Genotype–Environment Interaction and Horizontal and Vertical Distributions of Heartwood for Acacia melanoxylon R.Br
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site Information
2.2. Sampling and Measurements
2.3. Method of Stem Analysis
2.4. Data Analysis
3. Results
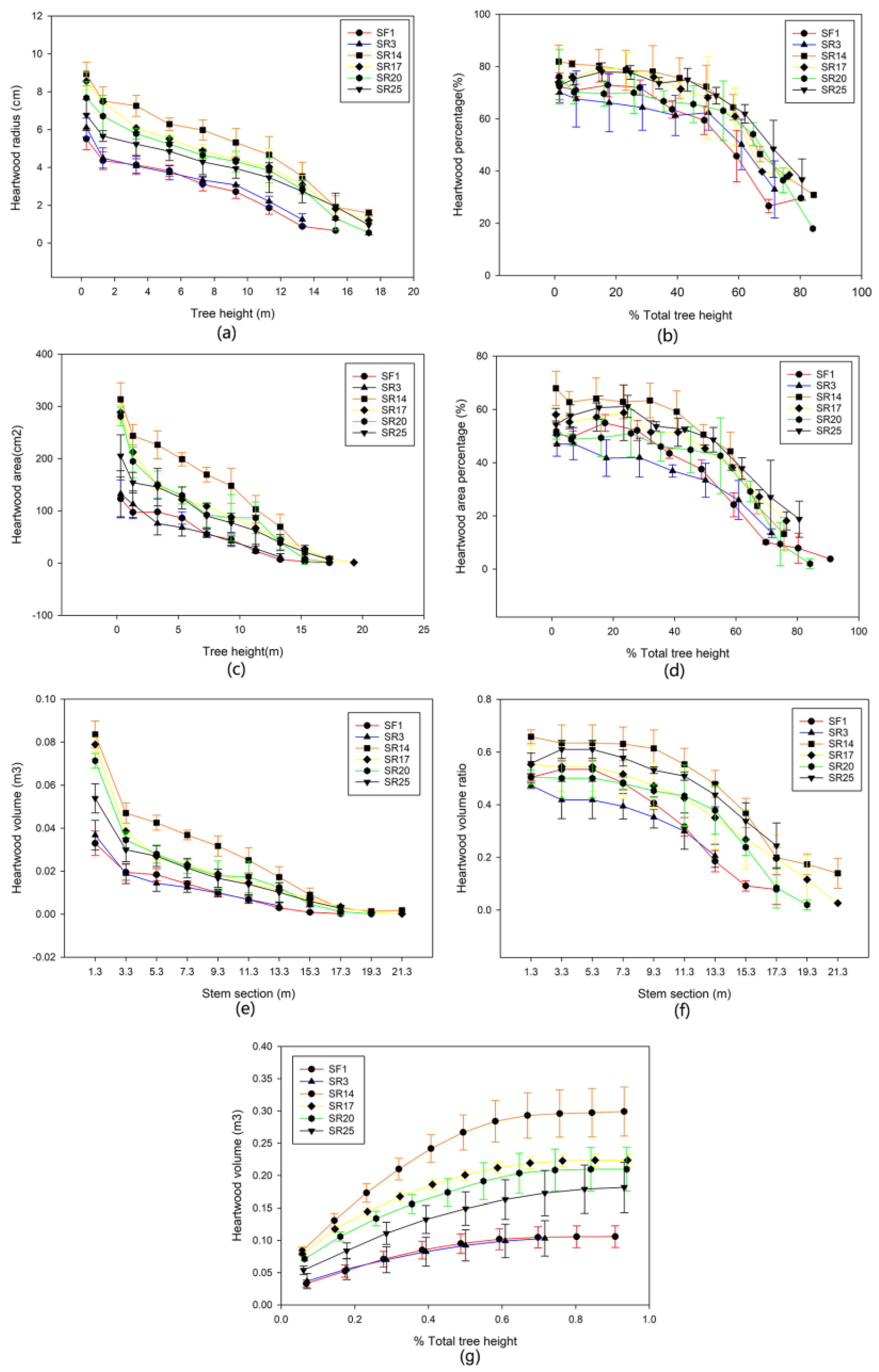
3.1. The Horizontal Wood Variation within and between Sites
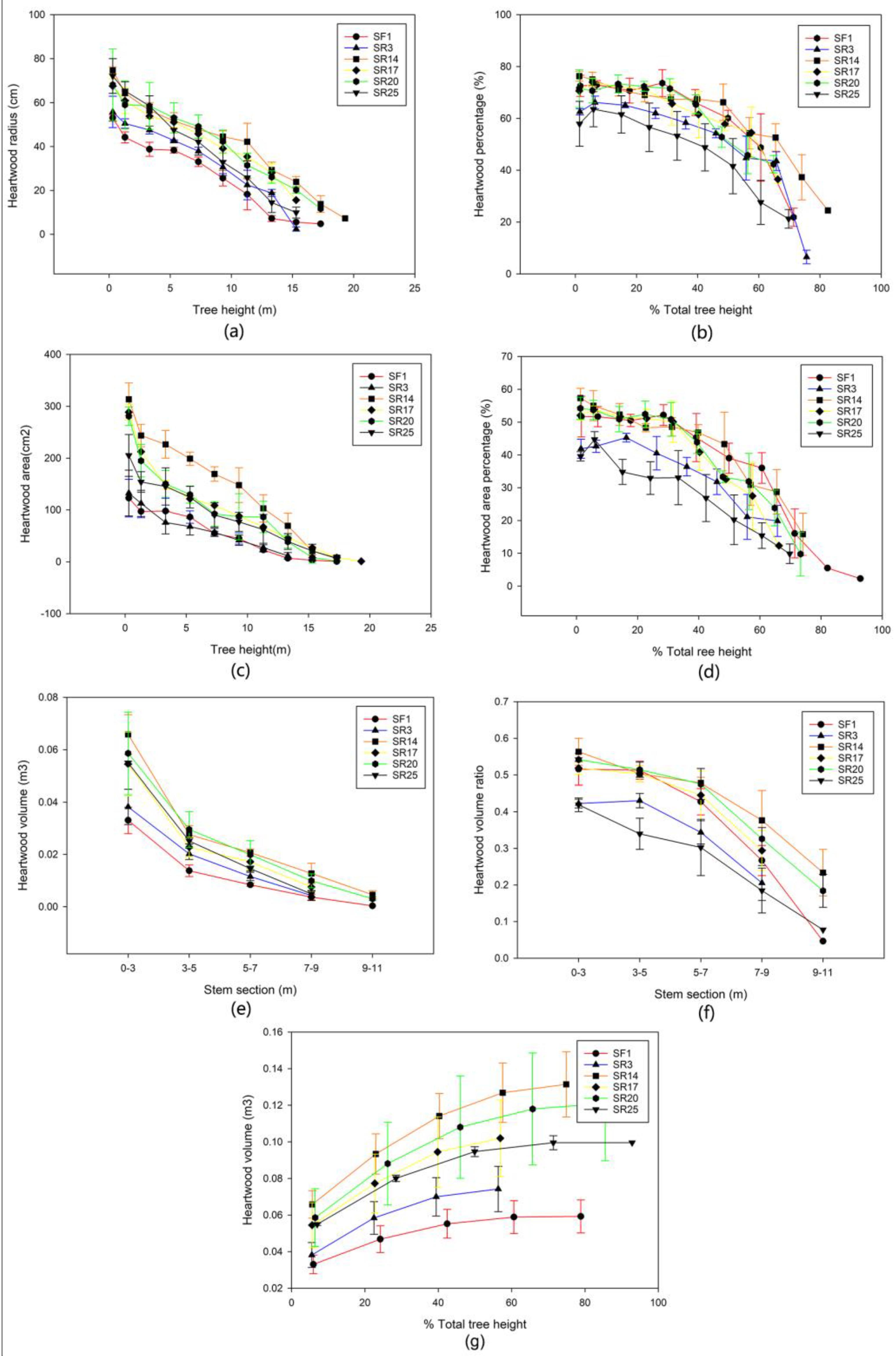
3.2. The Vertical Wood Variation within and between Sites
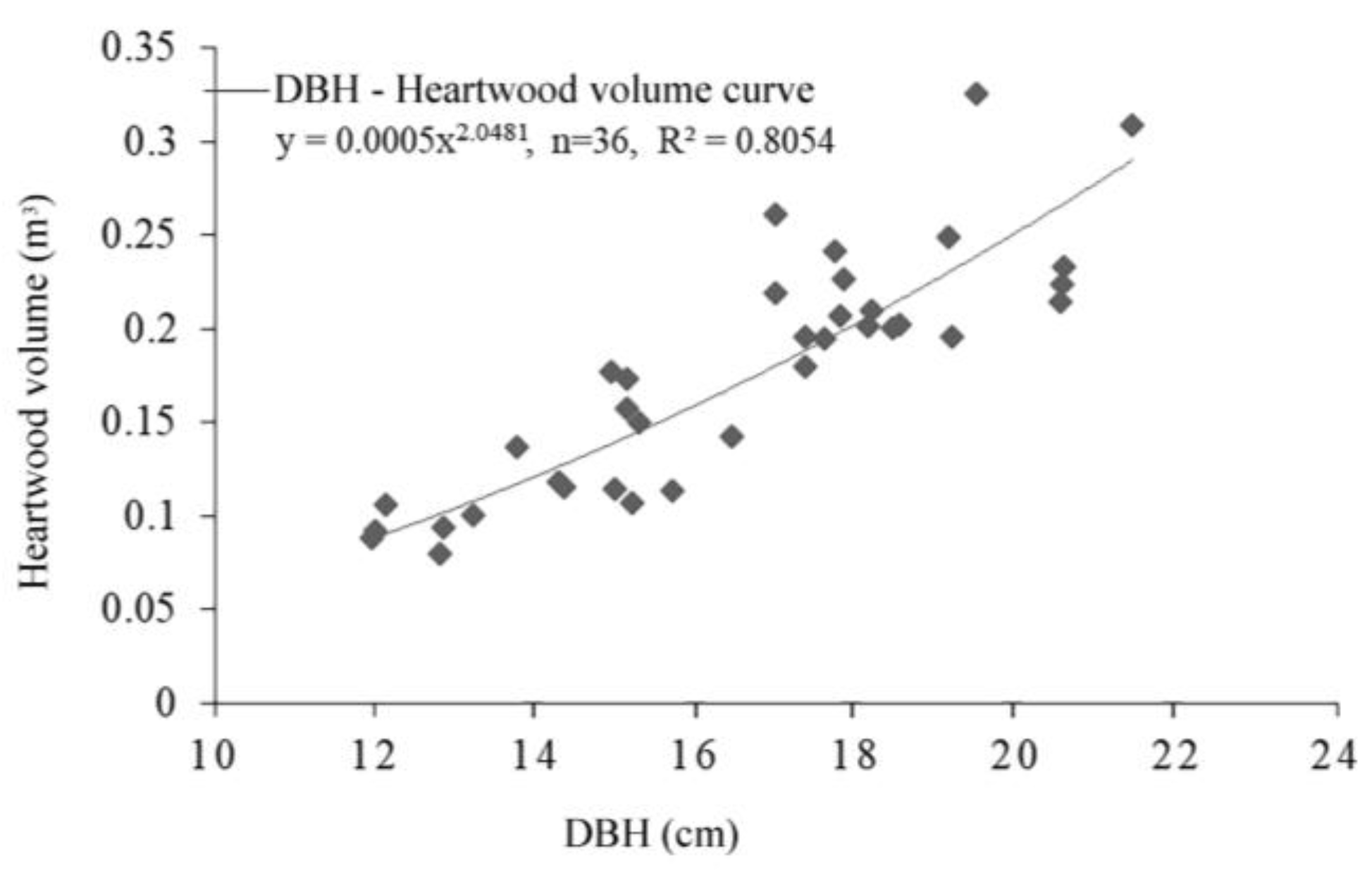
3.3. Relationship between Tree Growth Traits (DBH, DGH, and H) and Wood Attributes
3.4. Variation between Growth Traits and Clones
4. Discussion
4.1. Horizontal Variation between Clone × Site Interaction
4.2. Vertical Variation between Clone × Site Interaction
4.3. Correlation and Genetic Gains between Sites and Clones
4.4. Heritability and Repeatability of Clones
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Searle, S.D. Taylor & Francis Online: Acacia melanoxylon—A review of variation among planted tree. Aust. For. 2000, 63, 79–85. [Google Scholar] [CrossRef]
- Zhang, F.Q.; Chen, Z.X.; Searle, S.D.; Li, X.M.; Zhou, J.J. Temperate Australian Acacia species elimination trials in southern China. Aciar. Proc. 1997, 82, 36–44. [Google Scholar]
- Bradbury, G.; Potts, B.M.; Beadle, C.L.; Dutkowski, G.; Hamilton, M. Genetic and environmental variation in wood properties of Acacia melanoxylon. Ann. For. Sci. 2011, 68, 1363–1373. [Google Scholar] [CrossRef]
- Nicholas, I.; Brown, I. Blackwood: A Handbook for Growers and Users; Forest Research Bulletin No. 225; New Zealand Forest Research Institute: Rotorua, New Zealand, 2002. [Google Scholar]
