Regulatory Mechanisms of ArAux/IAA13 and ArAux/IAA16 in the Rooting Process of Acer rubrum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
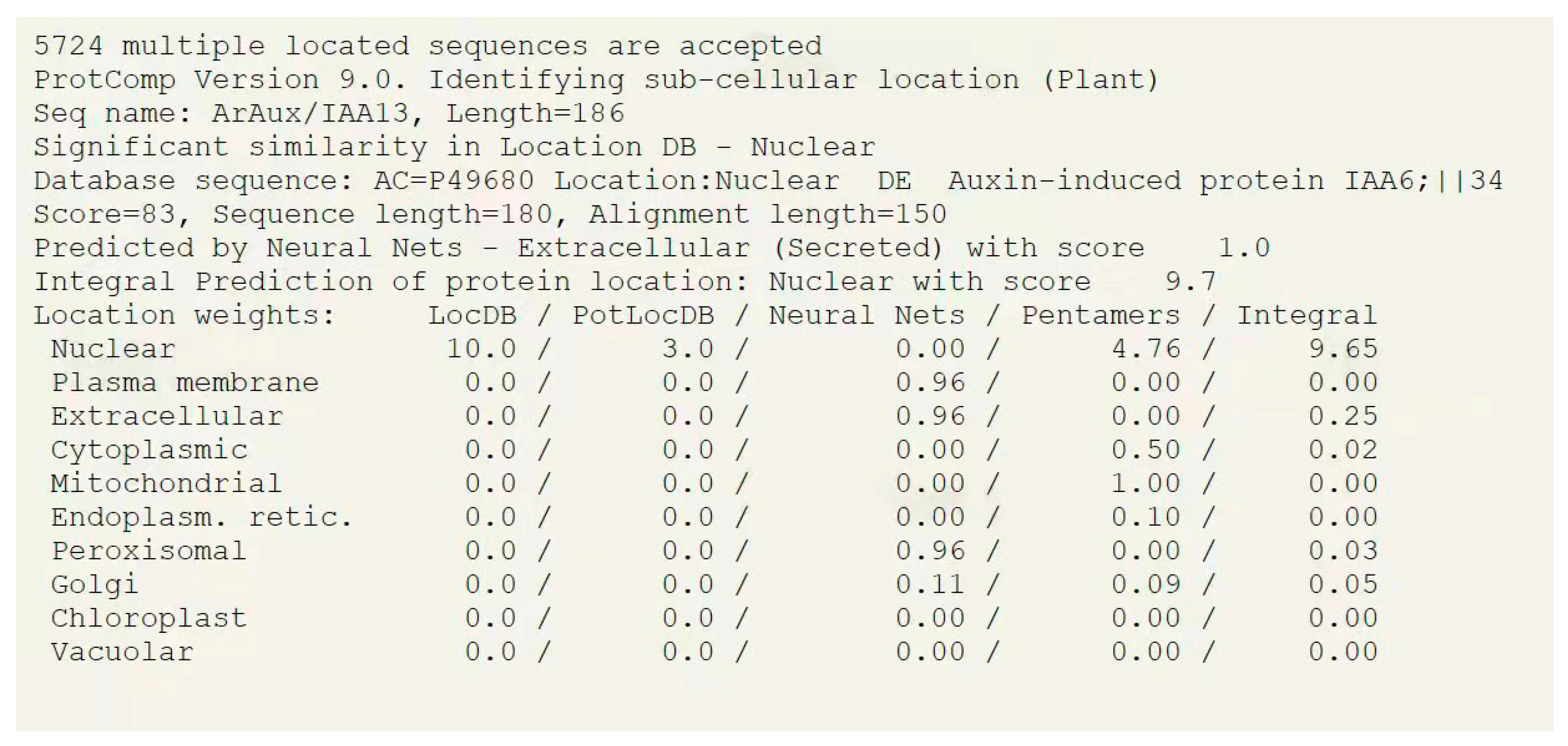
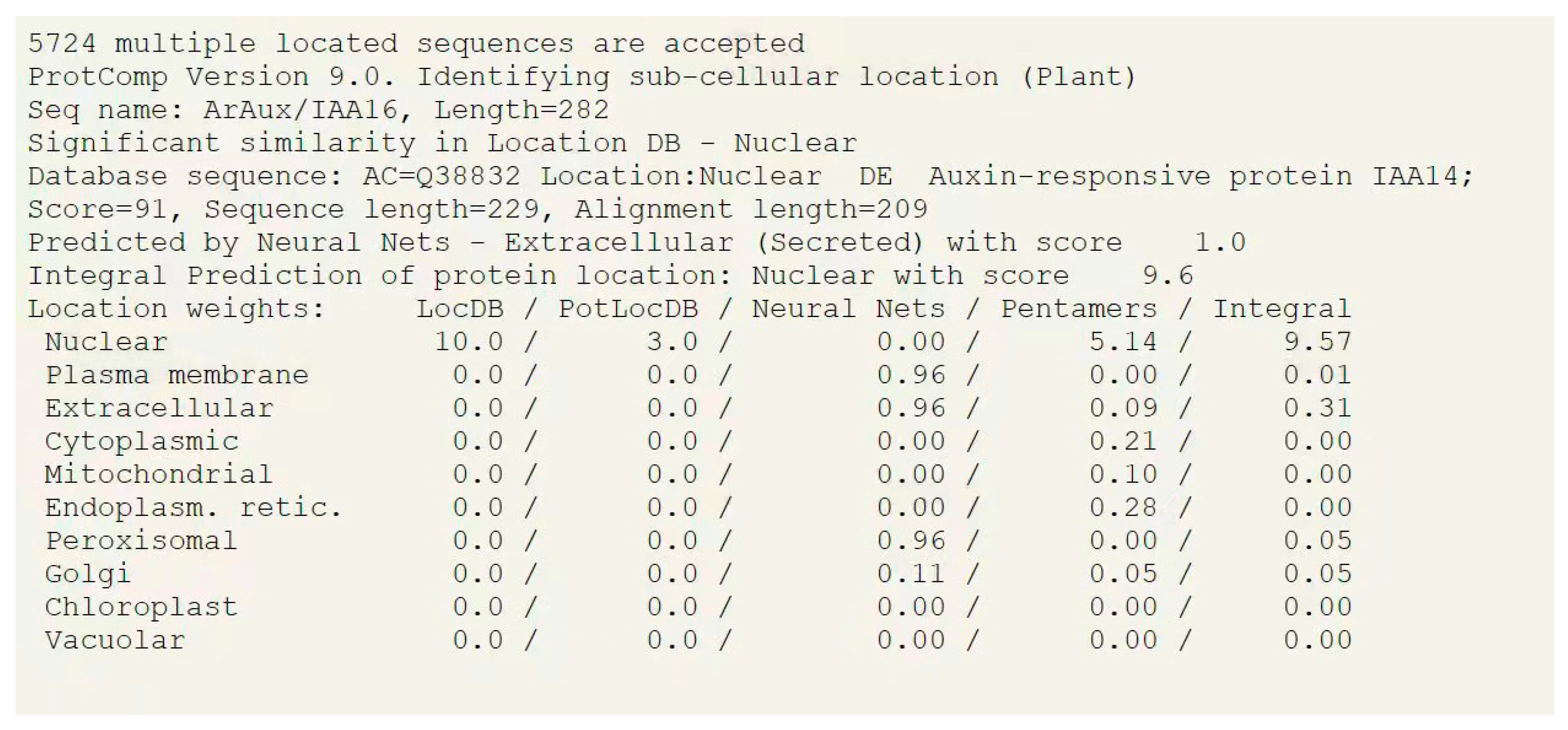
2.2. Bioinformatics Analysis of ArAux/IAA13 and ArAux/IAA16
2.3. Gene Cloning
2.4. Subcellular Localization
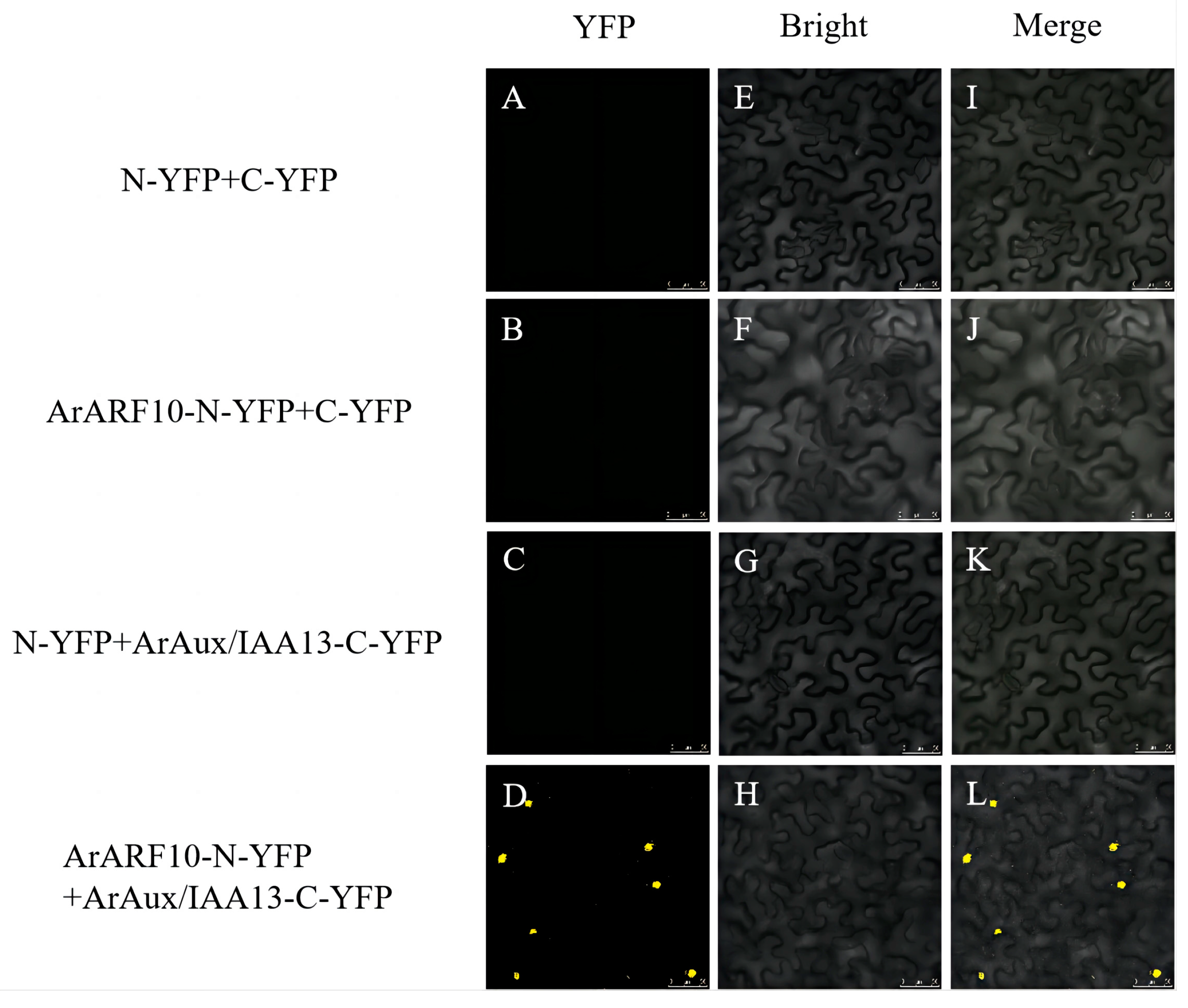
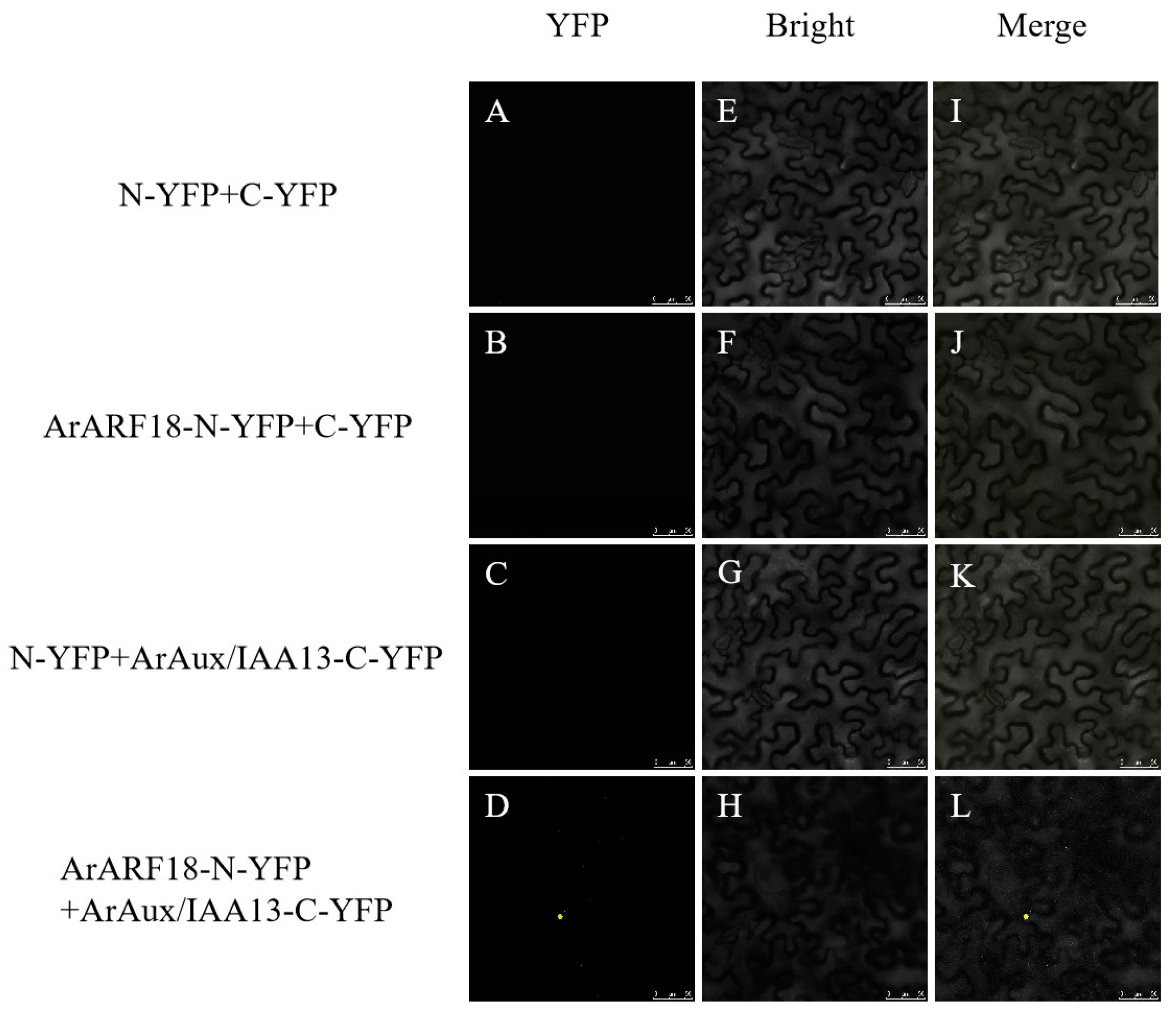
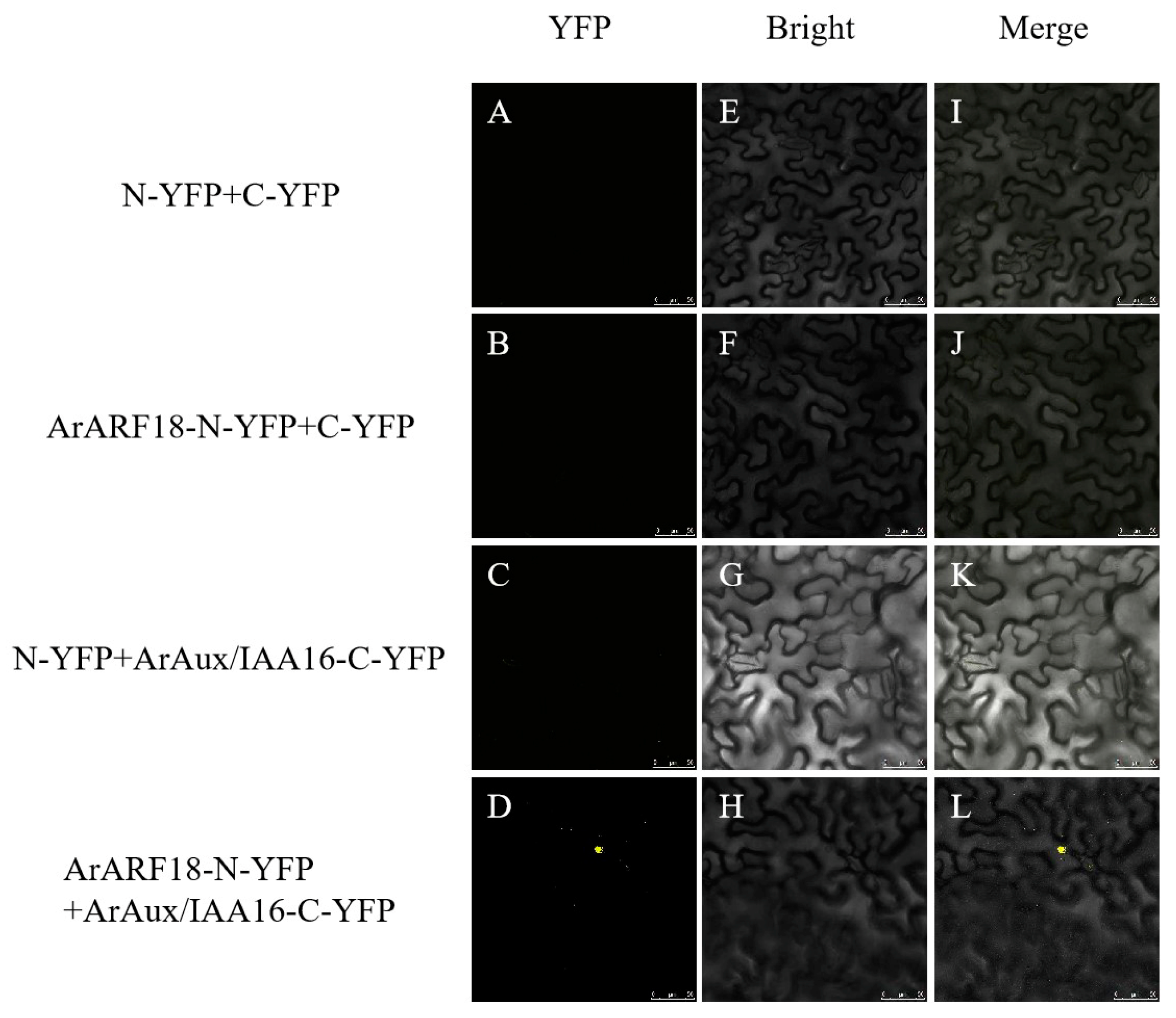
2.5. Bimolecular Fluorescence Complementation (BIFC) Assay
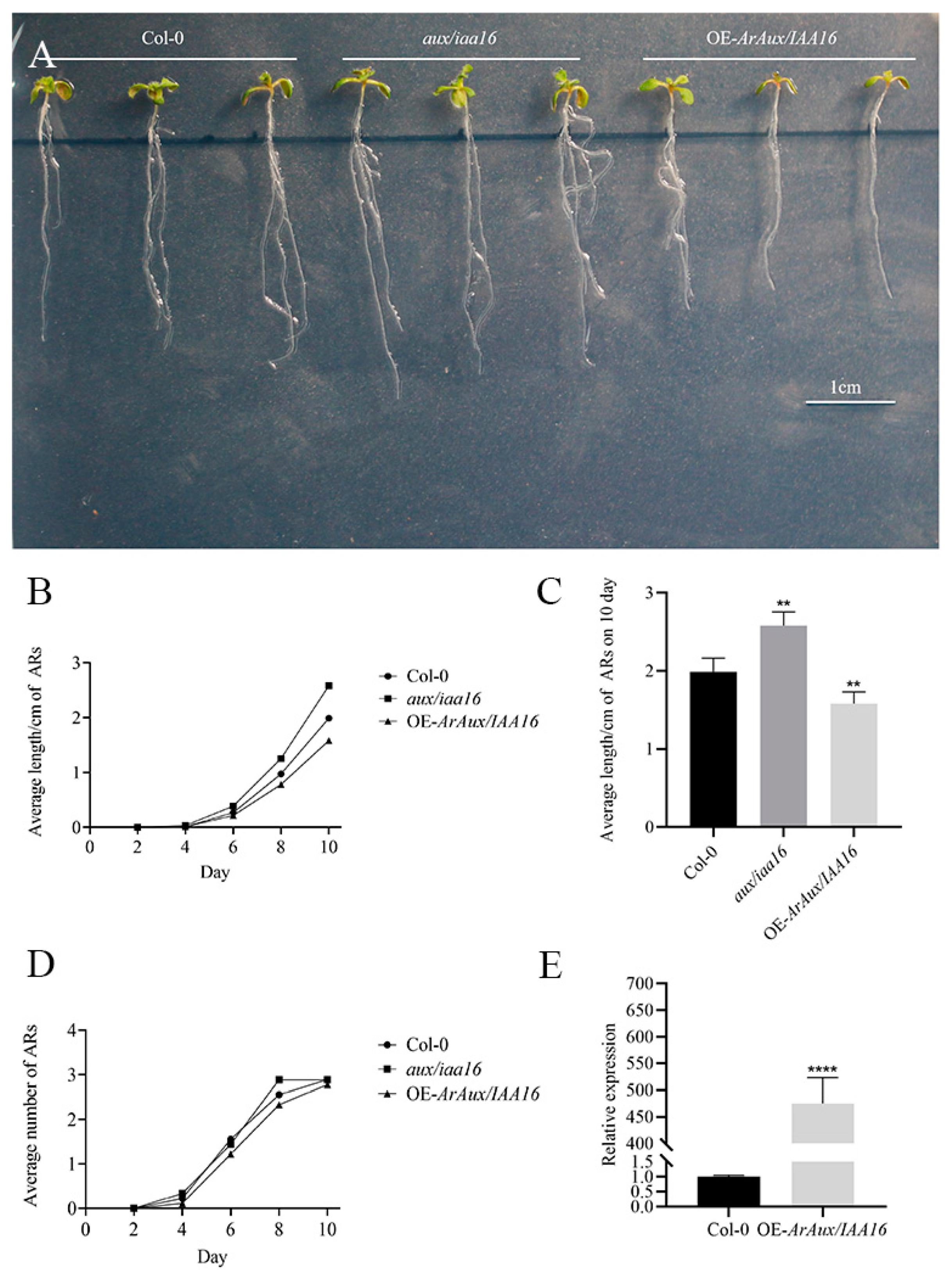
2.6. Transgenic Functional Validation
3. Results
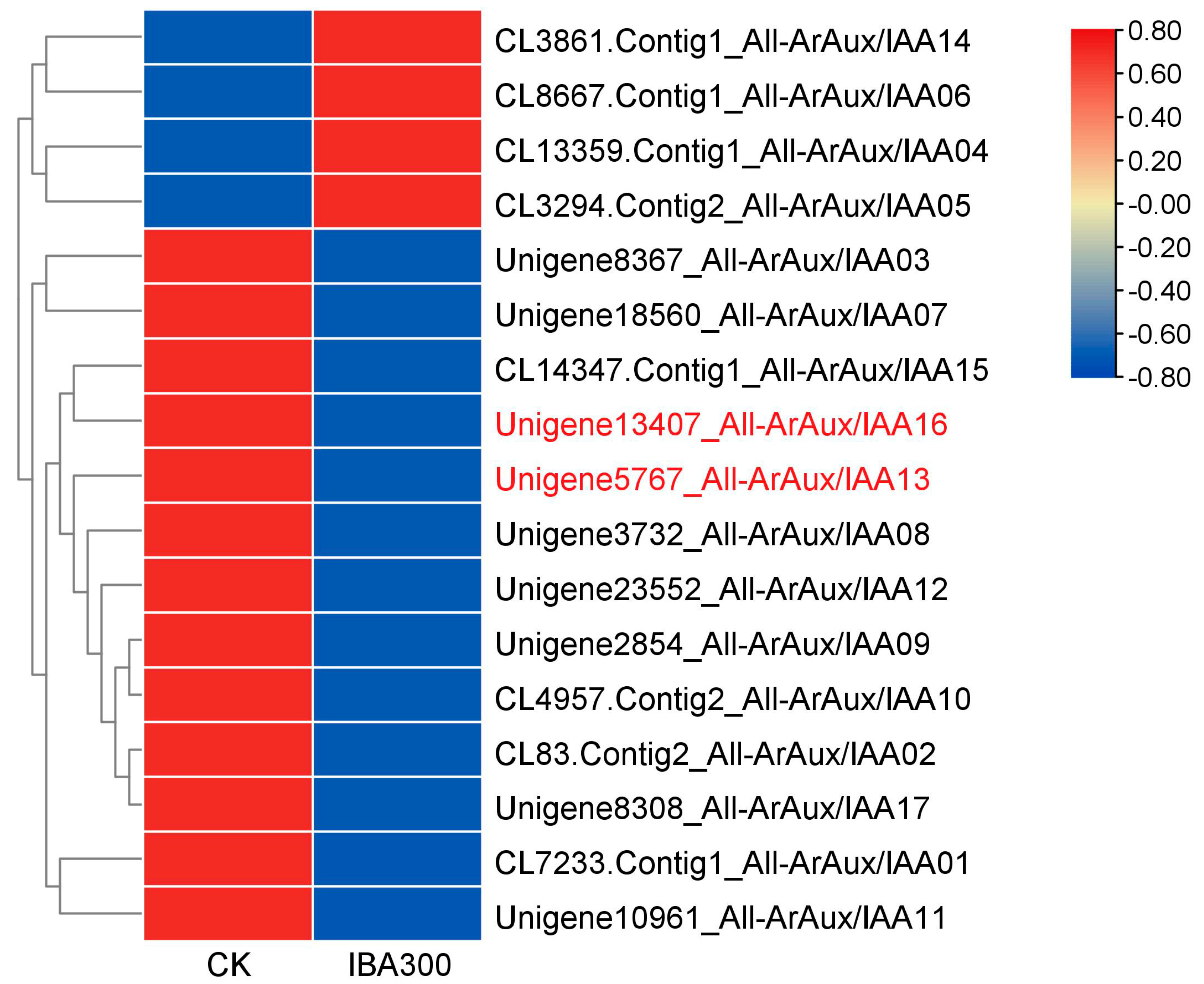
3.1. Bioinformatics Analysis of ArAux/IAAs
3.2. Subcellular Localization
3.3. Interaction of ArAux/IAA13 and ArAux/IAA16 with ArARF10 and ArARF18
3.4. Overexpression of ArAux/IAA13 and ArAux/IAA16 in A. thaliana Mutants
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, C.; Chen, Y.; Wang, Y.; Tan, F.; Ke, P.B.; Sha, W.F.; Li, Y.J. Sequence analysis of SSR in transcriptome of American red maple. J. Cent. South Univ. For. Technol. 2021, 41, 132–141. [Google Scholar]
- Zhang, Y.F. Study on the Adaptability of American Red Maple introduction and cultivation in the eastern Liaoning mountainous area. For. By-Prod. Spec. China 2022, 37, 41–42. [Google Scholar]
- Li, L. Current situation of fine varieties and breeding and cultivation techniques of American Red Maple. Heilongjiang Agric. Sci. 2018, 41, 158–160. [Google Scholar]
