Molecular and Clinical Characterization of CNGA3 and CNGB3 Genes in Brazilian Patients Affected with Achromatopsia
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Ophthalmic Examination
2.3. Genotyping
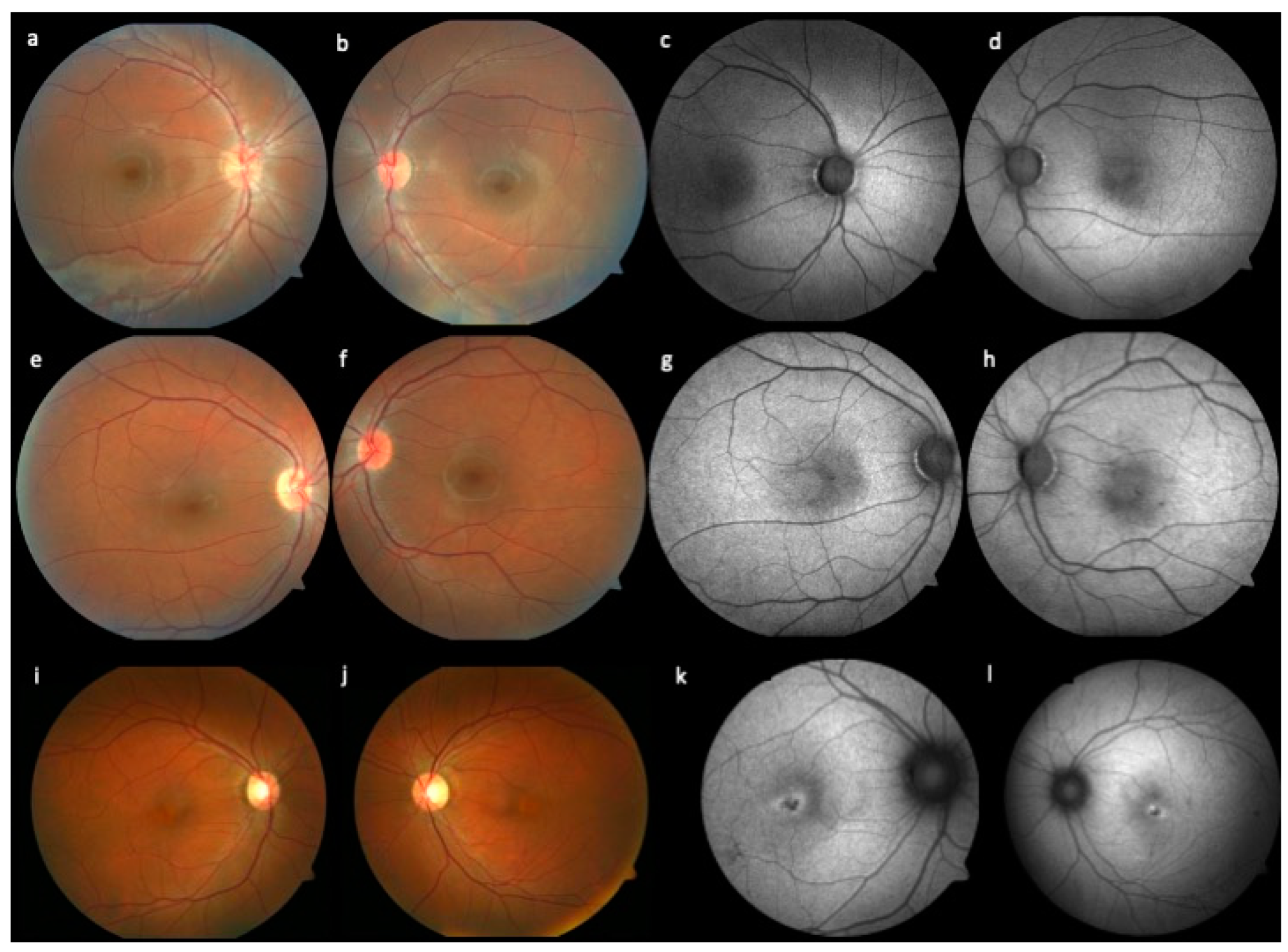
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michaelides, M.; Hunt, D.M.; Moore, A.T. The cone dysfunction syndromes. Br. J. Ophthalmol. 2004, 88, 291–297. [Google Scholar] [CrossRef]
- Aboshiha, J.; Dubis, A.M.; Carroll, J.; Hardcastle, A.; Michaelides, M. The cone dysfunction syndromes. Br. J. Ophthalmol. 2016, 100, 115–121. [Google Scholar] [CrossRef]
- Hirji, N.; Aboshiha, J.; Georgiou, M.; Bainbridge, J.; Michaelides, M. Achromatopsia: Clinical features, molecular genetics, animal models and therapeutic options. Ophthalmic Genet. 2018, 39, 149–157. [Google Scholar] [CrossRef]
- Brunetti-Pierri, R.; Karali, M.; Melillo, P.; Di Iorio, V.; De Benedictis, A.; Iaccarino, G.; Testa, F.; Banfi, S.; Simonelli, F. Clinical and Molecular Characterization of Achromatopsia Patients: A Longitudinal Study. Int. J. Mol. Sci. 2021, 22, 1681. [Google Scholar] [CrossRef]
- Thiadens, A.A.H.J.; Somervuo, V.; van den Born, L.I.; Roosing, S.; van Schooneveld, M.J.; Kuijpers, R.W.A.M.; van Moll-Ramirez, N.; Cremers, F.P.M.; Hoyng, C.B.; Klaver, C.C.W. Progressive Loss of Cones in Achromatopsia: An Imaging Study Using Spectral-Domain Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5952–5957. [Google Scholar] [CrossRef]
- Zobor, D.; Werner, A.; Stanzial, F.; Benedicenti, F.; Rudolph, G.; Kellner, U.; Hamel, C.; Andréasson, S.; Zobor, G.; Strasser, T.; et al. The Clinical Phenotype of CNGA3-Related Achromatopsia: Pretreatment Characterization in Preparation of a Gene Replacement Therapy Trial. Investig. Ophthalmol. Vis. Sci. 2017, 58, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Thiadens, A.A.; Slingerland, N.W.; Roosing, S.; Van Schooneveld, M.J.; Van Lith-Verhoeven, J.J.; Van Moll-Ramirez, N.; Born, L.I.V.D.; Hoyng, C.B.; Cremers, F.P.; Klaver, C.C. Genetic Etiology and Clinical Consequences of Complete and Incomplete Achromatopsia. Ophthalmology 2009, 116, 1984–1989.e1. [Google Scholar] [CrossRef] [PubMed]
- Kohl, S.; Varsanyi, B.; Antunes, G.A.; Baumann, B.; Hoyng, C.B.; Jägle, H.; Rosenberg, T.; Kellner, U.; Lorenz, B.; Salati, R.; et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur. J. Hum. Genet. 2005, 13, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.K.; Van Cauwenbergh, C.; Rother, C.; Baumann, B.; Reuter, P.; De Baere, E.; Wissinger, B.; Kohl, S. ACHM Study Group CNGB3 mutation spectrum including copy number variations in 552 achromatopsia patients. Hum. Mutat. 2017, 38, 1579–1591. [Google Scholar] [CrossRef]
