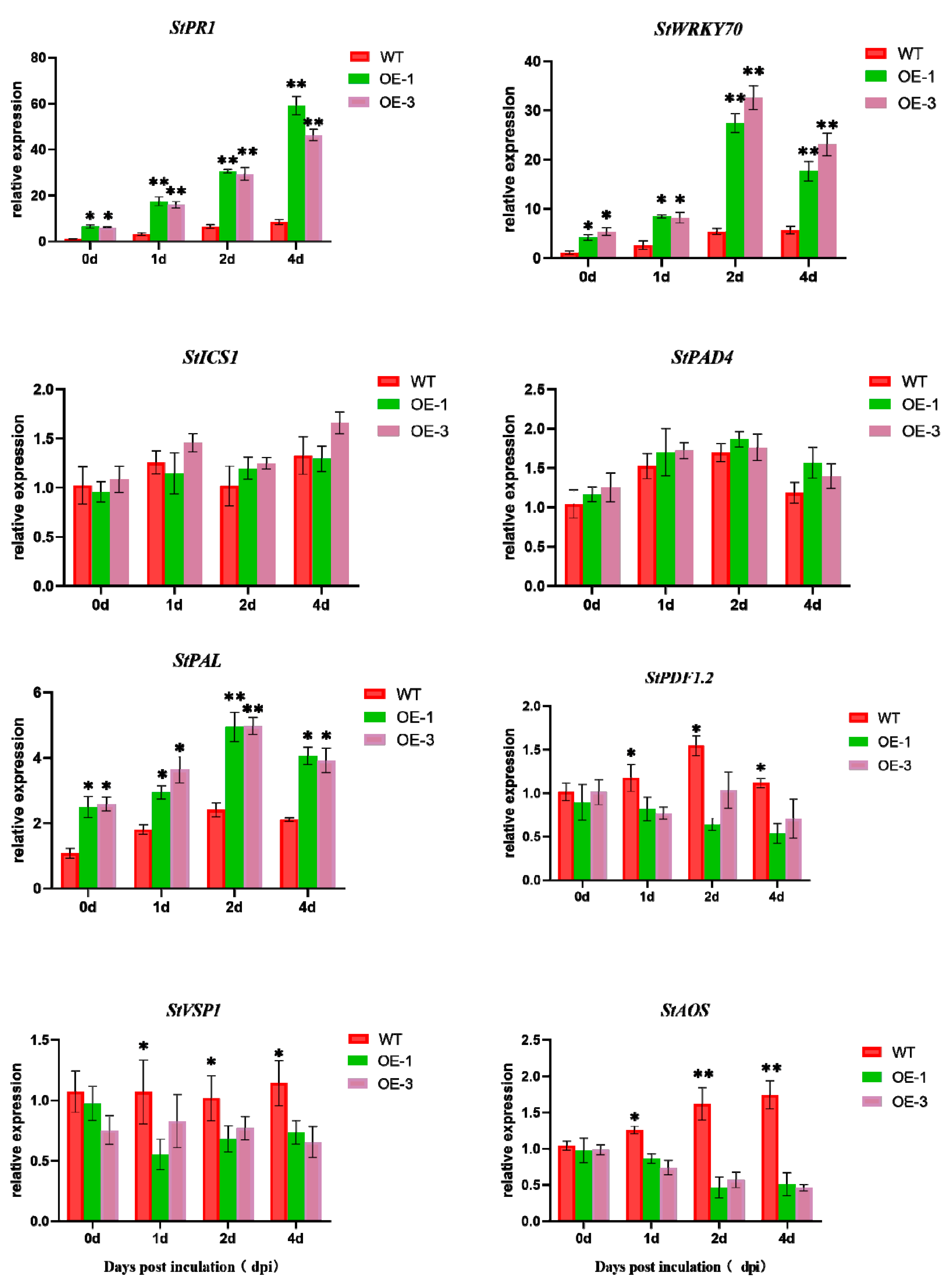
3.9. Effect of Transgenic Potato Overexpression of StNPR1 on SA/JA-Signaling-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahgoub, H.A.M.; Eisa, G.S.A.; Youssef, M.A.H. Molecular, biochemical and anatomical analysis of some potato (Solanum tuberosum L.) cultivars growing in Egypt. J. Genet. Eng. Biotechnol. 2015, 13, 39–49. [Google Scholar] [CrossRef]
- Pieterse, C.M.; van Wees, S.C.; van Pelt, J.A.; Knoester, M.; Laan, R.; Gerrits, H.; Weisbeek, P.J.; van Loon, L.C. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 1998, 10, 1571–1580. [Google Scholar] [CrossRef]
- Ferreira, V.; González, M.; Pianzzola, M.J.; Coll, N.S.; Siri, M.I.; Valls, M. Molecular Detection of Ralstonia solanacearum to Facilitate Breeding for Resistance to Bacterial Wilt in Potato. Methods Mol. Biol. 2021, 2354, 375–385. [Google Scholar] [PubMed]
- Jiang, G.; Wei, Z.; Xu, J.; Chen, H.; Zhang, Y.; She, X.; Macho, A.P.; Ding, W.; Liao, B. Bacterial Wilt in China: History, Current Status, and Future Perspectives. Front. Plant Sci. 2017, 8, 1549. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.S.; Liu, Q.P.; Zhang, S.T.; Tang, Y.M.; Jiang, G.F.; Li, S.L.; Ding, W. The sequevar distribution of Ralstonia solanacearum in tobacco-growing zones of China is structured by elevation. Eur. J. Plant Pathol. 2017, 147, 541–551. [Google Scholar] [CrossRef]
- Cao, H.; Glazebrook, J.; Clarke, J.D.; Volko, S.; Dong, X. The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 1997, 88, 57–63. [Google Scholar] [CrossRef]
- Mou, Z.; Fan, W.H.; Dong, X.N. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes. Cell 2003, 113, 935–944. [Google Scholar] [CrossRef]
- Chern, M.S.; Fitzgerald, H.A.; Yadav, R.C.; Canlas, P.E.; Dong, X.; Ronald, P.C. Evidence for a disease-resistance pathway in rice similar to the NPR1-mediated signaling pathway in Arabidopsis. Plant J. 2001, 27, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Bi, W.S.; Gao, J.; Yu, X.M.; Wang, H.Y.; Liu, D.Q. Systemic acquired resistance, NPR1, and pathogenesis-related genes in wheat and barley. J. Integr. Agric. 2018, 17, 2468–2477. [Google Scholar] [CrossRef]
