A Description of the Yield of Genetic Reinvestigation in Patients with Inherited Retinal Dystrophies and Previous Inconclusive Genetic Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Genetic Analysis
2.3. DNA Extraction
2.4. Ophthalmological Examinations
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jeffery, R.C.H.; Mukhtar, S.A.; McAllister, I.L.; Morgan, W.H.; Mackey, D.A.; Chen, F.K. Inherited retinal diseases are the most common cause of blindness in the working-age population in Australia. Ophthalmic Genet. 2021, 42, 431–439. [Google Scholar] [CrossRef]
- Glatz, M.; Riedl, R.; Glatz, W.; Schneider, M.; Wedrich, A.; Bolz, M.; Strauss, R.W. Blindness and visual impairment in Central Europe. PLoS ONE 2022, 17, e0261897. [Google Scholar] [CrossRef] [PubMed]
- Ziccardi, L.; Cordeddu, V.; Gaddini, L.; Matteucci, A.; Parravano, M.; Malchiodi-Albedi, F.; Varano, M. Gene Therapy in Retinal Dystrophies. Int. J. Mol. Sci. 2019, 20, 5722. [Google Scholar] [CrossRef]
- Nuzbrokh, Y.; Ragi, S.D.; Tsang, S.H. Gene therapy for inherited retinal diseases. Ann. Transl. Med. 2021, 9, 1278. [Google Scholar] [CrossRef]
- Velazquez, L.A.M.; Ballios, B.G. The Next Generation of Molecular and Cellular Therapeutics for Inherited Retinal Disease. Int. J. Mol. Sci. 2021, 22, 11542. [Google Scholar] [CrossRef]
- Chiu, W.; Lin, T.-Y.; Chang, Y.-C.; Lai, H.I.-A.M.; Lin, S.-C.; Ma, C.; Yarmishyn, A.A.; Lin, S.-C.; Chang, K.-J.; Chou, Y.-B.; et al. An Update on Gene Therapy for Inherited Retinal Dystrophy: Experience in Leber Congenital Amaurosis Clinical Trials. Int. J. Mol. Sci. 2021, 22, 4534. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.S.-Y.; Punzo, C. Update on Viral Gene Therapy Clinical Trials for Retinal Diseases. Hum. Gene Ther. 2022, 33, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Maguire, A.M.; Bennett, J.; Aleman, E.M.; Leroy, B.P.; Aleman, T.S. Clinical Perspective: Treating RPE65-Associated Retinal Dystrophy. Mol. Ther. 2021, 29, 442–463. [Google Scholar] [CrossRef]
- Cremers, F.P.; Lee, W.; Collin, R.W.; Allikmets, R. Clinical spectrum, genetic complexity and therapeutic approaches for retinal disease caused by ABCA4 mutations. Prog. Retin. Eye Res. 2020, 79, 100861. [Google Scholar] [CrossRef] [PubMed]
- Berger, W.; Kloeckener-Gruissem, B.; Neidhardt, J. The molecular basis of human retinal and vitreoretinal diseases. Prog. Retin. Eye Res. 2010, 29, 335–375. [Google Scholar] [CrossRef]
- Ali, M.U.; Rahman, M.S.U.; Cao, J.; Yuan, P.X. Genetic characterization and disease mechanism of retinitis pigmentosa; current scenario. 3 Biotech 2017, 7, 251. [Google Scholar] [CrossRef]
- Schneider, N.; Sundaresan, Y.; Gopalakrishnan, P.; Beryozkin, A.; Hanany, M.; Levanon, E.Y.; Banin, E.; Ben-Aroya, S.; Sharon, D. Inherited retinal diseases: Linking genes, disease-causing variants, and relevant therapeutic modalities. Prog. Retin. Eye Res. 2022, 89, 101029. [Google Scholar] [CrossRef]
- Hanany, M.; Rivolta, C.; Sharon, D. Worldwide carrier frequency and genetic prevalence of autosomal recessive inherited retinal diseases. Proc. Natl. Acad. Sci. USA 2020, 117, 2710–2716. [Google Scholar] [CrossRef] [PubMed]
- Musarella, M.A.; Burghes, A.; Anson-Cartwright, L.; Mahtani, M.M.; Argonza, R.; Tsui, L.C.; Worton, R. Localization of the gene for X-linked recessive type of retinitis pigmentosa (XLRP) to Xp21 by linkage analysis. Am. J. Hum. Genet. 1988, 43, 484–494. [Google Scholar] [PubMed]
- Bhattacharya, S.S.; Wright, A.F.; Clayton, J.F.; Price, W.H.; Phillips, C.I.; McKeown, C.M.E.; Jay, M.; Bird, A.C.; Pearson, P.L.; Southern, E.M.; et al. Close genetic linkage between X-linked retinitis pigmentosa and a restriction fragment length polymorphism identified by recombinant DNA probe L1.28. Nature 1984, 309, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Ankala, A.; Wilcox, W.R.; Hegde, M.R. Solving the molecular diagnostic testing conundrum for Mendelian disorders in the era of next-generation sequencing: Single-gene, gene panel, or exome/genome sequencing. Genet. Med. 2015, 17, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; MacLaren, R.E. Antisense oligonucleotide therapeutics in clinical trials for the treatment of inherited retinal diseases. Expert Opin. Investig. Drugs 2020, 29, 1163–1170. [Google Scholar] [CrossRef]
- Marmor, M.F.; Holder, G.E.; Seeliger, M.W.; Yamamoto, S. Standard for clinical electroretinography (2004 update). Doc. Ophthalmol. 2004, 108, 107–114. [Google Scholar] [CrossRef]
- Marmor, M.F.; Fulton, A.B.; Holder, G.E.; Miyake, Y.; Brigell, M.; Bach, M. ISCEV Standard for full-field clinical electroretinography (2008 update). Doc. Ophthalmol. 2009, 118, 69–77. [Google Scholar] [CrossRef]
- Hood, D.C.; Bach, M.; Brigell, M.; Keating, D.; Kondo, M.; Lyons, J.S.; Palmowski-Wolfe, A.M. ISCEV guidelines for clinical multifocal electroretinography (2007 edition). Doc. Ophthalmol. 2008, 116, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hood, D.C.; Bach, M.; Brigell, M.; Keating, D.; Kondo, M.; Lyons, J.S.; Marmor, M.F.; McCulloch, D.L.; Plamowski-Wolfe, A.M. ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc. Ophthalmol. 2012, 124, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reichel, F.F.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Sothilingam, V.; Kuehlewein, L.; Kahle, N.; et al. Three-year results of phase I retinal gene therapy trial for CNGA3-mutated achromatopsia: Results of a non randomised controlled trial. Br. J. Ophthalmol. 2022, 106, 1567–1572. [Google Scholar] [CrossRef] [PubMed]