- Sun, W.; Li, S.; Xiao, X.; Wang, P.; Zhang, Q. Genotypes and phenotypes of genes associated with achromatopsia: A reference for clinical genetic testing. Mol. Vis. 2020, 26, 588–602. [Google Scholar] [PubMed]
- Solaki, M.; Baumann, B.; Reuter, P.; Andreasson, S.; Audo, I.; Ayuso, C.; Balousha, G.; Benedicenti, F.; Birch, D.; Bitoun, P.; et al. Comprehensive variant spectrum of the CNGA3 gene in patients affected by achromatopsia. Hum. Mutat. 2022, 43, 832–858. [Google Scholar] [CrossRef] [PubMed]
- Michalakis, S.; Gerhardt, M.; Rudolph, G.; Priglinger, S.; Priglinger, C. Achromatopsia: Genetics and Gene Therapy. Mol. Diagn. Ther. 2022, 26, 51–59. [Google Scholar] [CrossRef]
- Motta, F.L.; Martin, R.P.; Filippelli-Silva, R.; Salles, M.V.; Sallum, J.M.F. Relative frequency of inherited retinal dystrophies in Brazil. Sci. Rep. 2018, 8, 15939. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef]
- Michalakis, S.; Becirovic, E.; Biel, M. Retinal cyclic nucleotide-gated channels: From pathophysiologyto therapy. Int. J. Mol. Sci. 2018, 19, 749. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.D.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Ochakovski, G.A.; Klein, R.; Schoen, C.; et al. Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial. JAMA Ophthalmol. 2020, 138, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Reichel, F.F.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Sothilingam, V.; Kuehlewein, L.; Kahle, N.; et al. Three-year results of phase I retinal gene therapy trial for CNGA3-mutated achromatopsia: Results of a non-randomized controlled trial. Br. J. Ophthalmol. 2021, 106, 1567–1572. [Google Scholar] [CrossRef]
- Sundaram, V.; Wilde, C.; Aboshiha, J.; Cowing, J.; Han, C.; Langlo, C.S.; Chana, R.; Davidson, A.E.; Sergouniotis, P.I.; Bainbridge, J.W.; et al. Retinal structure and function in achromatopsia: Implications for gene therapy. Ophthalmology 2014, 121, 234–245. [Google Scholar] [CrossRef]
- Aboshiha, J.; Dubis, A.M.; Cowing, J.A.; Fahy, R.T.A.; Sundaram, V.; Bainbridge, J.; Ali, R.; Dubra, A.; Nardini, M.; Webster, A.R.; et al. A Prospective Longitudinal Study of Retinal Structure and Function in Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5733–5743. [Google Scholar] [CrossRef]
- Triantafylla, M.; Papageorgiou, E.; Thomas, M.G.; McLean, R.; Kohl, S.; Sheth, V.; Tu, Z.; Proudlock, F.A.; Gottlob, I. Longitudinal Evaluation of Changes in Retinal Architecture Using Optical Coherence Tomography in Achromatopsia. Investig. Ophthalmol. Vis. Sci. 2022, 63, 6. [Google Scholar] [CrossRef]
