Genome-Wide Assessment of Runs of Homozygosity by Whole-Genome Sequencing in Diverse Horse Breeds Worldwide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sampling and Whole-Genome Sequencing
2.3. Quality Controls and SNP Genotyping
2.4. Runs of Homozygosity Detection
2.5. Inbreeding Coefficients
2.6. Detection of ROH Islands in Thoroughbreds and Candidate Genes
3. Results
3.1. Whole-Genome Sequencing
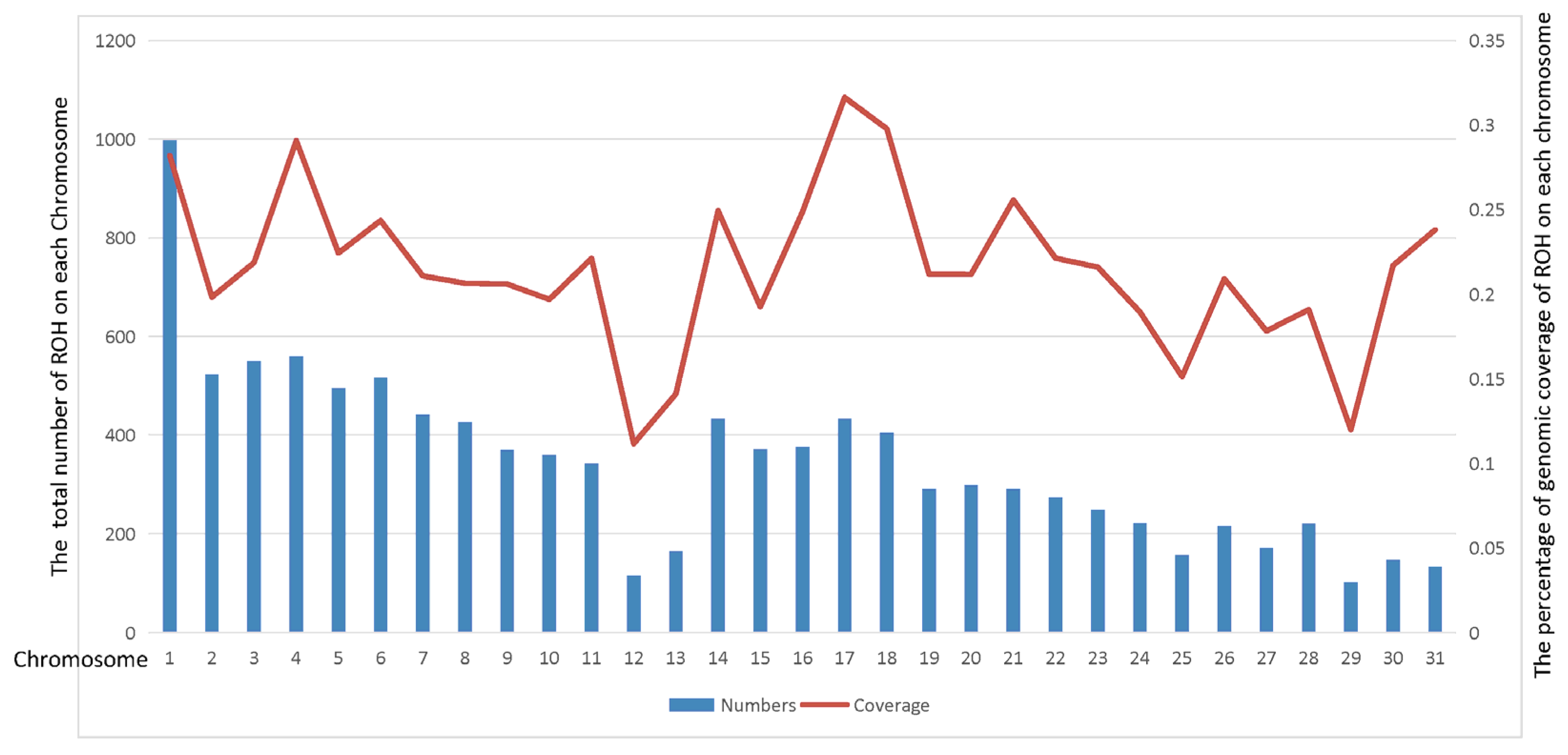
3.2. ROHs in the 16 Horse Breeds
3.3. Assessment of Inbreeding Coefficients
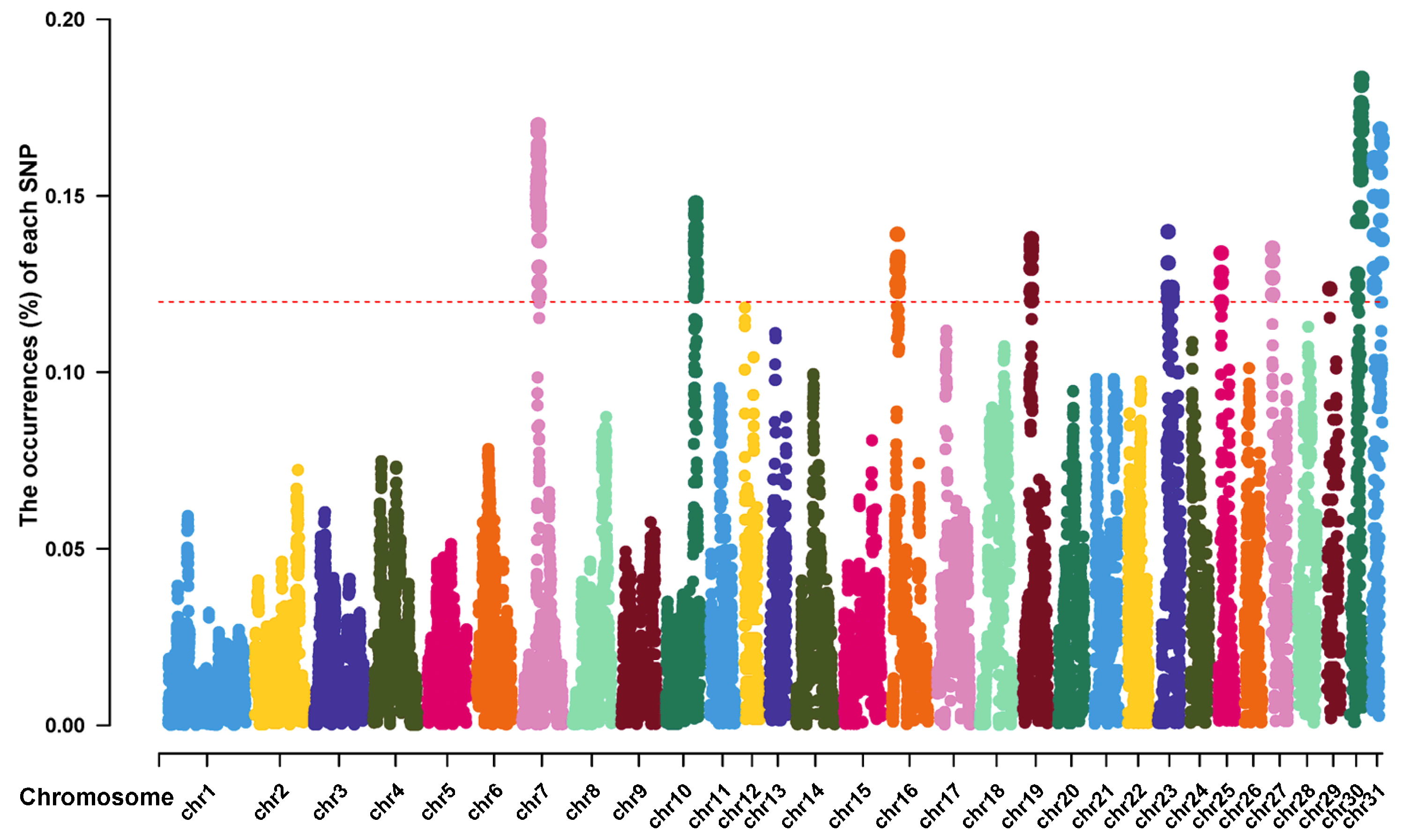
3.4. The ROH Islands and Candidate Genes in Thoroughbreds
4. Discussion
4.1. Distribution and Patterns of ROH in 16 Horse Populations
4.2. ROH-Based Genomic Inbreeding Coefficients
4.3. Candidate Genes in ROH Islands in Thoroughbreds Are Associated with Artificial Selection Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaunitz, C.; Fages, A.; Hanghoj, K.; Albrechtsen, A.; Khan, N.; Schubert, M.; Seguin-Orlando, A.; Owens, I.J.; Felkel, S.; Bignon-Lau, O.; et al. Ancient genomes revisit the ancestry of domestic and Przewalski’s horses. Science 2018, 360, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Fages, A.; Gaunitz, C.; Leonardi, M.; Wagner, S.; Khan, N.; Hanghoj, K.; Alquraishi, S.A.; Alfarhan, A.H.; Al-Rasheid, K.A.; et al. The Evolutionary Origin and Genetic Makeup of Domestic Horses. Genetics 2016, 204, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Outram, A.K.; Stear, N.A.; Bendrey, R.; Olsen, S.; Kasparov, A.; Zaibert, V.; Thorpe, N.; Evershed, R.P. The earliest horse harnessing and milking. Science 2009, 323, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Petersen, J.L.; Mickelson, J.R.; Cothran, E.G.; Andersson, L.S.; Axelsson, J.; Bailey, E.; Bannasch, D.; Binns, M.M.; Borges, A.S.; Brama, P.; et al. Genetic diversity in the modern horse illustrated from genome-wide SNP data. PLoS ONE 2013, 8, e54997. [Google Scholar] [CrossRef]
- Wade, C.M.; Giulotto, E.; Sigurdsson, S.; Zoli, M.; Gnerre, S.; Imsland, F.; Lear, T.L.; Adelson, D.L.; Bailey, E.; Bellone, R.R.; et al. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science 2009, 326, 865–867. [Google Scholar] [CrossRef]
- Kalbfleisch, T.S.; Rice, E.S.; DePriest, M.S., Jr.; Walenz, B.P.; Hestand, M.S.; Vermeesch, J.R.; BL, O.C.; Fiddes, I.T.; Vershinina, A.O.; Saremi, N.F.; et al. Improved reference genome for the domestic horse increases assembly contiguity and composition. Commun. Biol. 2018, 1, 197. [Google Scholar] [CrossRef]