- Bradbury, G.J.; Potts, B.M.; Beadle, C.L. Quantifying phenotypic variation in wood colour in Acacia melanoxylon R.Br. Forestry 2010, 83, 153–162. [Google Scholar] [CrossRef]
- Machado, J.S.; Louzada, J.L.; Santos, A.J.A.; Nunes, L.; Anjos, O.; Rodrigues, J.; Simoes, R.M.S.; Pereira, H. Variation of Wood Density and Mechanical Properties of Blackwood (Acacia melanoxylon R. Br.). Mater. Des. 2014, 56, 975–980. [Google Scholar] [CrossRef]
- Sellin, A. Sapwood-heartwood proportion related to tree diameter, age, and growth rate in Picea abies. Can. J. For. Res. 1994, 24, 1022–1028. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Zhang, Q.; Center, E.; University, N.F. Spatial Variations in Stem Heartwood and Sapwood for Trees with Various Differentiation Classes. Sci. Silvae Sin. 2014, 50, 114–121. [Google Scholar]
- Yang, B.; Jia, H.; Zhao, Z.; Pang, S.; Cai, D. Horizontal and Vertical Distributions of Heartwood for Teak Plantation. Forests 2020, 11, 225. [Google Scholar] [CrossRef]
- Woeste, K.E. Heartwood production in a 35-year-old black walnut progeny test. Can. J. For. Res. 2002, 32, 177–181. [Google Scholar] [CrossRef]
- Pigliucci, M. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zhou, B.; Hu, R.; Fang, Y. Inter- and intra-specific phenotypic variation of ecological stoichiometric traits in a mixed-oak secondary forest in China. J. For. Res. 2021, 32, 15. [Google Scholar] [CrossRef]
- Wu, H.b.; Lei, J.; Wang, X.Y.; Duan, H.; Zhang, J.G. Aggregation distributions across stand age in provenances of Cunninghamia lanceolata (Lamb.) Hook. For. Ecol. Manag. 2021, 494, 119317. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, Z.; Jin, G.; Zheng, Y.; Zhou, Z.; Sun, L.; Tong, H. Genetic parameters and genotype by environment interactions influencing growth and productivity in Masson pine in east and central China. For. Ecol. Manag. 2021, 487, 118991. [Google Scholar] [CrossRef]