- Li, X. Efficient breeding and cultivation techniques of Acer rubrum L. in northern Anhui. Mod. Agric. Sci. Technol. 2019, 48, 145–146. [Google Scholar]
- Ma, S.J.; Peng, T.L.; Yu, Y.; Liu, Y.; Li, C.; Wang, J. Effect of plant hormones and magnetic field on rooting of soft cuttings for Catalpa bungei. J. Northeast. For. Univ. 2020, 48, 21–24. [Google Scholar]
- Hao, A.L.; Wang, C.; Feng, X.B. Effects of growth regulators on softwood-cutting rooting and oxidase activities during rooting of Acer davidii Franch. Acta Bot. Boreali-Occident. Sin. 2009, 29, 2026–2030. [Google Scholar]
- Yuan, L.L.; Zhang, L.; Wang, H.X.; Sun, F.; Yv, Y.C.; Niu, T.; Li, C.X.; Wang, C.X. Changes of endogenous homones during Acer truncatum bunge cutting. Chin. Agric. Sci. Bull. 2012, 28, 61–64. [Google Scholar]
- Zhang, J.Q.; Zhang, D.Y.; Liu, D.H. Effects of different exogenous hormone treatments on rootage rate of Acer Tetramerum. Bull. Soil Water Conserv. 2017, 37, 207–210. [Google Scholar]
- Ma, J.; Xu, T.D. Non-canonical auxin signaling pathway in plants. Biotechnol. Bull. 2020, 36, 15–22. [Google Scholar]
- Maitra Majee, S.; Sharma, E.; Singh, B.; Khurana, J.P. Drought-induced protein (Di19-3) plays a role in auxin signaling by interacting with IAA14 in Arabidopsis. Plant Direct 2020, 4, e00234. [Google Scholar] [CrossRef]