- Petersen, J.L.; Coleman, S.J. Next-Generation Sequencing in Equine Genomics. Vet. Clin. N. Am. Equine Pract. 2020, 36, 195–209. [Google Scholar] [CrossRef]
- Wright, S. Coefficients of inbreeding and relationship. Am. Nat. 1922, 56, 330–338. [Google Scholar] [CrossRef]
- Oliehoek, P.A.; Bijma, P. Effects of pedigree errors on the efficiency of conservation decisions. Genet. Sel. Evol. 2009, 41, 9. [Google Scholar] [CrossRef]
- Hill, E.W.; Bradley, D.G.; Al-Barody, M.; Ertugrul, O.; Splan, R.K.; Zakharov, I.; Cunningham, E.P. History and integrity of thoroughbred dam lines revealed in equine mtDNA variation. Anim. Genet. 2002, 33, 287–294. [Google Scholar] [CrossRef]
- Ceballos, F.C.; Joshi, P.K.; Clark, D.W.; Ramsay, M.; Wilson, J.F. Runs of homozygosity: Windows into population history and trait architecture. Nat. Rev. Genet. 2018, 19, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Broman, K.W.; Weber, J.L. Long homozygous chromosomal segments in reference families from the centre d’Etude du polymorphisme humain. Am. J. Hum. Genet. 1999, 65, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Morton, N.E.; Collins, A. Extended tracts of homozygosity in outbred human populations. Hum. Mol. Genet. 2006, 15, 789–795. [Google Scholar] [CrossRef] [PubMed]
- McQuillan, R.; Leutenegger, A.L.; Abdel-Rahman, R.; Franklin, C.S.; Pericic, M.; Barac-Lauc, L.; Smolej-Narancic, N.; Janicijevic, B.; Polasek, O.; Tenesa, A.; et al. Runs of homozygosity in European populations. Am. J. Hum. Genet. 2008, 83, 359–372. [Google Scholar] [CrossRef]
- Curik, I.; Ferenčaković, M.; Sölkner, J. Inbreeding and runs of homozygosity: A possible solution to an old problem. Livest. Sci. 2014, 166, 26–34. [Google Scholar] [CrossRef]
- Falconer, D.S. Introduction to Quantitative Genetics; Chennai, India: Pearson Education India, 1996. [Google Scholar]
- Pemberton, T.J.; Absher, D.; Feldman, M.W.; Myers, R.M.; Rosenberg, N.A.; Li, J.Z. Genomic patterns of homozygosity in worldwide human populations. Am. J. Hum. Genet. 2012, 91, 275–292. [Google Scholar] [CrossRef]
- Bosse, M.; Megens, H.J.; Madsen, O.; Paudel, Y.; Frantz, L.A.; Schook, L.B.; Crooijmans, R.P.; Groenen, M.A. Regions of homozygosity in the porcine genome: Consequence of demography and the recombination landscape. PLoS Genet. 2012, 8, e1003100. [Google Scholar] [CrossRef]