- Malnoy, M.; Jin, Q.; Borejsza-Wysocka, E.E.; He, S.Y.; Aldwinckle, H.S. Overexpression of the apple MpNPR1 gene confers increased disease resistance in malus x domestica. Mol. Plant Microbe Interact. 2007, 20, 1568–1580. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Cheng, C.; Gao, Q.Q.; Liu, J.Y.; Guo, X.Q. Molecular cloning and characterization of GhNPR1, a gene implicated in pathogen responses from cotton (Gossypium hirsutum L.). Biosci. Rep. 2008, 28, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Backer, R.; Naidoo, S.; van den Berg, N. The Nonexpressor of Pathogenesis-Related Genes 1 (NPR1) and Related Family: Mechanistic Insights in Plant Disease Resistance. Front. Plant Sci. 2019, 10, 102. [Google Scholar] [CrossRef]
- Castello, M.J.; Medina-Puche, L.; Lamilla, J.; Tornero, P. NPR1 paralogs of Arabidopsis and their role in salicylic acid perception. PLoS ONE 2018, 13, e0209835. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.L.; Sun, T.J.; Ao, K.; Peng, Y.J.; Zhang, Y.X.; Li, X.; Zhang, Y.L. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454. [Google Scholar] [CrossRef] [PubMed]
- Rong, D.Y.; Luo, N.; Mollet, J.C.; Liu, X.M.; Yang, Z.B. Salicylic Acid Regulates Pollen Tip Growth through an NPR3/NPR4-Independent Pathway. Mol. Plant 2016, 9, 1478–1491. [Google Scholar] [CrossRef] [PubMed]
- Hepworth, S.R.; Zhang, Y.L.; McKim, S.; Li, X.; Haughn, G. BLADE-ON-PETIOLE-dependent signaling controls leaf and floral patterning in Arabidopsis. Plant Cell 2005, 17, 1434–1448. [Google Scholar] [CrossRef]
- Norberg, M.; Holmlund, M.; Nilsson, O. The BLADE ONPETIOLE genes act redundantly to control the growth and development of lateral organs. Development 2005, 132, 2203–2213. [Google Scholar] [CrossRef]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kogel, K.H.; Langen, G. Induced disease resistance and gene expression in cereals. Cell Microbiol. 2005, 7, 1555–1564. [Google Scholar] [CrossRef]
- Spoel, S.H.; Koornneef, A.; Claessens, S.M.C.; Korzelius, J.P.; Van Pelt, J.A.; Mueller, M.J.; Buchala, A.J.; Metraux, J.P.; Brown, R.; Kazan, K.; et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 2003, 15, 760–770. [Google Scholar] [CrossRef]
- Delaney, T.P.; Friedrich, L.; Ryals, J.A. Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc. Natl. Acad. Sci. USA 1995, 92, 6602–6606. [Google Scholar] [CrossRef]
- Cao, H.; Bowling, S.A.; Gordon, A.S.; Dong, X. Characterization of an Arabidopsis Mutant That Is Nonresponsive to Inducers of Systemic Acquired Resistance. Plant Cell 1994, 6, 1583–1592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008, 379, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, Y.; Zhang, M.; Zhou, S.; Kong, X.; Wang, W. Overexpression of the Wheat Expansin Gene TaEXPA2 Improved Seed Production and Drought Tolerance in Transgenic Tobacco Plants. PLoS ONE 2016, 11, e0153494. [Google Scholar] [CrossRef] [PubMed]