- Liu, H.B.; Li, L.; Li, C.; Huang, C.; ShangGuan, Y.; Chen, R.; Xiao, S.; Wen, W.; Xu, D. Identification and bioinformatic analysis of Aux/IAA family based on transcriptome data of Bletilla striata. Bioengineered 2019, 10, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Leyser, O. Auxin signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.P.; Zhang, M.Y.; Li, J.Y.; Zhao, H.W.; Ge, W.; Zhang, K.Z. Identification and analysis of Aux/IAA family in Acer rubrum. Evol. Bioinform. 2021, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Escobar, A.O.; Nic-Can, G.I.; Galaz Avalos, R.M.; Loyola-Vargas, V.M.; Gongora-Castillo, E. Transcriptome analysis of the induction of somatic embryogenesis in Coffea canephora and the participation of ARF and Aux/IAA genes. PeerJ 2019, 7, e7752. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.J.; Zhang, J.Z. Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, C.; Feng, S.; Zhong, S.; Li, H.; Zhong, J.; Shen, C.; Liu, J. Genome-wide analysis and characterization of Aux/IAA family genes related to fruit ripening in papaya (Carica papaya L.). BMC Genom. 2017, 18, 351. [Google Scholar] [CrossRef]
- Paul, P.; Dhandapani, V.; Rameneni, J.J.; Li, X.; Sivanandhan, G.; Choi, S.R.; Pang, W.; Im, S.; Lim, Y.P. Genome-wide analysis and characterization of Aux/IAA family genes in Brassica rapa. PLoS ONE 2016, 11, e0151522. [Google Scholar]
- Ali, S.; Wang, W.; Zhang, Z.; Xie, L.; Boer, D.R.; Khan, N. Genome-Wide Identification, Expression and Interaction Analysis of ARF and AUX/IAA Gene Family in Soybean. Front. Biosci. Landmark 2022, 27, 251. [Google Scholar] [CrossRef]