- Peripolli, E.; Munari, D.P.; Silva, M.; Lima, A.L.F.; Irgang, R.; Baldi, F. Runs of homozygosity: Current knowledge and applications in livestock. Anim. Genet. 2017, 48, 255–271. [Google Scholar] [CrossRef]
- Gorssen, W.; Meyermans, R.; Janssens, S.; Buys, N. A publicly available repository of ROH islands reveals signatures of selection in different livestock and pet species. Genet. Sel. Evol. 2021, 53, 2. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.E.; Hou, Y.; Zhu, B.; Cardone, M.F.; Jiang, L.; Cellamare, A.; Mitra, A.; Alexander, L.J.; Coutinho, L.L.; Dell’Aquila, M.E.; et al. Analysis of copy number variations among diverse cattle breeds. Genome Res. 2010, 20, 693–703. [Google Scholar] [CrossRef]
- Kirin, M.; McQuillan, R.; Franklin, C.S.; Campbell, H.; McKeigue, P.M.; Wilson, J.F. Genomic runs of homozygosity record population history and consanguinity. PLoS ONE 2010, 5, e13996. [Google Scholar] [CrossRef]
- Keller, M.C.; Visscher, P.M.; Goddard, M.E. Quantification of inbreeding due to distant ancestors and its detection using dense single nucleotide polymorphism data. Genetics 2011, 189, 237–249. [Google Scholar] [CrossRef]
- Visscher, P.M.; Medland, S.E.; Ferreira, M.A.; Morley, K.I.; Zhu, G.; Cornes, B.K.; Montgomery, G.W.; Martin, N.G. Assumption-free estimation of heritability from genome-wide identity-by-descent sharing between full siblings. PLoS Genet. 2006, 2, e41. [Google Scholar] [CrossRef]
- Zanella, R.; Peixoto, J.O.; Cardoso, F.F.; Cardoso, L.L.; Biegelmeyer, P.; Cantao, M.E.; Otaviano, A.; Freitas, M.S.; Caetano, A.R.; Ledur, M.C. Genetic diversity analysis of two commercial breeds of pigs using genomic and pedigree data. Genet. Sel. Evol. 2016, 48, 24. [Google Scholar] [CrossRef]
- Sackman, J.E.; Houpt, K.A. Equine Personality: Association With Breed, Use, and Husbandry Factors. J. Equine Vet. Sci. 2019, 72, 47–55. [Google Scholar] [CrossRef]
- Tabares-Seisdedos, R.; Rubenstein, J.L. Chromosome 8p as a potential hub for developmental neuropsychiatric disorders: Implications for schizophrenia, autism and cancer. Mol. Psychiatry 2009, 14, 563–589. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Yang, Z.; Cui, W.; Li, M.D. Crucial roles of the CHRNB3-CHRNA6 gene cluster on chromosome 8 in nicotine dependence: Update and subjects for future research. Transl. Psychiatry 2016, 6, e843. [Google Scholar] [CrossRef] [PubMed]