- Pompe, M.T.; Vrabič, N.; Volk, M.; Meglič, A.; Jarc-Vidmar, M.; Peterlin, B.; Hawlina, M.; Fakin, A. Disease Progression in CNGA3 and CNGB3 Retinopathy; Characteristics of Slovenian Cohort and Proposed OCT Staging Based on Pooled Data from 126 Patients from 7 Studies. Curr. Issues Mol. Biol. 2021, 43, 67. [Google Scholar] [CrossRef]

| Family | ID | Age of Onset | Sings/Symptoms Onset | Current BCVA | CS (logCS) | Gene | Nucleotide and Protein Changes | Zygosity | ACMG Classification |
|---|---|---|---|---|---|---|---|---|---|
| RE; LE | RE; LE | ||||||||
| 1 | 1.1 | 9 months | Nystagmus; photophobia | 20/100; 20/100 | N/A | CNGA3 | c.67C > T (p.Arg23*) c.1687C > T (p.Arg563Cys) | heterozygous heterozygous | pathogenic pathogenic |
| 2 | 2.1 | 5 months | Nystagmus; photophobia | 20/400; 20/400 | 1.35; 1.50 | CNGA3 | c.1775C > T (p.Pro592Leu) c.829C > T (p.Arg277Cys) | heterozygous heterozygous | pathogenic pathogenic |
| 3 | 3.1 | N/A | Photophobia, color blindness | N/A | N/A | CNGA3 | c.1717T > C (p.Tyr573His) | homozygous | pathogenic |
| 4 | 4.1 | 3 months | Nystagmus; photophobia | 20/125; 20/125 | 1.50; 1.45 | CNGA3 | c.572G > A (p.Cys191Tyr) c.811C > G (p.Pro271Ala) | heterozygous heterozygous | pathogenic likely pathogenic |
| 5 | 5.1 | 3 months | Nystagmus; photophobia | 5/400; 5/400 | N/A | CNGA3 | c.1775C > T (p.Pro592Leu) | homozygous | pathogenic |
| 6 | 6.1 | Since birth | Nystagmus | 20/70; 20/70 | 1.60; 1.65 | CNGA3 | c.1669G > A (p.Gly557Arg) | homozygous | likely pathogenic |
| 7 | 7.1 | Childhood | Low central vision | 20/50; 20/150 | N/A | CNGA3 | c.1669G > A (p.Gly557Arg) c.1981C > A (p.Arg661Ser) | heterozygous heterozygous | likely pathogenic pathogenic |
| 8 | 8.1 | Since birth | Nystagmus; photophobia | 20/200; 20/200 | 1.30; 1.35 | CNGA3 | c.1585G > A (p.Val529Met) c.1319G > A (p.Trp440*) | heterozygous heterozygous | pathogenic pathogenic |
| 9 | 9.1 | 3 months | Nystagmus; photophobia | 20/200; 20/160 | 1.30; 1.25 | CNGA3 | c.1669G > A (p.Gly557Arg) c.967G > C (p.Ala323Pro) | heterozygous heterozygous | likely pathogenic likely pathogenic |
| 10 | 10.1 | Childhood | Photophobia; color blindness | 20/100; 20/100 | 1.30; 1.00 | CNGA3 | c.1279C > T (p.Arg427Cys) c.1717T > C (p.Tyr573His) | heterozygous heterozygous | pathogenic pathogenic |
| 11 | 11.1 | 2 months | Nystagmus | N/A | N/A | CNGA3 | c.1641C > A (p.Phe547Leu) | homozygous | pathogenic |
| 12 | 12.1 | 4 months | Nystagmus; photophobia | 20/125; 20/125 | N/A | CNGA3 | c.1981C > A (p.Arg661Ser) c.778G > A (p.Asp260Asn) | heterozygous heterozygous | pathogenic pathogenic |
| 13 | 13.1 | Since birth | Nystagmus | 20/400; 20/400 | 1.15; 1.30 | CNGA3 | c.1495C > T (p.Arg499*) c.572G > A (p.Cys191Tyr) | heterozygous heterozygous | pathogenic pathogenic |