- Guan, D.; Hu, X.; Diao, D.; Wang, F.; Liu, Y.P. Genome-wide analysis and identification of the Aux/IAA gene family in peach. Int. J. Mol. Sci. 2019, 20, 4703. [Google Scholar] [CrossRef]
- Xie, Y.; Ying, J.; Tang, M.; Wang, Y.; Xu, L.; Liu, M.; Liu, L. Genome-wide identification of AUX/IAA in radish and functional characterization of RsIAA33 gene during taproot thickening. Gene 2021, 795, 145782. [Google Scholar] [CrossRef]
- Yamauchi, T.; Tanaka, A.; Inahashi, H.; Nishizawa, N.K.; Tsutsumi, N.; Inukai, Y.; Nakazono, M. Fine control of aerenchyma and lateral root development through AUX/IAA- and ARF-dependent auxin signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 20770–20775. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Sugita, K.; Qin, Y.; Rahman, A. Aux/IAA14 regulates microRNA-mediated cold stress response in Arabidopsis roots. Int. J. Mol. Sci. 2020, 21, 8441. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.P.; Zhang, M.Y.; Li, J.Y.; Zhao, H.W.; Zhang, K.Z.; Ge, W. Key regulatory pathways, microRNAs, and target genes participate in adventitious root formation of Acer rubrum l. Sci. Rep. 2022, 12, 12057. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Zeng, Y.; Shen, W.; Tuo, D.; Li, X.; Zhou, P. Nimble Cloning: A Simple, Versatile, and Efficient System for Standardized Molecular Cloning. Front. Bioeng Biotechnol. 2020, 7, 460. [Google Scholar] [CrossRef]