- Pennesi, M.E.; Weleber, R.G.; Yang, P.; Whitebirch, C.; Thean, B.; Flotte, T.R.; Humphries, M.; Chegarnov, E.; Beasley, K.N.; Stout, J.T.; et al. Results at 5 Years after Gene Therapy for RPE65-Deficient Retinal Dystrophy. Hum. Gene Ther. 2018, 29, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.A.; Erker, L.R.; Audo, I.; Choi, D.; Mohand-Said, S.; Sestakauskas, K.; Benoit, P.; Appelqvist, T.; Krahmer, M.; Ségaut-Prévost, C.; et al. Three-Year Safety Results of SAR422459 (EIAV-ABCA4) Gene Therapy in Patients with ABCA4-Associated Stargardt Disease: An Open-Label Dose-Escalation Phase I/IIa Clinical Trial, Cohorts 1–5. Am. J. Ophthalmol. 2022, 240, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Russell, S.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Drack, A.V.; Simonelli, F.; Leroy, B.P.; Reape, K.Z.; High, K.A.; et al. Durability of Voretigene Neparvovec for Biallelic RPE65-Mediated Inherited Retinal Disease: Phase 3 Results at 3 and 4 Years. Ophthalmology 2021, 128, 1460–1468. [Google Scholar] [CrossRef]
- Lopes, V.S.; Boye, S.E.; Louie, C.M.; Dyka, F.; Chiodo, V.; Fofo, H.; Hauswirth, W.W.; Williams, D.S. Retinal gene therapy with a large MYO7A cDNA using adeno-associated virus. Gene Ther. 2013, 20, 824–833. [Google Scholar] [CrossRef]
- Fischer, M.D.; Ochakovski, G.A.; Beier, B.; Seitz, I.P.; Vaheb, Y.; Kortuem, C.; Reichel, F.F.L.; Kuehlewein, L.; Kahle, N.A.; Peters, T.; et al. Efficacy and Safety of Retinal Gene Therapy Using Adeno-Associated Virus Vector for Patients with Choroideremia: A Randomized Clinical Trial. JAMA Ophthalmol. 2019, 137, 1247–1254. [Google Scholar] [CrossRef]
- Fischer, M.D.; Michalakis, S.; Wilhelm, B.; Zobor, D.; Muehlfriedel, R.; Kohl, S.; Weisschuh, N.; Ochakovski, G.A.; Klein, R.; Schoen, C.; et al. Safety and Vision Outcomes of Subretinal Gene Therapy Targeting Cone Photoreceptors in Achromatopsia: A Nonrandomized Controlled Trial. JAMA Ophthalmol. 2020, 138, 643–651. [Google Scholar] [CrossRef]
- Cukras, C.; Wiley, H.E.; Jeffrey, B.G.; Sen, H.N.; Turriff, A.; Zeng, Y.; Vijayasarathy, C.; Marangoni, D.; Ziccardi, L.; Kjellstrom, S.; et al. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol. Ther. 2018, 26, 2282–2294. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Xue, K.; de la Camara, C.M.-F.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.R.; Drack, A.V.; Cideciyan, A.V.; Jacobson, S.G.; Leroy, B.P.; Van Cauwenbergh, C.; Ho, A.C.; Dumitrescu, A.V.; Han, I.C.; Martin, M.; et al. Intravitreal antisense oligonucleotide sepofarsen in Leber congenital amaurosis type 10: A phase 1b/2 trial. Nat. Med. 2022, 28, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Cideciyan, A.V.; Jacobson, S.G.; Ho, A.C.; Krishnan, A.K.; Roman, A.J.; Garafalo, A.V.; Wu, V.; Swider, M.; Sumaroka, A.; Van Cauwenbergh, C.; et al. Restoration of Cone Sensitivity to Individuals with Congenital Photoreceptor Blindness within the Phase 1/2 Sepofarsen Trial. Ophthalmol. Sci. 2022, 2, 100133. [Google Scholar] [CrossRef] [PubMed]
- Farrar, G.J.; Carrigan, M.; Dockery, A.; Millington-Ward, S.; Palfi, A.; Chadderton, N.; Humphries, M.; Kiang, A.S.; Kenna, P.F.; Humphries, P. Toward an elucidation of the molecular genetics of inherited retinal degenerations. Hum. Mol. Genet. 2017, 26, R2–R11. [Google Scholar] [CrossRef] [PubMed]
- Dockery, A.; Whelan, L.; Humphries, P.; Farrar, G.J. Next-Generation Sequencing Applications for Inherited Retinal Diseases. Int. J. Mol. Sci. 2021, 22, 5684. [Google Scholar] [CrossRef]
- Garafalo, A.V.; Cideciyan, A.V.; Héon, E.; Sheplock, R.; Pearson, A.; Yu, C.W.; Sumaroka, A.; Aguirre, G.D.; Jacobson, S.G. Progress in treating inherited retinal diseases: Early subretinal gene therapy clinical trials and candidates for future initiatives. Prog. Retin. Eye Res. 2020, 77, 100827. [Google Scholar] [CrossRef]
- Motta, F.L.; Martin, R.P.; Filippelli-Silva, R.; Salles, M.V.; Sallum, J.M.F. Relative frequency of inherited retinal dystrophies in Brazil. Sci. Rep. 2018, 8, 15939. [Google Scholar] [CrossRef]
- Chen, T.-C.; Huang, D.-S.; Lin, C.-W.; Yang, C.-H.; Yang, C.-M.; Wang, V.Y.; Lin, J.-W.; Luo, A.C.; Hu, F.-R.; Chen, P.-L. Genetic characteristics and epidemiology of inherited retinal degeneration in Taiwan. NPJ Genom Med. 2021, 6, 16. [Google Scholar] [CrossRef]
- Karali, M.; Testa, F.; Di Iorio, V.; Torella, A.; Zeuli, R.; Scarpato, M.; Romano, F.; Onore, M.E.; Pizzo, M.; Melillo, P.; et al. Genetic epidemiology of inherited retinal diseases in a large patient cohort followed at a single center in Italy. Sci. Rep. 2022, 12, 20815. [Google Scholar] [CrossRef]
- Chen, C.; Sun, Q.; Gu, M.; Qian, T.; Luo, D.; Liu, K.; Xu, X.; Yu, S. Multimodal imaging and genetic characteristics of Chinese patients with USH2A-associated nonsyndromic retinitis pigmentosa. Mol. Genet. Genom. Med. 2020, 8, e1479. [Google Scholar] [CrossRef]