| 14 | 14.1 | 2 years | Nystagmus; photophobia | 20/150; 20/150 | N/A | CNGA3 | c.2T > G (p.Met1?) c.1306C > T (p.Arg436Trp) | heterozygous heterozygous | likely pathogenic pathogenic |
| 15 | 15.1 | Since birth | Nystagmus | 20/160; 20/250 | 1.60; 1.45 | CNGA3 | c.1279C > T (p.Arg427Cys) c.1495C > T (p.Arg499*) | heterozygous heterozygous | pathogenic pathogenic |
| 15 | 15.2 | Since birth | Nystagmus; photophobia | HM; 20/640 | 0.0; 0.15 | CNGA3 | c.1279C > T (p.Arg427Cys) c.1495C > T (p.Arg499*) | heterozygous heterozygous | pathogenic pathogenic |
| 16 | 16.1 | Since birth | Nystagmus; photophobia | N/A | N/A | CNGA3 | c.1201T > C (p.Ser401Pro) | homozygous | likely pathogenic |
| 17 | 17.1 | 1 year | Nystagmus | 20/80; 20/100 | 1.15; 1.35 | CNGA3 | c.1279C > T (p.Arg427Cys) c.1495C > T (p.Arg499*) | heterozygous heterozygous | pathogenic pathogenic |
| 18 | 18.1 | Since birth | Photophobia | 20/200; 20/200 | 1.35; 1.35 | CNGA3 | c.1585G > A (p.Val529Met) c.847C > T (p.Arg238Trp) | heterozygous heterozygous | pathogenic pathogenic |
| Family | ID | Age of Onset | Sings/Symptoms Onset | Current BCVA | CS (logCS) | Gene | Nucleotide and Protein Changes | Zygosity | ACMG Classification |
|---|---|---|---|---|---|---|---|---|---|
| 19 | 19.1 | 3 months | Nystagmus; photophobia | 10/400; 10/400 | N/A | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| 20 | 20.1 | Since birth | Nystagmus; photophobia | 20/200; 20/150 | 1.60; 1.45 | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| 21 | 21.1 | Since birth | Nystagmus; photophobia | 20/400; 20/400 | N/A | CNGB3 | c.1148delC (p.Thr383Ilefs*13) c.1285delT (p.Ser429Leufs*9) | heterozygous heterozygous | pathogenic pathogenic |
| 22 | 22.1 | Since birth | Photophobia | 20/200; 20/400 | 1.35; 1.35 | CNGB3 | c.1148delC (p.Thr383Ilefs*13) c.2T > C (p.Met1?) | heterozygous heterozygous | pathogenic pathogenic |
| 23 | 23.1 | Since birth | Photophobia | 20/400; 20/200 | 1.15; 1.20 | CNGB3 | c.1148delC (p.Thr383Ilefs*13) c.903 + 1G > A (p.?) | heterozygous heterozygous | pathogenic pathogenic |
| 24 | 24.1 | Since birth | Nystagmus | 20/250; 20/250 | 1.30; 1.60 | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| 24 | 24.2 | Since birth | Nystagmus | 20/160; 20/250 | 1.45; 1.10 | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| 24 | 24.3 | Since birth | Nystagmus | 20/250; 20/250 | 1.30; 1.35 | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| 25 | 25.1 | 2 years | Nystagmus | N/A | N/A | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| 25 | 25.2 | 3 months | Nystagmus | N/A | N/A | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| 26 | 26.1 | Since birth | Nystagmus; photophobia | 20/150; 20/150 | 1.35; 1.20 | CNGB3 | c.566G > A (p.Trp189*) | homozygous | pathogenic |