- Blauwendraat, C.; Nalls, M.A.; Singleton, A.B. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020, 19, 170–178. [Google Scholar] [CrossRef]
- Barone, R.; Cirnigliaro, L.; Saccuzzo, L.; Valdese, S.; Pettinato, F.; Prato, A.; Bernardini, L.; Fichera, M.; Rizzo, R. PARK2 microdeletion in a multiplex family with autism spectrum disorder. Int. J. Dev. Neurosci. 2023, 83, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Lu, W.; Tian, Y.; Hou, Q.; Man, H.Y. Prkn knockout mice show autistic-like behaviors and aberrant synapse formation. iScience 2022, 25, 104573. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.I.; Vaccari, C.M.; Terracciano, A.; Doria-Lamba, L.; Facchinetti, S.; Priolo, M.; Ayuso, C.; De Jorge, L.; Gimelli, S.; Santorelli, F.M.; et al. The metabotropic glutamate receptor 1, GRM1: Evaluation as a candidate gene for inherited forms of cerebellar ataxia. J. Neurol. 2010, 257, 598–602. [Google Scholar] [CrossRef]
- Ohmura, H.; Matsui, A.; Hada, T.; Jones, J.H. Physiological responses of young thoroughbred horses to intermittent high-intensity treadmill training. Acta Vet. Scand. 2013, 55, 59. [Google Scholar] [CrossRef]
- Ohmura, H.; Mukai, K.; Takahashi, Y.; Takahashi, T.; Jones, J.H. Hypoxic training increases maximal oxygen consumption in Thoroughbred horses well-trained in normoxia. J. Equine Sci. 2017, 28, 41–45. [Google Scholar] [CrossRef]
- Davis, R.L.; Turner, D.L. Vertebrate hairy and Enhancer of split related proteins: Transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene 2001, 20, 8342–8357. [Google Scholar] [CrossRef]
- Gerrard, D.T.; Berry, A.A.; Jennings, R.E.; Piper Hanley, K.; Bobola, N.; Hanley, N.A. An integrative transcriptomic atlas of organogenesis in human embryos. eLife 2016, 5, e15657. [Google Scholar] [CrossRef]
- Chopra, N.; Yang, T.; Asghari, P.; Moore, E.D.; Huke, S.; Akin, B.; Cattolica, R.A.; Perez, C.F.; Hlaing, T.; Knollmann-Ritschel, B.E.; et al. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc. Natl. Acad. Sci. USA 2009, 106, 7636–7641. [Google Scholar] [CrossRef] [PubMed]