| ABCA4 ABCC6 ABHD12 ACO2 ADAM9 ADAMTS18 ADGRV1 ADIPOR1 AGBL5 AHI1 AIPL1 ALMS1 ARHGEF18 ARL13B ARL2BP ARL3 ARL6 ARMC9 ARSG ATF6 ATOH7 B9D1 B9D2 BBIP1 BBS1 BBS10 BBS12 BBS2 BBS4 BBS5 BBS7 BBS9 BEST1 C1QTNF5 C21ORF2 C2ORF71 C5ORF42 C8ORF37 CA4 CABP4 | CACNA1F CACNA2D4 CAPN5 CC2D2A CDH23 CDH3 CDHR1 CEP104 CEP120 CEP164 CEP19 CEP250 CEP290 CEP41 CEP78 CERK CHM CIB2 CISD2 CLN3 CLRN1 CNGA1 CNGA3 CNGB1 CNGB3 CNNM4 COL11A1 COL11A2 COL18A1 COL2A1 COL9A1 COL9A2 COL9A3 CPE CRB1 CRX CSPP1 CTC1 CTNNA1 CTNNB1 | CWC27 CYP4V2 DFNB31 DHDDS DHX38 DRAM2 DTHD1 EFEMP1 ELOVL4 EMC1 ESPN EYS FAM161A FDXR FLVCR1 FRMD7 FZD4 GNAT1 GNAT2 GNB3 GNPTG GPR179 GRK1 GRM6 GUCA1A GUCY2D HAR HGSNAT HK1 HMX IDH3A IDH3B IFT140 IFT172 IFT27 IFT81 IMPDH1 IMPG1 IMPG2 INPP5E | INVS IQCB1 JAG1 KCNJ13 KCNV2 KIAA0556 KIAA0586 KIAA0753 KIAA154 KIF11 KIF7 KIZ KLHL7 LCA5 LRAT LRIT3 LRP2 LRP5 LZTFL1 MAK MERTK MFN2 MFRP MFSD8 MKKS MKS1 MMACHC MT-ATP6 MT-ATP8 MT-CO1 MT-CO2 MT-CO3 MT-CYB MT-ND1 MT-ND2 MT-ND3 MT-ND4 MT-ND4L MT-ND5 MT-ND6 | MT-RNR1 MT-RNR2 MT-TA MT-TC MT-TD MT-TE MT-TF MT-TG MT-TH MT-TI MT-TK MT-TL1 MT-TL2 MT-TM MT-TN MT-TP MT-TQ MT-TR MT-TS1 MT-TS2 MT-TT MT-TV MT-TW MT-TY MTTP MVK MYO7A NDP NEK2 NMNAT1 NPHP1 NPHP3 NPHP4 NR2E3 NR2F1 NRL NYX OAT OFD1 OPA1 | OPA3 OTX2 P3H2 PANK2 PAX2 PCDH15 PCYT1A PDE6A PDE6B PDE6C PDE6D PDE6G PDE6H PDZD7 PEX1 PEX10 PEX11B PEX12 PEX13 PEX14 PEX16 PEX19 PEX2 PEX26 PEX3 PEX5 PEX6 PEX7 PHYH PISD PITPNM3 PLA2G5 PNPLA6 POC1B POMGNT1 PRCD PRDM13 PROM1 PRPF3 PRPF31 | PRPF4 PRPF6 PRPF8 PRPH2 PRPS1 RAB28 RAX2 RBP3 RBP4 RCBTB1 RD3 RDH11 RDH12 RDH5 REEP6 RGR RGS9 RGS9BP RHO RIMS1 RLBP1 ROM1 RP1 RP1L1 RP2 RPE65 RPGR RPGRIP1 RPGRIP1L RS1 RTN4IP1 SAG SAMD11 SCAPER SCLT1 SDCCAG8 SEMA4A SLC24A1 SLC25A46 SLC7A14 SNRNP200 | SPATA7 SPP2 SRD5A3 TCTN1 TCTN2 TCTN3 TEAD1 TIMM8A TIMP3 TMEM107 TMEM126A TMEM138 TMEM216 TMEM231 TMEM237 TMEM67 TOPORS TRAF3IP1 TREX1 TRIM32 TRPM1 TSPAN12 TTC21B TTC8 TTLL5 TTPA TUB TUBB4B TULP1 USH1C USH1G USH2A VCAN VPS13B WDPCP WDR19 WFS1 YME1L1 ZNF408 ZNF423 ZNF513 |
| Patient | Gender | Age at First Examination | Genotype | Description of Pathogenic Variants | Phenotype at Initial Examination |
|---|---|---|---|---|---|
| RP145LU | K | 12 | ABC4A | heterozygous for the missense variant, ABCA4 c.2915C > A, p.(Thr972Asn), which is pathogenic and heterozygous for the frameshift variant, ABCA4 c.4042del, p.(Thr1348Hisfs*41), which is likely pathogenic. | STGD |
| RP146LU | M | 8 | ABC4A | heterozygous for ABCA4 c.2915C > A, p.(Thr972Asn), which is pathogenic and heterozygous for ABCA4 c.4042del, p.(Thr1348Hisfs*41), which is likely pathogenic | STGD |
| RP173LU | K | 14 | ABCA4 | heterozygous for ABCA4 c.2894A > G, p.(Asn965Ser), which is pathogenic heterozygous for ABCA4 c.768G > T, p.(Val256=), which is pathogenic. | CD |
| RP125LU | M | 4 | ABCA4 | heterozygous for ABCA4 c.2588G > C, p.(Gly863Ala) classified as pathogenic and heterozygous for ABCA4 c.5603A > T, p.(Asn1868Ile) classified as a risk factor | CRD |
| RP135LU | M | 15 | ABCA4 | homozygous for a deletion, ABCA4 c.2918 + 11_3522 + 86del which encompasses exons 20–23 classified as pathogenic | CRD |
| RP209LU | K | 8 | ABCA4 | homozygous for ABCA4 c.768G > T, p.(Val256=), which is pathogenic | CRD |
| RP253LU | K | 10 | ABCA4 | homozygous for ABCA4 c.319C > T, p.(Arg107*), which is pathogenic | CRD |
| RP5LU | K | 7 | ABCA4 | homozygous for ABCA4 c.768G > T, p.(Val256=) which is pathogenic | CRD |
| RP161LU | K | 20 | ABCA4 | heterozygous for ABCA4 c.4773 + 3A > G, which is pathogenic and heterozygous for ABCA4 c.768G > T, p.(Val256=), which is pathogenic | STGD |
| RP162LU | M | 10 | ABCA4 | heterozygous for ABCA4 c.6286G > A, p.(Glu2096Lys), which is pathogenic and heterozygous for ABCA4 c.5461–10T > C, which is pathogenic and for ABCA4 c.5603A > T, p.(Asn1868Ile), which is risk factor | STGD |
| RP170LU | K | 14 | ABCA4 | heterozygous for ABCA4 c.4139C > T, p.(Pro1380Leu), which is pathogenic. heterozygous for ABCA4 c.2894A > G, p.(Asn965Ser), which is pathogenic. heterozygous for ABCA4 c.5603A > T, p.(Asn1868Ile), which is a risk factor | STGD |
| RP171LU | K | 15 | ABCA4 | heterozygous for ABCA4 c.6181_6184del, p.(Thr2061Serfs*53), which is pathogenic, heterozygous for ABCA4 c.3322C > T, p.(Arg1108Cys), which is pathogenic and heterozygous for ABCA4 c.5603A > T, p.(Asn1868Ile), which is a risk factor | STGD |
| RP18LU | K | 12 | ABCA4 | heterozygous for ABCA4 c.2894A > G, p.(Asn965Ser), which is pathogenic and heterozygous for ABCA4 c.319C > T, p.(Arg107*), which is pathogenic | STGD |
| RP200LU | K | 17 | ABCA4 | heterozygous for ABCA4 c.3322C > T, p.(Arg1108Cys), which is pathogenic and heterozygous for ABCA4 c.768G > T, p.(Val256=), which is pathogenic | STGD |
| RP206LU | K | 18 | ABCA4 | heterozygous for ABCA4 c.4601del, p.(Leu1534Trpfs*2), which is pathogenic, heterozygous for ABCA4 c.2588G > C, p.(Gly863Ala), which is pathogenic and heterozygous for ABCA4 c.5603A > T, p.(Asn1868Ile), which is risk factor | STGD |
| RP215LU | K | 19 | ABCA4 | heterozygous for ABCA4 c.5461-10T > C, which is pathogenic, heterozygous for ABCA4 c.2894A > G, p.(Asn965Ser), which is pathogenic and heterozygous for ABCA4 c.5603A > T, p.(Asn1868Ile), which is a risk factor | STGD |
| RP224LU | M | 11 | ABCA4 | heterozygous for ABCA4 c.4139C > T, p.(Pro1380Leu), which is pathogenic heterozygous for ABCA4 c.2599del, p.(Thr867Profs*34), which is likely pathogenic. | STGD |
| RP242LU | K | 13 | ABCA4 | heterozygous for ABCA4 c.2894A > G, p.(Asn965Ser), which is pathogenic and heterozygous for ABCA4 c.1610G > A, p.(Arg537His), which is likely pathogenic | STGD |
| RP261LU | M | 10 | ABCA4 | homozygous for ABCA4 c.5584G > C, p.(Gly1862Arg), which is likely pathogenic | STGD |
| RP287LU | K | 19 | ABCA4 | heterozygous for ABCA4 c.6088C > T, p.(Arg2030*), which is pathogenic and heterozygous for ABCA4 c.5882G > A, p.(Gly1961Glu), which is pathogenic | STGD |