- Wang, X.; Fu, Y.; Ban, L.; Wang, Z.; Feng, G.; Li, J.; Gao, H. Selection of reliable reference genes for quantitative real-time RT-PCR in alfalfa. Genes Genet. Syst. 2015, 90, 175–180. [Google Scholar] [CrossRef]
- Yu, H.; Soler, M.; San Clemente, H.; Mila, I.; Paiva, J.A.; Myburg, A.A.; Bouzayen, M.; Grima-Pettenati, J.; Cassan-Wang, H. Comprehensive genome-wide analysis of the Aux/IAA gene family in Eucalyptus: Evidence for the role of EgrIAA4 in wood formation. Plant Cell Physiol. 2015, 56, 700–714. [Google Scholar] [CrossRef]
- Gan, D.; Zhuang, D.; Ding, F.; Yu, Z.; Zhao, Y. Identification and expression analysis of primary auxin-responsive Aux/IAA gene family in cucumber (Cucumis sativus). J. Genet. 2013, 92, 513–521. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, D.; Bian, Y.; Lv, Y.; Xie, Q. Genome-wide analysis of primary auxin-responsive Aux/IAA gene family in maize (Zea mays. L.). Mol. Biol. Rep. 2010, 37, 3991–4001. [Google Scholar] [CrossRef]
- Singh, V.K.; Jain, M. Genome-wide survey and comprehensive expression profiling of Aux/IAA gene family in chickpea and soybean. Front. Plant Sci. 2015, 6, 918. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, K.; Luo, L.; Lv, Y.Y.; Li, Y.Y.; Zhu, S.Q.; Luo, B.; Wan, Y.S.; Zhang, X.S.; Liu, F.Z. Identification of peanut Aux/IAA genes and functional prediction during seed development and maturation. Plants 2022, 11, 472. [Google Scholar] [CrossRef]