- Bentzer, B.G.; Foster, G.S.; Hellberg, A.R.; Podzorski, A.C. Genotype × environment interaction in Norway spruce involving three levels of genetic control: Seed source, clone mixture, and clone. Can. J. For. Res. 1988, 18, 1172–1181. [Google Scholar] [CrossRef]
- Nawrot, M.; Pazdrowski, W.; Szymański, M. Dynamics of heartwood formation and axial and radial distribution of sapwood and heartwood in stems of European larch (Larix decidua Mill.). J. For. Sci. 2008, 54, 409–417. [Google Scholar] [CrossRef]
- Wang, X.; Wang, C.; Zhang, Q.; Quan, X. Heartwood and sapwood allometry of seven Chinese temperate tree species. Ann. For. Sci. 2010, 67, 410. [Google Scholar] [CrossRef]
- Almeida, M.; Vidaurre, G.B.; Pezzopane, J.; Lousada, J.; Oliveira, R.F. Heartwood variation of Eucalyptus urophylla is influenced by climatic conditions. For. Ecol. Manag. 2019, 458, 1–10. [Google Scholar] [CrossRef]
- Tewari, V.P.; Mariswamy, K.M. Heartwood, sapwood and bark content of teak trees grown in Karnataka, India. For. Res. Engl. Version 2013, 24, 5. [Google Scholar] [CrossRef]
- Ištok, I.; Bogoslav, Š.; Hasan, M.; Popović, G.; Sedlar, T. Fiber Characteristics of White Poplar (Populus alba L.) Juvenile Wood along the Drava River. Drv. Ind. 2017, 68, 241–247. [Google Scholar] [CrossRef]