| RP94LU | M | 11 | ABCA4 | homozygous for ABCA4 c.868C > T, p.(Arg290Trp), which is pathogenic | STGD |
| RP48LU | K | 25 | ABCA4 | heterozygous for ABCA4 c.3322C > T, p.(Arg1108Cys), which is pathogenic and heterozygous for ABCA4 c.2894A > G, p.(Asn965Ser), which is pathogenic | STGD |
| RP85LU | K | 25 | ABCA4 | homozygous for ABCA4 c.5882G > A, p.(Gly1961Glu), which is pathogenic | STGD |
| RP191LU | K | 16 | ABCA4 | heterozygous for ABCA4 c.1804C > T, p.(Arg602Trp), which is pathogenic, heterozygous for ABCA4 c.3113C > T, p.(Ala1038Val), which is pathogenic, heterozygous for ABCA4 c.1622T > C, p.(Leu541Pro), which is pathogenic and heterozygous for ABCA4 c.5603A > T, p.(Asn1868Ile), which is a risk factor | CD |
| RP21LU | K | 18 | ABCA4 | homozygous ABCA4c.5882G > A, p.(Gly1961Glu pathogenic. homozygous for ABCA4 c.634C > T, p.(Arg212Cys), which is pathogenic | CD |
| RP22LU | K | 10 | ABCA4 | heterozygous for ABCA4 c.4773 + 1G > A, which is pathogenic and heterozygous for ABCA4 c.53G > A, p.(Arg18Gln), which is pathogenic | CRD |
| RP172LU | K | 8 | ABCA4 | homozygous for ABCA4 c.3113C > T, p.(Ala1038Val), which is pathogenic and homozygous for ABCA4 c.1622T > C, p.(Leu541Pro), which is pathogenic | CRD |
| RP20LU | K | 14 | ABCA4 | heterozygous for ABCA4 c.5413A > G, p.(Asn1805Asp), which is pathogenic and heterozygous for ABCA4 c.6159G > A, p.(Trp2053*), which is likely pathogenic | STGD |
| RP34LU | M | 18 | ABCA4 | heterozygous for ABCA4 c.5461-10T > C, which is pathogenic, heterozygous for ABCA4 c.5196 + 1137G > A, which is pathogenic and heterozygous for ABCA4 c.5603A > T, p.(Asn1868Ile), which is risk factor | STGD |
| RP41LU | M | 15 | ABCA4 | heterozygous for ABCA4 c.6079C > T, p.(Leu2027Phe), which is pathogenic and heterozygous for ABCA4 c.4139C > T, p.(Pro1380Leu), which is pathogenic | STGD |
| RP273LU | M | 1 | AIPL1 | heterozygous for AIPL1 c.834G > A, p.(Trp278*), which is pathogenic and heterozygous for AIPL1 c.537del, p.(Val180Serfs*29), which is likely pathogenic | LCA |
| RP181LU | K | 18 | BBS1 | homozygous for BBS1 c.1169T > G, p.(Met390Arg), which is pathogenic | RP |
| RP12LU | M | 3 | BBS10 | homozygous BBS10 c.271dup, p.(Cys91Leufs*5), which is pathogenic | Bardet–Biedl |
| RP154LU | K | 13 | BBS10 | homozygous for BBS10 c.1244del, p.(His415Leufs*16), which is pathogenic | Bardet–Biedl |
| RP155LU | K | 14 | BBS10 | homozygous for BBS10 c.271dup, p.(Cys91Leufs*5), which is pathogenic | Bardet–Biedl |
| RP190LU | K | 8 | BBS10 | homozygous for BBS10 c.271dup, p.(Cys91Leufs*5), which is pathogenic | Bardet–Biedl |
| RP236LU | M | 12 | BBS5 | homozygous for BBS5 c.790G > A, p.(Gly264Arg), which is pathogenic | achromatopsia |
| RP30LU | K | 8 | BBS5 | homozygous for BBS5 c.790G > A, p.(Gly264Arg), which is pathogenic | achromatopsia |
| RP76LU | M | 16 | BBS9 | homozygous for BBS9 c.1561C > T, p.(Arg521*), which is pathogenic | Bardet–Biedl |
| RP1LU | M | 10 | CACNA1F | hemizygous for CACNA1F c.4156C > T, p.(Gln1386*) which is likely pathogenic | CHM |
| RP205LU | M | 6 | CACNA1F | hemizygous for CACNA1F c.3895C > T, p.(Arg1299*), which is pathogenic | CSNB |
| RP195LU | M | 7 | CACNA1F | hemizygous for CACNA1F c.4134–1G > C, which is pathogenic | XLRS |
| RP23LU | M | 8 | CACNA1F | hemizygous for CACNA1F c.3542_3548del, p.(Tyr1181Cysfs*5), which is likely pathogenic | XLRS |
| RP166LU | M | 8 | CACNA1F | hemizygous for CACNA1F c.952_954del, p.(Phe318del), which is pathogenic | XLRS |
| RP50LU | M | 2 | CACNA1F | hemizygous for CACNA1F c.2071C > T, p.(Arg691*), which is pathogenic | XLRS |
| RP65LU | M | 19 | CACNA2D4 | homozygous for CACNA2D4 c.1564C > T, p.(Arg522*), which is likely pathogenic | CD |
| RP123LU | K | 6 | CDH23 | homozygous for CDH2 c.8733del, p.(Asp2911Glufs*41), which is likely pathogenic | Usher |
| RP108LU | M | 12 | CDH3 | heterozygous for CDH3 c.1795 + 1G > A, which is likely pathogenic and heterozygous for CDH3 c.1643C > G, p.(Pro548Arg), which is a VUS; however, this variant is absent in control populations and predicted to be deleterious via in silico tools and NGS data strongly suggest that these variants are in trans, thus interpreted as causative | macular dystrophy |
| RP221LU | K | 19 | CDHR1 | heterozygous for CDHR1 c.783G > A, p.(Pro261=), which is pathogenic and heterozygous for CDHR1 c.2522_2528del, p.(Ile841Serfs*119), which is pathogenic | RP |
| RP106LU | M | 1 | CEP290 | heterozygous for CEP290 c.4661_4663del, p.(Glu1554del), which is pathogenic and heterozygous for CEP290 c.2052 + 1_2052 + 2del, which is pathogenic | LCA |
| RP116LU | K | 3 | CEP290 | heterozygous for CEP290 c.4661_4663del, p.(Glu1554del), pathogenic. heterozygous for CEP290 c.955del, p.(Ser319Leufs*16), likely pathogenic | LCA |
| RP137LU | K | 1 | CEP290 | heterozygous for CEP290 c.2991 + 1655A > G, which is pathogenic and heterozygous for CEP290 c.1992del, p.(Pro665Leufs*10), which is pathogenic | LCA |
| RP150LU | M | 1 | CEP290 | homozygous for CEP290 c.2991 + 1655A > G, which is pathogenic | LCA |
| RP156LU | K | 1 | CEP290 | heterozygous for CEP290 c.2991 + 1655A > G, which is pathogenic and heterozygous for CEP290 c.384_387del, p.(Asp128Glufs*34), which is pathogenic | LCA |
| RP157LU | K | 0 | CEP290 | heterozygous for CEP290 c.2991 + 1655A > G, which is pathogenic. heterozygous for CEP290 c.170C > A, p.(Ser57*), which is likely pathogenic | LCA |