| 27 | 27.1 | Since birth | Nystagmus; photophobia | 20/100; 20/100 | N/A | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| 28 | 28.1 | Since birth | Nystagmus; photophobia | 20/160; 20/160 | 1.40; 1.30 | CNGB3 | c.1148delC (p.Thr383Ilefs*13) c.566G > A (p.Trp189*) | heterozygous heterozygous | pathogenic pathogenic |
| 29 | 29.1 | N/A | N/A | 20/160; 20/160 | 1.20; 1.10 | CNGB3 | c.852 + 1G > T (p.?) | homozygous | pathogenic |
| 30 | 30.1 | N/A | Nystagmus; photophobia | CF; 20/80 | N/A | CNGB3 | c.446_447insT (p.Lys149Asnfs*30) | homozygous | pathogenic |
| 31 | 31.1 | 6 months | Photophobia | 20/400; 20/320 | 0.55; 1.45 | CNGB3 | c.1432C > T (p.Arg478*) | homozygous | pathogenic |
| 32 | 32.1 | Since birth | Nystagmus; photophobia | 20/160; 20/160 | 1.50; 1.55 | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| 33 | 33.1 | 4 months | Nystagmus | 20/160; 20/200 | 1.30; 1.30 | CNGB3 | c.1810C > T (p.Arg604*) | homozygous | pathogenic |
| 34 | 34.1 | Since birth | Photophobia | 20/125; 20/200 | 1.40; 1.20 | CNGB3 | c.1148delC (p.Thr383Ilefs*13) c.991-3T > G (p.?) | heterozygous heterozygous | pathogenic likely pathogenic |
| 35 | 35.1 | 2 months | Nystagmus | N/A | N/A | CNGB3 | c.566G > A (p.Trp189*) | homozygous | pathogenic |
| 36 | 36.1 | Since birth | Nystagmus, photophobia | 20/125; 20/100 | N/A | CNGB3 | c.1148delC (p.Thr383Ilefs*13) c.1893T > A (p.Tyr631*) | heterozygous heterozygous | pathogenic likely pathogenic (novel) |
| 37 | 37.1 | N/A | Photophobia; color blindness | 20/400; 20/400 | N/A | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| 38 | 38.1 | 4 months | Nystagmus | 20/250; 20/200 | 1.30; 1.35 | CNGB3 | c.1148delC (p.Thr383Ilefs*13) | homozygous | pathogenic |
| Causative Gene | Transcript | Nucleotide Change | Consequence | Patients Evaluated | gnomAD Allele Frequency (%) | |
|---|---|---|---|---|---|---|
| Allele Count | Number of Homozygotes | |||||
| CNGA3 | NM_001298.3 | c.67C > T | (p.Arg23*) | 1 | 0 | 0.003540 |
| CNGA3 | NM_001298.3 | c.1687C > T | (p.Arg563Cys) | 1 | 0 | 0.002122 |
| CNGA3 | NM_001298.2 | c.1775C > T | (p.Pro592Leu) | 3 | 1 | 0.0003980 |
| CNGA3 | NM_001298.2 | c.829C > T | (p.Arg277Cys) | 1 | 0 | 0.009548 |
| CNGA3 | NM_001298.2 | c.1717T > C | (p.Tyr573His) | 3 | 1 | 0.003187 |
| CNGA3 | NM_001298.2 | c.572G > A | (p.Cys191Tyr) | 2 | 0 | 0.002121 |
| CNGA3 | NM_001298.2 | c.811C > G | (p.Pro271Ala) | 1 | 0 | 0.01202 |
| CNGA3 | NM_001298.2 | c.1669G > A | (p.Gly557Arg) | 4 | 1 | 0.01415 |
| CNGA3 | NM_001298.2 | c.1981C > A | (p.Arg661Ser) | 2 | 0 | 0.03084 |