- Imanifard, Z.; Vandelle, E.; Bellin, D. Measurement of Hypersensitive Cell Death Triggered by Avirulent Bacterial Pathogens in Arabidopsis. Methods Mol. Biol. 2018, 1743, 39–50. [Google Scholar]
- Lee, H.I.; Leon, J.; Raskin, I. Biosynthesis and metabolism of salicylic acid. Proc. Natl. Acad. Sci. USA 1995, 92, 4076–4079. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Palva, E.T. The WRKY70 transcription factor: A node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 2004, 16, 319–331. [Google Scholar] [CrossRef]
- Morris, K.; MacKerness, S.A.; Page, T.; John, C.F.; Murphy, A.M.; Carr, J.P.; Buchanan-Wollaston, V. Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J. 2000, 23, 677–685. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E.; et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120. [Google Scholar] [CrossRef]
- Rojo, E.; Leon, J.; Sanchez-Serrano, J.J. Cross-talk between wound signalling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J. 1999, 2, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Von Malek, B.; van der Graaff, E.; Schneitz, K.; Keller, B. The Arabidopsis male-sterile mutant dde2-2 is defective in the ALLENE OXIDE SYNTHASE gene encoding one of the key enzymes of the jasmonic acid biosynthesis pathway. Planta 2002, 216, 187–192. [Google Scholar] [CrossRef]
- Zheng, Y.; Sheng, J.P.; Zhao, R.R.; Zhang, J.; Lv, S.N.; Liu, L.Y.; Shen, L. Preharvest L-Arginine Treatment Induced Postharvest Disease Resistance to Botrysis cinerea in Tomato Fruits. J. Agric. Food Chem. 2011, 59, 6543–6549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, Z.Q.; Qin, G.Z.; Tian, S.P. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biol. Technol. 2010, 56, 50–55. [Google Scholar] [CrossRef]
- Cao, H.; Li, X.; Dong, X. Generation of broad-spectrum disease resistance by overexpression of an essential regulatory gene in systemic acquired resistance. Proc. Natl. Acad. Sci. USA 1998, 95, 6531–6536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, Y.T.; Qu, N.; Zhao, Q.; Bi, D.; Li, X. Negative regulation of defense responses in Arabidopsis by two NPR1 paralogs. Plant J. 2006, 48, 647–656. [Google Scholar] [CrossRef]
- Peraza-Echeverria, S.; Santamaria, J.M.; Fuentes, G.; Menendez-Ceron, M.D.; Vallejo-Reyna, M.A.; Herrera-Valencia, V.A. The NPR1 family of transcription cofactors in papaya: Insights into its structure, phylogeny and expression. Genes Genomics. 2012, 34, 379–390. [Google Scholar] [CrossRef]