- Wu, J.; Peng, Z.; Liu, S.Y.; He, Y.; Cheng, L.; Kong, F.; Wang, J.; Lu, G. Genome-wide analysis of Aux/IAA gene family in Solanaceae species using tomato as a model. Mol. Genet. Genom. 2012, 287, 295–311. [Google Scholar] [CrossRef]
- Hochholdinger, F.; Yu, P.; Marcon, C. Genetic control of root system development in maize. Trends Plant Sci. 2018, 23, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Q.; Zhang, Z.; Zhang, C.X.; Wang, Y.L.; Liu, C.B.; Wu, J.C.; Han, M.L.; Wang, Q.X.; Chao, D.Y. Phytochrome-interacting factors orchestrate hypocotyl adventitious root initiation in Arabidopsis. Development 2022, 149, dev200362. [Google Scholar] [CrossRef] [PubMed]
- Lakehal, A.; Chaabouni, S.; Cavel, E.; Le Hir, R.; Ranjan, A.; Raneshan, Z.; Novak, O.; Pacurar, D.I.; Perrone, I.; Jobert, F.; et al. A molecular framework for the control of adventitious rooting by TIR1/AFB2-Aux/IAA-dependent auxin signaling in Arabidopsis. Mol. Plant 2019, 12, 1499–1514. [Google Scholar] [CrossRef]
- Ludwig, Y.; Berendzen, K.W.; Xu, C.; Piepho, H.P.; Hochholdinger, F. Diversity of stability, localization, interaction and control of downstream gene activity in the maize Aux/IAA protein family. PLoS ONE 2014, 9, e107346. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wu, M.; Liu, H.; Gao, Y.; Xiang, Y. Systematic identification and expression pattern analysis of the Aux/IAA and ARF gene families in moso bamboo (Phyllostachys edulis). Plant Physiol. Biochem. 2018, 130, 431–444. [Google Scholar] [CrossRef]