| RP249LU | K | 7 | CEP290 | heterozygous for CEP290 c.3249dup, p.(Arg1084Thrfs*11), which is pathogenic and heterozygous for CEP290 c.1065 + 1G > A, which is likely pathogenic | LCA |
| RP294LU | M | 19 | CEP290 | heterozygous for CEP290 c.2991 + 1655A > G, which is pathogenic and heterozygous for CEP290 c.384_387del, p.(Asp128Glufs*34), which is pathogenic | LCA |
| RP66LU | K | 0 | CEP290 | heterozygous for CEP290 c.2991 + 1655A > G, which is pathogenic and heterozygous for CEP290 c.1681C > T, p.(Gln561*), which is likely pathogenic. | LCA |
| RP82LU | M | 1 | CEP290 | heterozygous for CEP290 c.2991 + 1655A > G, which is pathogenic and heterozygous for CEP290 c.1992del, p.(Pro665Leufs*10), which is pathogenic | LCA |
| RP262LU | M | 5 | CEP290 | heterozygous for CEP290 c.4438–3del, which is pathogenic and heterozygous for CEP290 c.164_167del, p.(Thr55Serfs*3), which is pathogenic | RP |
| RP258LU | K | 5 | CFAP410 | homozygous for CFAP410 c.33_34insAGCTGCACAGCGTGCA, p.(Ala12Serfs*60), which is pathogenic | CD |
| RP59LU | K | 5 | CFAP410 | homozygous for CFAP410 c.218G > C, p.(Arg73Pro) which is pathogenic | CD |
| RP64LU | K | 11 | CFAP410 | homozygous for deletion CFAP410 c.(?_-1)_(*1_?)del, which is pathogenic | CD |
| RP80LU | K | 14 | CFAP410 | homozygous for a deletion CFAP410 c.(?_-1)_(*1_?)del, which encompasses the whole CFAP410 gene and is pathogenic | CD |
| RP256LU | K | 10 | CFAP410 | homozygous for CFAP410 c.218G > C, p.(Arg73Pro), which is pathogenic | RP |
| RP288LU | M | 24 | CHM | hemizygous for CHM c.1244 + 1G > C, which is likely pathogenic | CHM |
| RP49LU | M | 16 | CHM | hemizygous for CHM c.1144G > T, p.(Glu382*), which is pathogenic | CHM |
| RP257LU | M | 18 | CHM | hemizygous for a deletion CHM c.(314 + 1_315 − 1)_(1166 + 1_1167 − 1)del, which encompasses exons 5–8 of CHM, classified as pathogenic | RP |
| RP264LU | M | 10 | CHM | hemizygous for a 6 Mb deletion, seq[GRCh37] del(X)(q21.1q21.2), chrX:g.79270061–85302755del, encompassing the entire panel gene CHM and classified as pathogenic. | RP |
| RP129LU | K | 8 | CHM | heterozygous for CHM c.1411del, p.(Gln471Argfs*5), which is likely pathogenic | CHM carrier |
| RP42LU | K | 13 | CHM | heterozygous for CHM c.1144G > T, p.(Glu382*), which is pathogenic | CHM carrier |
| RP227LU | K | 7 | CLN3 | homozygous for deletion CLN3 c.(460 + 1_461 − 1)_(677 + 1_678 − 1)del, which encompasses exons 8–9 of CLN3 and is classified as pathogenic | CLN3 |
| RP234LU | M | 7 | CLN3 | homozygous for deletion CLN3 c.(460 + 1_461 − 1)_(677 + 1_678 − 1)del, which encompasses exons 8–9 of CLN3 and is classified as pathogenic | CLN3 |
| RP220LU | M | 6 | CLN3 | homozygous for a deletion CLN3 c.(460 + 1_461 − 1)_(677 + 1_678 − 1)del, which encompasses exons 8–9 of CLN3 and is classified as pathogenic | RP |
| RP68LU | M | 18 | CNGB1 | heterozygous for CNGB1 c.2957A > T, p.(Asn986Ile), which is pathogenic and heterozygous for CNGB1 c.2293C > T, p.(Arg765Cys), which is likely pathogenic | RP |
| RP26LU | M | 10 | CNGB3 | heterozygous for CNGB3 c.1285dup, p.(Ser429Phefs*33), which is pathogenic and heterozygous for CNGB3 c.819_826del, p.(Arg274Valfs*13), which is pathogenic | achromatopsia |
| RP27LU | M | 1 | CNGB3 | heterozygous for CNGB3 c.1148del, p.(Thr383Ilefs*13), which is pathogenic and heterozygous for CNGB3 c.1643G > T, p.(Gly548Val), which is VUS, however, CNGB3 c.1643G > T, p.(Gly548Val) is absent in control populations and predicted to be deleterious by insilico tools and thus compound heterozygosity of the variants would explain the phenotype | achromatopsia |
| RP15LU | K | 6 | COL18A1 | homozygous for COL18A1 c.2157 + 2T > C, which is likely pathogenic | Knobloch syndrome |
| RP132LU | M | 2 | COL18A1 | homozygous for COL18A1 c.3514_3515del, p.(Leu1172Valfs*72), which is pathogenic | LCA |
| RP244LU | K | 12 | COL18A1 | homozygous for COL18A1 c.874del, p.(Glu292Lysfs*17), which is likely pathogenic | macular dystrophy |
| RP207LU | M | 10 | COL18A1 | heterozygous for COL18A1 c.3666_3682del, p.(Ala1223Glnfs*19), which is likely pathogenic and heterozygous for COL18A1 c.3809 + 2T > C, which is likely pathogenic | vitreoretinal dystrophy |
| RP140LU | M | 13 | CRX | heterozygous for CRX c.413del, p.(Ile138Thrfs*49), which is pathogenic | CRD |
| RP92LU | K | 5 | CRX | heterozygous for CRX c.413del, p.(Ile138Thrfs*49), which is pathogenic | CRD |
| RP117LU | M | 4 | CRX | heterozygous frameshift variant CRX c.413del, p.(Ile138Thrfs*49) which is pathogenic | RP |
| RP114LU | K | 1 | GUCA1A | heterozygous for GUCA1A c.332A > T, p.(Glu111Val), which is likely pathogenic | CRD |
| RP134LU | M | 13 | GUCY2D | heterozygous for GUCY2D c.2377del, p.(Glu793Asnfs*42), which is likely pathogenic and heterozygous for GUCY2D c.1567-17T > A, which is a VUS, however, these GUCY2D variants are consistent with the patient’s phenotype, and GUCY2D c.1567-17T > A is rare in control populations and predicted to affect splicing by in silico tools, thus compound heterozygosity of the variants could explain the phenotype | CRD |
| RP148LU | M | 3 | GUCY2D | heterozygous for GUCY2D c.2944 + 1del, which is pathogenic and heterozygous for GUCY2D c.2965G > C, p.(Val989Leu), which is a VUS, however, these GUCY2D variants are consistent with the patient’s phenotype, and GUCY2D c.2965G > C, p.(Val989Leu) is absent in control populations and predicted to be deleterious by in silico tools, NGS data suggests that these variants are in trans in thispatient, which could explain the patient’s clinical presentation | CRD |