- Chopra, N.; Knollmann, B.C. Triadin regulates cardiac muscle couplon structure and microdomain Ca(2+) signalling: A path towards ventricular arrhythmias. Cardiovasc. Res. 2013, 98, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Stupka, N.; Kintakas, C.; White, J.D.; Fraser, F.W.; Hanciu, M.; Aramaki-Hattori, N.; Martin, S.; Coles, C.; Collier, F.; Ward, A.C.; et al. Versican processing by a disintegrin-like and metalloproteinase domain with thrombospondin-1 repeats proteinases-5 and -15 facilitates myoblast fusion. J. Biol. Chem. 2013, 288, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- van der Veer, E.P.; de Bruin, R.G.; Kraaijeveld, A.O.; de Vries, M.R.; Bot, I.; Pera, T.; Segers, F.M.; Trompet, S.; van Gils, J.M.; Roeten, M.K.; et al. Quaking, an RNA-binding protein, is a critical regulator of vascular smooth muscle cell phenotype. Circ. Res. 2013, 113, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, Y.; Chen, Q.; Niu, B. Advances in Antidiabetic Drugs Targeting Insulin Secretion. Curr. Pharm. Des. 2018, 24, 3990–3997. [Google Scholar] [CrossRef]
- Thompson, B.; Satin, L.S. Beta-Cell Ion Channels and Their Role in Regulating Insulin Secretion. Compr. Physiol. 2021, 11, 1–21. [Google Scholar] [CrossRef]
- Hiriart, M.; Velasco, M.; Larque, C.; Diaz-Garcia, C.M. Metabolic syndrome and ionic channels in pancreatic beta cells. Vitam. Horm. 2014, 95, 87–114. [Google Scholar] [CrossRef]
- Breuhaus, B.A. Glucose and Insulin Responses to an Intravenous Glucose Load in Thoroughbred and Paso Fino Horses. J. Equine Vet. Sci. 2019, 81, 102793. [Google Scholar] [CrossRef]
- Urschel, K.L.; Escobar, J.; McCutcheon, L.J.; Geor, R.J. Insulin infusion stimulates whole-body protein synthesis and activates the upstream and downstream effectors of mechanistic target of rapamycin signaling in the gluteus medius muscle of mature horses. Domest. Anim. Endocrinol. 2014, 47, 92–100. [Google Scholar] [CrossRef]
- Cartier-Michaud, A.; Bailly, A.L.; Betzi, S.; Shi, X.; Lissitzky, J.C.; Zarubica, A.; Serge, A.; Roche, P.; Lugari, A.; Hamon, V.; et al. Genetic, structural, and chemical insights into the dual function of GRASP55 in germ cell Golgi remodeling and JAM-C polarized localization during spermatogenesis. PLoS Genet. 2017, 13, e1006803. [Google Scholar] [CrossRef]
- Li, W.; Tang, W.; Teves, M.E.; Zhang, Z.; Zhang, L.; Li, H.; Archer, K.J.; Peterson, D.L.; Williams, D.C., Jr.; Strauss, J.F., 3rd; et al. A MEIG1/PACRG complex in the manchette is essential for building the sperm flagella. Development 2015, 142, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Stratton, C.J.; Bao, J.; Zheng, H.; Bhetwal, B.P.; Yanagimachi, R.; Yan, W. Spata6 is required for normal assembly of the sperm connecting piece and tight head-tail conjunction. Proc. Natl. Acad. Sci. USA 2015, 112, E430–E439. [Google Scholar] [CrossRef] [PubMed]