- Mori, H.; Ueno, S.; Ujino-Ihara, T.; Fujiwara, T.; Yamashita, K.; Kanetani, S.; Endo, R.; Matsumoto, A.; Uchiyama, K.; Yoshida, T.; et al. Genotype-by-environment interaction and genetic dissection of heartwood color in Cryptomeria japonica based on multiple common gardens and quantitative trait loci mapping. PLoS ONE 2022, 17, e0270522. [Google Scholar] [CrossRef]
- Bon, P.V.; Harwood, C.E.; Chi, N.Q.; Thinh, H.H.; Kien, N.D. Comparing wood density, heartwood proportion and bark thickness of diploid and triploid acacia hybrid clones in Vietnam. J. Trop. For. Sci. 2020, 32, 206–216. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Vanderklein, D.; Mencuccini, M. Tree height and age-related decline in growth in Scots pine (Pinus sylvestris L.). Oecologia 2007, 150, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Pliura, A.; Zhang, S.Y.; Mackay, J.; Bousquet, J. Genotypic variation in wood density and growth traits of poplar hybrids at four clonal trials. For. Ecol. Manag. 2007, 238, 92–106. [Google Scholar] [CrossRef]
- Wu, S.; Xu, J.; Li, G.; Risto, V.; Du, Z.; Lu, Z.; Wang, L.W. Genotypic variation in wood properties and growth traits of Eucalyptus hybrid clones in southern China. New For. 2011, 42, 35–50. [Google Scholar] [CrossRef]
- Zhang, P.D.; Pei, J.C.; Kang, X.Y. Genotypic parameters of wood density and fiber traits in triploid hybrid clones of Populus tomentosa at five clonal trials. Ann. For. Sci. 2013, 70, 751–759. [Google Scholar] [CrossRef]
- Wu, Q.; Zeng, B.S.; Chen, K.K.; Qiu, Z.F.; Fan, C.J.; Kang, H.H.; Chen, Z.X. Early selection of Acacia melanoxylon clones. For. Environ. Sci. 2014, 30, 40–44. [Google Scholar]
- Luo, R.; Chen, K.K.; Qu, B.P.; Zeng, B.S.; Kang, H.H.; Chen, Z.X. Growth performance and early selection of Acacia melanoxylon clones at three sites. For. Environ. Sci. 2012, 28, 57–61. [Google Scholar] [CrossRef]