| RP176LU | K | 18 | GUCY2D | heterozygous for GUCY2D c.2944 + 1del, which is pathogenic. heterozygous for GUCY2D c.1982G > T, p.(Gly661Val), which is a VUS, however, these GUCY2D variants are consistent with the patient’s phenotype, and GUCY2D c.1982G > T, p.(Gly661Val) is absent in control populations and predicted to be deleterious by in silico tools, compound heterozygosity of the variants would explain the patient’s clinical presentation | CRD |
| RP24LU | M | 6 | GUCY2D | heterozygous for GUCY2D c.2302C > T, p.(Arg768Trp), which is pathogenic and heterozygous for GUCY2D c.1567-17T > A, which is a VUS, however, these GUCY2D variants are consistent with the patient’s phenotype, and GUCY2D c.1567-17T > A is rare in control populations and predicted to affect splicing by in silico tools, compound heterozygosity of the variants could explain the patient’s clinical presentation | CRD |
| RP217LU | M | 16 | IMPDH1 | heterozygous for IMPDH1 c.931G > A, p.(Asp311Asn), which is pathogenic | RP |
| RP40LU | K | 2 | IQCB1 | heterozygous for IQCB1 c.1332G > A, p.(Trp444*), which is pathogenic and heterozygous for IQCB1 c.424_425del, p.(Phe142Profs*5), which is pathogenic | Senior–Loken |
| RP104LU | M | 16 | KCNV2 | homozygous for the nonsense variant KCNV2 c.427G > T, p.(Glu143*), which is pathogenic | CRD |
| RP112LU | K | 13 | KCNV2 | homozygous for KCNV2 c.427G > T, p.(Glu143*), which is pathogenic | CRD |
| RP98LU | M | 11 | KCNV2 | homozygous for KCNV2 c.427G > T, p.(Glu143*), which is pathogenic | CRD |
| RP43LU | K | 1 | KIF11 | heterozygous for KIF11 c.1985T > A, p.(Leu662*), which is pathogenic | microcephaly and RD |
| RP78LU | K | 20 | KLHL7 | heterozygous for KLHL7 c.422T > C, p.(Val141Ala) which is likely pathogenic | RP |
| RP91LU | K | 21 | LRAT | homozygous for LRAT c.470T > C, p.(Leu157Pro), which is likely pathogenic | EORD |
| RP266LU | M | 17 | LRAT | homozygous for LRAT c.470T > C, p.(Leu157Pro), which is likely pathogenic | RP |
| RP121LU | M | 9 | MERTK | homozygous for MERTK c.2302G > A, p.(Ala768Thr), which is pathogenic | RP |
| RP290LU | M | 13 | MERTK | homozygous for MERTK c.1960 + 1G > A, which is likely pathogenic | RP |
| RP71LU | M | 12 | MERTK | heterozygous for MERTK c.345C > G, p.(Cys115Trp), which is pathogenic and heterozygous for MERTK c.1377_1379delinsAGCC, p.(Arg460Alafs*15), which is likely pathogenic | RP |
| RP238LU | M | 19 | MFN2 | heterozygous for deletion MFN2 c.(474 + 1_475 − 1)_(816 + 1_817 − 1)del, which encompasses exons 6–8 of MFN2. This alteration is classified as likely pathogenic | macular dystrophy |
| RP4LU | K | 17 | MFRP | homozygous for MFRP c.1090_1091del, p.(Thr364Glnfs*27), which is pathogenic | RP |
| RP203LU | M | 4 | MYO7A | heterozygous for MYO7A c.1556G > A, p.(Gly519Asp), which is pathogenic and heterozygous for MYO7A c.3719G > A, p.(Arg1240Gln), which is pathogenic | Usher |
| RP300LU | M | 2 | MYO7A | heterozygous for MYO7A c.401T > A, p.(Ile134Asn), which is pathogenic. heterozygous for MYO7A c.6558 + 1G > T, which is likely pathogenic | Usher |
| RP115LU | K | 1 | NMNAT1 | heterozygous for NMNAT1 c.196C > T, p.(Arg66Trp) and heterozygous for NMNAT1 c.769G > A, p.(Glu257Lys), which are both pathogenic | LCA |
| RP90LU | K | 9 | NPHP1 | homozygous for a deletion NPHP1 c.(?_-1)_(*1_?)del, which encompasses the whole NPHP1 gene, which is classified as pathogenic | RP and renal failure |
| RP194LU | K | 11 | NR2E3 | heterozygous for NR2E3 c.119-2A > C and heterozygous for NR2E3 c.349 + 5G > C, which are both pathogenic | RP |
| RP216LU | M | 5 | NR2E3 | heterozygous for NR2E3 c.119-2A > C, and NR2E3 c.932G > A, p.(Arg311Gln), which are both pathogenic | RP |
| RP136LU | M | 6 | NYX | hemizygous for NYX c.85_108del, p.(Arg29_Ala36del), which is pathogenic | CSNB |
| RP138LU | M | 9 | NYX | hemizygous for NYX c.559_560delinsAA, p.(Ala187Lys), which is likely pathogenic | CSNB |
| RP84LU | M | 2 | NYX | hemizygous for NYX c.559_560delinsAA, p.(Ala187Lys), which is likely pathogenic | CSNB |
| RP185LU | M | 8 | NYX | hemizygous for NYX c.559_560delinsAA, p.(Ala187Lys), which is likely pathogenic | CSNB |
| RP201LU | M | 6 | NYX | hemizygous for NYX c.559_560delinsAA, p.(Ala187Lys), which is likely pathogenic | CSNB |
| RP233LU | M | 5 | NYX | hemizygous for NYX c.559_560delinsAA, p.(Ala187Lys), which is likely pathogenic | CSNB |
| RP160LU | M | 4 | OPA1 | heterozygous for OPA1 c.983A > G, p.(Lys328Arg), which is pathogenic | optic atrophy |
| RP184LU | M | 8 | OPA1 | heterozygous for a deletion OPA1 c.(?_-1)_(*1_?)del, which encompasses the whole OPA1 gene | optic atrophy |
| RP286LU | K | 24 | OPA1 | heterozygous for OPA1 c.2497-4_2557del, which is likely pathogenic | optic atrophy |
| RP107LU | M | 2 | OPA1 | heterozygous for OPA1 c.703C > T, p.(Arg235*), which is pathogenic | RP |
| RP149LU | M | 13 | OTX2 | heterozygous for OTX2 c.483dup, p.(Asp162Argfs*25), which is likely pathogenic | EORD |
| RP131LU | K | 9 | PANK2 | heterozygous for PANK2 c.981 + 1G > C, which is likely pathogenic and heterozygous for PANK2 c.1512dup, p.(Ala505Serfs*7), which is likely pathogenic | RP and neurological symptoms |
| RP44LU | M | 9 | PCARE | homozygous for PCARE c.1541del, p.(Pro514Hisfs*27), which is pathogenic | RP |