- Novak, S.; Smith, T.A.; Paradis, F.; Burwash, L.; Dyck, M.K.; Foxcroft, G.R.; Dixon, W.T. Biomarkers of in vivo fertility in sperm and seminal plasma of fertile stallions. Theriogenology 2010, 74, 956–967. [Google Scholar] [CrossRef]
- Gibb, Z.; Lambourne, S.R.; Aitken, R.J. The paradoxical relationship between stallion fertility and oxidative stress. Biol. Reprod. 2014, 91, 77. [Google Scholar] [CrossRef]
- Nath, L.C.; Anderson, G.A.; McKinnon, A.O. Reproductive efficiency of Thoroughbred and Standardbred horses in north-east Victoria. Aust. Vet. J. 2010, 88, 169–175. [Google Scholar] [CrossRef] [PubMed]

| Classification of ROH by Length | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Horse Population | No. of Samples | Total No. a | Mean No. b | Total Length (Mb) c | Total Mean Length (Mb) d | Max. Length (Mb) e | <1 Mb | 1–5 Mb | 5–10 Mb | >10 Mb |
| Friesian | 5 | 3183 | 637 | 3178.47 | 635.69 | 7.82 | 2116 | 1060 | 7 | 0 |
| Thoroughbred | 22 | 12,490 | 568 | 13,526.86 | 614.86 | 13.89 | 8233 | 4130 | 117 | 10 |
| Arabian | 5 | 3104 | 621 | 3013.17 | 602.63 | 7.34 | 2119 | 970 | 15 | 0 |
| Shetland pony | 3 | 1435 | 478 | 1545.37 | 515.12 | 8.18 | 918 | 504 | 13 | 0 |
| Andalusian | 4 | 2102 | 526 | 2047.94 | 511.99 | 8.47 | 1428 | 667 | 7 | 0 |
| Akhal-Teke | 5 | 2112 | 422 | 1997.03 | 399.41 | 8.37 | 1508 | 599 | 5 | 0 |
| Standardbred | 4 | 1549 | 387 | 1550.21 | 387.55 | 11.38 | 1094 | 437 | 17 | 1 |
| Hanoverian | 4 | 1282 | 321 | 1207.67 | 301.92 | 7.32 | 918 | 360 | 4 | 0 |
| American Quarter Horse | 7 | 2173 | 310 | 1997.14 | 285.31 | 8.99 | 1587 | 575 | 11 | 0 |
| Franches-Montagnes | 6 | 1476 | 246 | 1649.65 | 274.94 | 10.56 | 939 | 520 | 16 | 1 |
| Criollo | 2 | 491 | 246 | 403.68 | 201.84 | 4.12 | 390 | 101 | 0 | 0 |
| Jeju | 2 | 501 | 251 | 369.51 | 184.76 | 4.13 | 417 | 84 | 0 | 0 |
| Przewalskii | 10 | 1802 | 180 | 1591.60 | 159.16 | 8.74 | 1378 | 423 | 1 | 0 |
| Mongolian | 5 | 993 | 199 | 785.24 | 157.05 | 4.70 | 809 | 184 | 0 | 0 |
| Debao | 5 | 836 | 167 | 593.08 | 118.62 | 5.06 | 712 | 123 | 1 | 0 |
| Yakutian | 7 | 711 | 102 | 459.81 | 65.69 | 2.91 | 639 | 72 | 0 | 0 |
| ROH Length Category (Mb) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Horse Population | Horse Population | No. of Samples | FROH (<1 Mb) | FROH (1–5 Mb) | FROH (5–10 Mb) | FROH (>10 Mb) | FROH all | SD |