| CNGA3 | NM_001298.2 | c.1585G > A | (p.Val529Met) | 2 | 0 | 0.006726 |
| CNGA3 | NM_001298.2 | c.1319G > A | (p.Trp440*) | 1 | 0 | 0.0003986 |
| CNGA3 | NM_001298.2 | c.967G > C | (p.Ala323Pro) | 1 | 0 | 0.009544 |
| CNGA3 | NM_001298.2 | c.1279C > T | (p.Arg427Cys) | 4 | 0 | 0.03902 |
| CNGA3 | NM_001298.3 | c.1641C > A | (p.Phe547Leu) | 2 | 1 | 0.01592 |
| CNGA3 | NM_001298.2 | c.778G > A | (p.Asp260Asn) | 1 | 0 | 0.003182 |
| CNGA3 | NM_001298.2 | c.1495C > T | (p.Arg499*) | 4 | 0 | 0.001063 |
| CNGA3 | NM_001298.2 | c.2T > G | (p.Met1?) | 1 | 0 | - |
| CNGA3 | NM_001298.3 | c.1306C > T | (p.Arg436Trp) | 1 | 0 | 0.009574 |
| CNGA3 | NM_001298.3 | c.1201T > C | (p.Ser401Pro) | 1 | 0 | 0.0003995 |
| CNGA3 | NM_001298.2 | c.847C > T | (p.Arg238Trp) | 1 | 0 | 0.009948 |
| CNGB3 | NM_019098.4 | c.1148delC | (p.Thr383Ilefs*13) | 28 | 11 | 0.1750 |
| CNGB3 | NM_019098.5 | c.1285delT | (p.Ser429Leufs*9) | 1 | 0 | 0.000399 |
| CNGB3 | NM_019098.5 | c.2T > C | (p.Met1?) | 1 | 0 | - |
| CNGB3 | NM_019098.4 | c.903 + 1G > A | (p.?) | 1 | 0 | - |
| CNGB3 | NM_019098.4 | c.566G > A | (p.Trp189*) | 5 | 2 | 0.0003977 |
| CNGB3 | NM_019098.4 | c.852 + 1G > T | (p.?) | 2 | 1 | - |
| CNGB3 | NM_019098.4 | c.446_447insT | (p.Lys149Asnfs*30) | 2 | 1 | 0.0003977 |
| CNGB3 | NM_019098.4 | c.1432C > T | (p.Arg478*) | 2 | 1 | 0.001991 |
| CNGB3 | NM_019098.5 | c.1810C > T | (p.Arg604*) | 2 | 1 | 0.0007969 |
| CNGB3 | NM_019098.4 | c.991-3T > G | (p.?) | 1 | 0 | 0.001338 |
| CNGB3 | NM_019098.5 | c.1893T > A | (p.Tyr631*) | 1 | 0 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, R.A.S.; Motta, F.L.; Zin, O.A.; da Palma, M.M.; Rodrigues, G.D.; Sallum, J.M.F. Molecular and Clinical Characterization of CNGA3 and CNGB3 Genes in Brazilian Patients Affected with Achromatopsia. Genes 2023, 14, 1296. https://doi.org/10.3390/genes14061296
Amaral RAS, Motta FL, Zin OA, da Palma MM, Rodrigues GD, Sallum JMF. Molecular and Clinical Characterization of CNGA3 and CNGB3 Genes in Brazilian Patients Affected with Achromatopsia. Genes. 2023; 14(6):1296. https://doi.org/10.3390/genes14061296
Chicago/Turabian StyleAmaral, Rebeca A. S., Fabiana L. Motta, Olivia A. Zin, Mariana M. da Palma, Gabriela D. Rodrigues, and Juliana M. F. Sallum. 2023. "Molecular and Clinical Characterization of CNGA3 and CNGB3 Genes in Brazilian Patients Affected with Achromatopsia" Genes 14, no. 6: 1296. https://doi.org/10.3390/genes14061296
APA StyleAmaral, R. A. S., Motta, F. L., Zin, O. A., da Palma, M. M., Rodrigues, G. D., & Sallum, J. M. F. (2023). Molecular and Clinical Characterization of CNGA3 and CNGB3 Genes in Brazilian Patients Affected with Achromatopsia. Genes, 14(6), 1296. https://doi.org/10.3390/genes14061296