| RP83LU | K | 18 | PCDH15 | heterozygous for PCDH15 c.310del, p.(Asp104Ilefs*6) and PCDH15 c.3761dup, p.(Asn1254Lysfs*54), which are likely pathogenic | Usher |
| RP99LU | M | 2 | PCDH15 | homozygous for PCDH15 c.3441dup, p.(Phe1148Ilefs*8), which is pathogenic | Usher |
| RP120LU | M | 12 | PDE6B | heterozygous for PDE6B c.1580T > C, p.(Leu527Pro) and PDE6B c.2193 + 1G > A which are both pathogenic | RP |
| RP235LU | M | 2 | PDE6C | heterozygous for PDE6C c.826C > T, p.(Arg276*) and PDE6C c.2457T > A, p.(Tyr819*), which are both likely pathogenic | CD |
| RP2LU | M | 8 | PNPLA6 | heterozygous for PNPLA6 c.(2143 + 1_2144-1)_(2351 + 1_2352 − 1)del, which is likely pathogenic and heterozygous for PNPLA6 c.3625T > C, p.(Trp1209Arg), which is a VUS; however, these PNPLA6 variants are consistent with the patient’s phenotype, and PNPLA6 c.3625T > C, p.(Trp1209Arg) is rare in control populations and predicted to be deleterious by in silico tools, compound heterozygosity of the variants would explain the patient’s clinical presentation | RP |
| RP37LU | K | 12 | POC1B | heterozygous for POC1B c.1331_1332dup, p.(Thr445Argfs*10), which is pathogenic and heterozygous for POC1B c.52A > T, p.(Lys18*), which is likely pathogenic | achromatopsia |
| RP177LU | M | 4 | POMGNT1 | homozygous for POMGNT1 c.1539 + 1G > A, which is pathogenic | Muscle–Eye–Brain Disease |
| RP67LU | K | 10 | PROM1 | heterozygous for a deletion PROM1 c.(?-1)_(220 + 1_221 − 1)del, which encompasses exon 1 of PROM1 and is classified as likely pathogenic | CD |
| RP198LU | M | 12 | PROM1 | heterozygous for PROM1 c.2050C > T, p.(Arg684*), which is pathogenic and heterozygous for PROM1 c.1632G > T, p.(Gly544=), which is pathogenic | CRD |
| RP29LU | M | 18 | PROM1 | heterozygous for a deletion PROM1 c.(?-1)_(220 + 1_221 − 1)del, which encompasses exon 1 of PROM1 and is classified as likely pathogenic | RP |
| RP32LU | M | 23 | PROM1 | heterozygous for a deletion PROM1 c.(?-1)_(220 + 1_221 − 1)del, which encompasses exon 1 of PROM1 and is likely pathogenic | RP |
| RP31LU | K | 10 | PROM1 | homozygous for PROM1 c.1909C > T, p.(Gln637*), which is likely pathogenic | RP |
| RP47LU | K | 18 | PROM1 | homozygous for PROM1 c.1909C > T, p.(Gln637*), which is likely pathogenic | RP |
| RP245LU | M | 15 | PRPF31 | heterozygous for a deletion PRPF31 c.(?_-396)_(*1_?)del, which encompasses the whole PRPF31 gene and is classified as pathogenic | RP |
| RP270LU | K | 22 | PRPF31 | heterozygous for a deletion PRPF31 c.(?_-396)_(*1_?)del, which encompasses the entire PRPF31 gene and is classified as pathogenic | RP |
| RP103LU | M | 12 | PRPF8 | heterozygous for PRPF8 c.5804G > A, p.(Arg1935His), which is pathogenic | RP |
| RP188LU | K | 20 | PRPF8 | heterozygous for PRPF8 c.6901C > T, p.(Pro2301Ser), which is pathogenic | RP |
| RP248LU | M | 5 | PRPF8 | heterozygous for PRPF8 c.6926A > T, p.(His2309Leu), which is likely pathogenic | RP |
| RP179LU | M | 14 | PRPH2 | heterozygous for PRPH2 c.633C > G, p.(Phe211Leu), which is pathogenic | RP |
| RP8LU | M | 19 | RDH12 | homozygous for RDH12 c.481C > T, p.(Arg161Trp), which pathogenic | CRD |
| RP7LU | K | 9 | RDH5 | homozygous for RDH5 c.382G > A, p.(Asp128Asn), which is pathogenic | Fundus albipunctatus |
| RP11LU | M | 10 | RHO | heterozygous for RHO c.541G > A, p.(Glu181Lys), which is pathogenic | Aaland eye disease |
| RP74LU | K | 19 | RLBP1 | Homozygous for RLBP1 c.286_297del p.(Phe96_Phe99del), which is pathogenic | RP with maculopathy |
| RP292LU | K | 8 | RP1 | heterozygous for RP1 c.1498_1499del, p.(Met500Valfs*7), which is pathogenic and heterozygous for RP1 c.1601_1604del, p.(Lys534Argfs*11), which is likely pathogenic | RP |
| RP189LU | M | 13 | RP1 | heterozygous for RP1 c.271del, p.(Ser91Alafs*25), and RP1 c.753C > A, p.(Tyr251*), which are both likely pathogenic | RP |
| RP124LU | M | 13 | RP1L1 | heterozygous for RP1L1 c.133C > T, p.(Arg45Trp), which is pathogenic | macular dystrophy |
| RP219LU | M | 16 | RP2 | hemizygous for RP2 c.400C > T, p.(Gln134*), which is pathogenic | RP |
| RP86LU | K | 1 | RPE65 | heterozygous for RPE65 c.886dup, p.(Arg296Lysfs*7), which is pathogenic and heterozygous for RPE65 c.612C > A, p.(Tyr204*), which is likely pathogenic | RP |
| RP16LU | K | 16 | RPGR | heterozygous for RPGR c.2641G > T, p.(Glu881*), which is likely pathogenic | RP |
| RP225LU | M | 15 | RPGR | hemizygous for RPGR c.764C > T, p.(Thr255Ile), which is likely pathogenic | RP |
| RP60LU | M | 20 | RPGR | hemizygous for RPGR c.2730_2731del, p.(Glu911Glyfs*167), which is pathogenic | RP |
| RP63LU | M | 16 | RPGR | hemizygous for RPGR c.2405_2406del, p.(Glu802Glyfs*32), which is pathogenic | RP |
| RP113LU | M | 8 | RPGR | hemizygous for RPGR c.2252_2255del, p.(Lys751Argfs*63), which is pathogenic | RP |
| RP192LU | M | 10 | RPGR | hemizygous for the deletion RPGR c.(1572 + 1_1573 − 1)_(*1_?)del, which encompasses exons 14–19 of RPGR and is classified as pathogenic | RP |
| RP199LU | M | 12 | RPGR | hemizygous for RPGR c.1573-8A > G, which is likely pathogenic | RP |
| RP222LU | M | 15 | RPGR | hemizygous for RPGR c.2426_2427del, p.(Glu809Glyfs*25), which is pathogenic | RP |
| RP251LU | M | 20 | RPGR | hemizygous for a deletion RPGR c.(1414 + 1_1415 − 1)_(*1_?)del, which encompasses exons 12–15 of RPGR and is classified as pathogenic | RP |