- Abel, S.; Oeller, P.W.; Theologis, A. Early auxin-induced genes encode short-lived nuclear proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 326–330. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Y.X.; Wang, Y.Q.; Li, H.M.; Liu, J.X.; Tan, J.X.; He, J.D.; Bai, J.W.; Ma, H.L. Evolution analysis of the Aux/IAA gene family in plants shows dual origins and variable nuclear localization signals. Int. J. Mol. Sci. 2017, 18, 2107. [Google Scholar] [CrossRef]
- Ori, N. Dissecting the biological functions of ARF and Aux/IAA genes. Plant Cell 2019, 31, 1210–1211. [Google Scholar] [CrossRef]
- Wang, H.; Jones, B.; Li, Z.; Frasse, P.; Delalande, C.; Regad, F.; Chaabouni, S.; Latche, A.; Pech, J.C.; Bouzayen, M. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell 2005, 17, 2676–2692. [Google Scholar] [CrossRef]
- Rinaldi, M.A.; Liu, J.; Enders, T.A.; Bartel, B.; Strader, L.C. A gain-of-function mutation in IAA16 confers reduced responses to auxin and abscisic acid and impedes plant growth and fertility. Plant Mol. Biol. 2012, 79, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.M.; Li, H.X.; Zhai, L.L.; Xie, X.; Li, X.Y.; Bian, S.M. Identification and functional characterization of the Aux/IAA gene VcIAA27 in blueberry. Plant Signal. Behav. 2020, 15, 1700327. [Google Scholar] [CrossRef] [PubMed]