- Backer, R.; Mahomed, W.; Reeksting, B.J.; Engelbrecht, J.; Ibarra-Laciette, E.; van den Berg, N. Phylogenetic and expression analysis of the NPR1-like gene family from Persea americana (Mill.). Front. Plant Sci. 2015, 6, 300. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z.; Niu, X.; Xu, Q.; Yang, L. Genome-Wide Identification and Analysis of the NPR1-Like Gene Family in Bread Wheat and Its Relatives. Int. J. Mol. Sci. 2019, 20, 5974. [Google Scholar] [CrossRef]
- Prasath, D.; Karthika, R.; Habeeba, N.T.; Suraby, E.J.; Rosana, O.B.; Shaji, A.; Eapen, S.J.; Deshpande, U.; Anandaraj, M. Comparison of the Transcriptomes of Ginger (Zingiber officinale Rosc.) and Mango Ginger (Curcuma amada Roxb.) in Response to the Bacterial Wilt Infection. PLoS ONE 2014, 9, e99731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Zhu, Z.; Song, Y.; Wang, X.Y.; Wang, X.L.; Zhou, X.; Gao, J.; Kuai, B.K. MKK4/MKK5-MPK1/MPK2 cascade mediates SA-activated leaf senescence via phosphorylation of NPR1 in Arabidopsis. Plant Mol. Biol. 2020, 104, 217. [Google Scholar] [CrossRef]
- Uwamahoro, F.; Berlin, A.; Bucagu, C.; Bylund, H.; Yuen, J. Ralstonia solanacearum causing potato bacterial wilt: Host range and cultivars’ susceptibility in Rwanda. Mol. Plant Pathol. 2020, 69, 559–568. [Google Scholar] [CrossRef]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef]
- Thomma, B.P.; Eggermont, K.; Penninckx, I.A.; Mauch-Mani, B.; Vogelsang, R.; Cammue, B.P.; Broekaert, W.F. Separate jasmonate-dependent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, F.; Kong, D.; Feng, Z.; Xu, Y.; Yuan, Q.; Liu, D.; Wang, X.; Feng, X.; Li, F. Genome-Wide Identification of the NPR1-like Gene Family in Solanum tuberosum and Functional Characterization of StNPR1 in Resistance to Ralstonia solanacearum. Genes 2023, 14, 1170. https://doi.org/10.3390/genes14061170
He F, Kong D, Feng Z, Xu Y, Yuan Q, Liu D, Wang X, Feng X, Li F. Genome-Wide Identification of the NPR1-like Gene Family in Solanum tuberosum and Functional Characterization of StNPR1 in Resistance to Ralstonia solanacearum. Genes. 2023; 14(6):1170. https://doi.org/10.3390/genes14061170
Chicago/Turabian StyleHe, Fumeng, Dexing Kong, Zhe Feng, Yongqing Xu, Qiang Yuan, Dan Liu, Xue Wang, Xu Feng, and Fenglan Li. 2023. "Genome-Wide Identification of the NPR1-like Gene Family in Solanum tuberosum and Functional Characterization of StNPR1 in Resistance to Ralstonia solanacearum" Genes 14, no. 6: 1170. https://doi.org/10.3390/genes14061170