| RP100LU | M | 8 | RPGR | hemizygous for a deletion RPGR c.(778 + 1_779 − 1)_(1245 + 1_1246 − 1)del, which encompasses exons 8–10 of RPGR and is classified as pathogenic. | RP |
| RP52LU | M | 16 | RPGR | hemizygous for RPGR c.3300_3301del, p.(His1100Glnfs*10), which is pathogenic | macular dystrophy |
| RP167LU | M | 9 | RS1 | hemizygous for RS1 c.416del, p.(Gln139Argfs*10) which is classified as pathogenic | XLRS |
| RP168LU | M | 5 | RS1 | hemizygous for a deletion RS1 c.(?_-1)_(52 + 1_53 − 1)del, encompassing exon 1 of RS1, which is classified as pathogenic | XLRS |
| RP193LU | M | 18 | RS1 | hemizygous for RS1 c.214G > A, p.(Glu72Lys), which is pathogenic | XLRS |
| RP223LU | M | 10 | RS1 | hemizygous for deletion RS1 c.(?_-1)_(52 + 1_53 − 1)del, which encompasses exon 1 of RS1 and is pathogenic | XLRS |
| RP226LU | M | 19 | RS1 | hemizygous for a deletion RS1 c.(?_-1)_(52 + 1_53 − 1)del, encompassing exon 1 of RS1, classified as pathogenic | XLRS |
| RP33LU | M | 10 | RS1 | hemizygous for RS1 c.149G > A, p.(Trp50*), which is pathogenic | XLRS |
| RP35LU | M | 10 | RS1 | hemizygous for RS1 c.366G > A, p.(Trp122*), which is pathogenic | XLRS |
| RP36LU | M | 11 | RS1 | hemizygous for a deletion RS1 c.(?_-1)_(52 + 1_53 − 1)del, encompassing exon 1 of RS1, which is pathogenic | XLRS |
| RP79LU | M | 19 | RS1 | hemizygous for a deletion RS1 c.(?_-1)_(52 + 1_53 − 1)del, encompassing exon 1 of RS1, which pathogenic | XLRS |
| RP95LU | M | 6 | RS1 | hemizygous for a deletion RS1 c.(?_-1)_(52 + 1_53 − 1)del, encompassing exon 1 of RS1, which is pathogenic | XLRS |
| RP232LU | K | 15 | TIMM8A | heterozygous for TIMM8A c.116del, p.(Met39Argfs*26), which is pathogenic | carrier of Mohr–Tranebjaerg syndrome |
| RP126LU | K | 1 | TRPM1 | heterozygous for the deletion TRPM1 c.(−64 + 1_−63 − 1)_(899 + 1_900 − 1)del, encompassing exons 2 (first coding exon) to 7, which is classified as pathogenic and heterozygous for TRPM1 c.3607_3608del, p.(Glu1203Asnfs*11), which is likely pathogenic | CSNB |
| RP13LU | K | 7 | TRPM1 | homozygous for TRPM1 c.2629C > T, p.(Arg877*), which is pathogenic | CSNB |
| RP243LU | M | 8 | TRPM1 | homozygous for TRPM1 c.2629C > T, p.(Arg877*), which is pathogenic | CSNB |
| RP169LU | M | 3 | TULP1 | homozygous for TULP1 c.148del, p.(Glu50Asnfs*59), which is pathogenic | LCA |
| RP75LU | K | 11 | TULP1 | homozygous for TULP1 c.1153G > A, p.(Gly385Arg), which is pathogenic | RP |
| RP61LU | K | 7 | USH1C | heterozygous for USH1C c.496 + 1G > T, and USH1C c.238dup, p.(Arg80Profs*69), which are pathogenic | Usher |
| RP197LU | M | 8 | USH2A | heterozygous for USH2A c.10450C > T, p.(Arg3484*), and USH2A c.779T > G, p.(Leu260*), whichare pathogenic | Usher |
| RP291LU | M | 1 | USH2A | heterozygous for USH2A c.8682-9A > G, which is pathogenic and heterozygous for USH2A c.1070_1071del, p.(Asn357Serfs*9), which is likely pathogenic | Usher |
| RP159LU | K | 13 | WFS1 | heterozygous for WFS1 c.1673G > A, p.(Arg558His), which is pathogenic and heterozygous for WFS1 c.2149G > A, p.(Glu717Lys), which is pathogenic | optic atrophy |
| RP9LU | K | 17 | WFS1 | heterozygous for WFS1 c.1673G > A, p.(Arg558His), and WFS1 c.2149G > A, p.(Glu717Lys), which are pathogenic | optic atrophy |
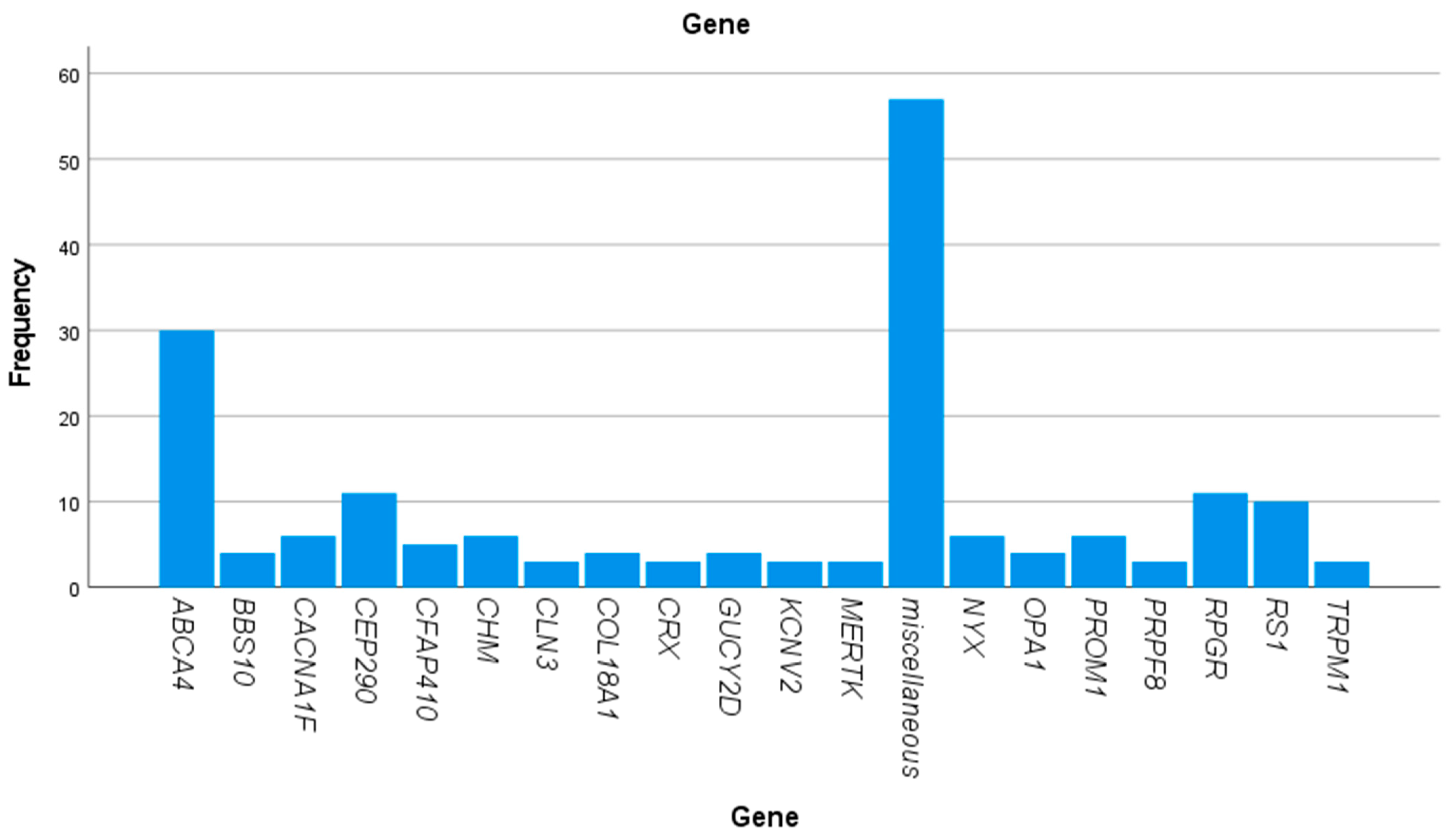
| Gene | Number of Patients |
|---|---|
| ABCA4 | 30 |
| AIPL1 | 1 |
| BBS1 | 1 |
| BBS10 | 4 |
| BBS5 | 2 |
| BBS9 | 1 |
| CACNA1F | 6 |
| CACNA2D4 | 1 |
| CDH23 | 1 |
| CDH3 | 1 |
| CDHR1 | 1 |
| CEP290 | 11 |
| CFAP410 | 5 |
| CHM | 6 |
| CLN3 | 3 |
| CNGB1 | 1 |
| CNGB3 | 2 |
| COL18A1 | 4 |
| CRX | 3 |
| GUCA1A | 1 |
| GUCY2D | 4 |
| IMPDH1 | 1 |
| IQCB1 | 1 |
| KCNV2 | 3 |
| KIF11 | 1 |
| KLHL7 | 1 |
| LRAT | 2 |
| MERTK | 3 |
| MFN2 | 1 |
| MFRP | 1 |
| MYO7A | 2 |
| NMNAT1 | 1 |
| NPHP1 | 1 |
| NR2E3 | 2 |
| NYX | 6 |
| OPA1 | 4 |
| OTX2 | 1 |
| PANK2 | 1 |
| PCARE | 1 |
| PCDH15 | 2 |
| PDE6B | 1 |
| PDE6C | 1 |
| PNPLA6 | 1 |
| POC1B | 1 |
| POMGNT1 | 1 |
| PROM1 | 6 |
| PRPF31 | 2 |
| PRPF8 | 3 |
| PRPH2 | 1 |
| RDH12 | 1 |
| RDH5 | 1 |
| RHO | 1 |
| RLBP1 | 1 |
| RP1 | 2 |
| RP1L1 | 1 |
| RP2 | 1 |
| RPE65 | 1 |
| RPGR | 11 |
| RS1 | 10 |
| TIMM8A | 1 |
| TRPM1 | 3 |
| TULP1 | 2 |
| USH1C | 1 |
| USH2A | 2 |
| WFS1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Areblom, M.; Kjellström, S.; Andréasson, S.; Öhberg, A.; Gränse, L.; Kjellström, U. A Description of the Yield of Genetic Reinvestigation in Patients with Inherited Retinal Dystrophies and Previous Inconclusive Genetic Testing. Genes 2023, 14, 1413. https://doi.org/10.3390/genes14071413
Areblom M, Kjellström S, Andréasson S, Öhberg A, Gränse L, Kjellström U. A Description of the Yield of Genetic Reinvestigation in Patients with Inherited Retinal Dystrophies and Previous Inconclusive Genetic Testing. Genes. 2023; 14(7):1413. https://doi.org/10.3390/genes14071413
Chicago/Turabian StyleAreblom, Maria, Sten Kjellström, Sten Andréasson, Anders Öhberg, Lotta Gränse, and Ulrika Kjellström. 2023. "A Description of the Yield of Genetic Reinvestigation in Patients with Inherited Retinal Dystrophies and Previous Inconclusive Genetic Testing" Genes 14, no. 7: 1413. https://doi.org/10.3390/genes14071413
APA StyleAreblom, M., Kjellström, S., Andréasson, S., Öhberg, A., Gränse, L., & Kjellström, U. (2023). A Description of the Yield of Genetic Reinvestigation in Patients with Inherited Retinal Dystrophies and Previous Inconclusive Genetic Testing. Genes, 14(7), 1413. https://doi.org/10.3390/genes14071413