- Estrella-Maldonado, H.; Chan-Leon, A.; Fuentes, G.; Giron-Ramirez, A.; Desjardins, Y.; Santamaria, J.M. The interaction between exogenous IBA with sucrose, light and ventilation alters the expression of ARFs and Aux/IAA genes in Carica papaya plantlets. Plant Mol. Biol. 2022, 110, 107–130. [Google Scholar] [CrossRef] [PubMed]



| Serial Number | ENN-Linked Genes | ECN-Linked Genes |
|---|---|---|
| 1 | None | ArAux/IAA13 |
| 2 | None | ArAux/IAA16 |
| 3 | ArARF10 | None |
| 4 | ArARF18 | None |
| 5 | None | None |
| 6 | ArARF10 | ArAux/IAA13 |
| 7 | ArARF18 | ArAux/IAA13 |
| 8 | ArARF10 | ArAux/IAA16 |
| 9 | ArARF18 | ArAux/IAA16 |
| Primer Names | Primer Sequences |
|---|---|
| 18S-F | CCTGAGAAACGGCTACCACAT |
| 18S-R | CACCAGACTTGCCCTCCA |
| ArAux/IAA13-F | GAAAGTCCAGACGAATGAGAGC |
| ArAux/IAA13-R | TCCAACGTCATGGCAAGATC |
| ArAux/IAA16-F | AACAAGAAGAAAGGAGGCACAG |
| ArAux/IAA16-R | CCAGAGAAAACCCACTTGCTAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, H.; Li, H.; Yu, J.; Zhao, H.; Zhang, K.; Ge, W. Regulatory Mechanisms of ArAux/IAA13 and ArAux/IAA16 in the Rooting Process of Acer rubrum. Genes 2023, 14, 1206. https://doi.org/10.3390/genes14061206
Zhu H, Li H, Yu J, Zhao H, Zhang K, Ge W. Regulatory Mechanisms of ArAux/IAA13 and ArAux/IAA16 in the Rooting Process of Acer rubrum. Genes. 2023; 14(6):1206. https://doi.org/10.3390/genes14061206
Chicago/Turabian StyleZhu, Huiyu, Huiju Li, Jiayu Yu, Hewen Zhao, Kezhong Zhang, and Wei Ge. 2023. "Regulatory Mechanisms of ArAux/IAA13 and ArAux/IAA16 in the Rooting Process of Acer rubrum" Genes 14, no. 6: 1206. https://doi.org/10.3390/genes14061206