| FS | Friesian | 5 | 1.16 × 10−1 | 1.59 × 10−1 | 3.81 × 10−3 | 0 | 2.79 × 10−1 | 3.11 × 10−4 |
| AB | Arabian | 5 | 1.15 × 10−1 | 1.41 × 10−1 | 7.50 × 10−3 | 0 | 2.64 × 10−1 | 3.12 × 10−4 |
| TB | Thoroughbred | 23 | 9.73 × 10−2 | 1.44 × 10−1 | 1.42 × 10−2 | 2.27 × 10−3 | 2.58 × 10−1 | 4.25 × 10−4 |
| AL | Andalusian | 4 | 9.69 × 10−2 | 1.23 × 10−1 | 4.87 × 10−3 | 0 | 2.24 × 10−1 | 3.13 × 10−4 |
| ST | Shetland pony | 3 | 8.26 × 10−2 | 1.31 × 10−1 | 1.19 × 10−2 | 0 | 2.26 × 10−1 | 3.93 × 10−4 |
| AT | Akhal-Teke | 5 | 8.14 × 10−2 | 9.06 × 10−2 | 3.06 × 10−3 | 0 | 1.75 × 10−1 | 3.17 × 10−4 |
| STD | Standardbred | 4 | 7.33 × 10−2 | 8.39 × 10−2 | 1.15 × 10−2 | 1.25 × 10−3 | 1.70 × 10−1 | 3.93 × 10−4 |
| HAN | Hanoverian | 4 | 6.00 × 10−2 | 6.95 × 10−2 | 2.79 × 10−3 | 0 | 1.32 × 10−1 | 3.25 × 10−4 |
| QT | American Quarter Horse | 7 | 5.94 × 10−2 | 6.15 × 10−2 | 4.22 × 10−3 | 0 | 1.25 × 10−1 | 3.26 × 10−4 |
| JEJU | Jeju | 2 | 5.41 × 10−2 | 2.69 × 10−2 | 0 | 0 | 8.10 × 10−2 | ND |
| CR | Criollo | 2 | 5.19 × 10−2 | 3.66 × 10−2 | 0 | 0 | 8.85 × 10−2 | ND |
| MON | Franches-Montagnes | 5 | 4.96 × 10−2 | 8.53 × 10−2 | 8.80 × 10−3 | 9.26 × 10−4 | 1.45 × 10−1 | 4.43 × 10−4 |
| MG | Mongolian | 5 | 4.26 × 10−2 | 2.63 × 10−2 | 0 | 0 | 6.89 × 10−2 | 2.21 × 10−4 |
| DB | Debao | 5 | 3.54 × 10−2 | 1.62 × 10−2 | 4.44 × 10−4 | 0 | 5.20 × 10−2 | 1.90 × 10−4 |
| PRZ | Przewalskii | 10 | 3.50 × 10−2 | 3.44 × 10−2 | 3.83 × 10−4 | 0 | 6.98 × 10−2 | 3.10 × 10−4 |
| YAK | Yakutian | 7 | 2.26 × 10−2 | 6.20 × 10−3 | 0 | 0 | 2.88 × 10−2 | 1.33 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Zhu, B.; Tang, X.; Chen, B.; Liu, M.; Gao, N.; Li, S.; Gu, J. Genome-Wide Assessment of Runs of Homozygosity by Whole-Genome Sequencing in Diverse Horse Breeds Worldwide. Genes 2023, 14, 1211. https://doi.org/10.3390/genes14061211
Chen C, Zhu B, Tang X, Chen B, Liu M, Gao N, Li S, Gu J. Genome-Wide Assessment of Runs of Homozygosity by Whole-Genome Sequencing in Diverse Horse Breeds Worldwide. Genes. 2023; 14(6):1211. https://doi.org/10.3390/genes14061211
Chicago/Turabian StyleChen, Chujie, Bo Zhu, Xiangwei Tang, Bin Chen, Mei Liu, Ning Gao, Sheng Li, and Jingjing Gu. 2023. "Genome-Wide Assessment of Runs of Homozygosity by Whole-Genome Sequencing in Diverse Horse Breeds Worldwide" Genes 14, no. 6: 1211. https://doi.org/10.3390/genes14061211