- Sta Cruz, M.T.L.G.; Chujoy, E. Heritability estimates of some root characters in sweetpotatoes. Philipp. J. Crop Sci. 1994, 19, 27–32. [Google Scholar]
- Wu, J.; Zhou, Q.; Sang, Y.; Kang, X.; Zhang, P. Genotype-environment interaction and stability of fiber properties and growth traits in triploid hybrid clones of Populus tomentosa. BMC Plant Biol. 2021, 21, 405. [Google Scholar] [CrossRef]
- Okuyama, P. Heartwood colour variation in home garden teak (Tectona grandis) from wet and dry localities of Kerala, India. J. Trop. For. Sci. 2006, 18, 1. [Google Scholar]
- Tavares, S.K.F.; Pereira, H. Heartwood and sapwood variation in Acacia melanoxylon R. Br. trees in Portugal. Forestry 2006, 79, 371–380. [Google Scholar] [CrossRef]
- Dzifa, A.; Alexia, K.; Nomessi, S.; Kokutse, K.; Kokou, K. Which factors most influence heartwood distribution and radial growth in plantation teak? Ann. For. Sci. 2010, 67, 407. [Google Scholar] [CrossRef]
- Pérez, D.; Pérez, M. Effect of thinning on stem form and wood characteristics of teak (Tectona grandis) in a humid tropical site in Costa Rica. Silva Fenn. 2005, 39, 217–225. [Google Scholar] [CrossRef]
- Mcdowell, N.; Barnard, H.; Bond, B.J.; Hinckley, T.; Hubbard, R.; Ishii, H.; Köstner, B.; Magnani, F.; Marshall, J.; Meinzer, F. The relationship between tree height and leaf area: Sapwood area ratio. Oecologia 2002, 132, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Björklund, L. Identifying heartwood-rich stands or stems of Pinus sylvestris by using inventory data. Silva Fenn. 1999, 33, 119–129. [Google Scholar] [CrossRef]
- Mossman, S. The Reel Life: Adventures in Travel Fishing. Maderas Cienc. Y Tecnol. 2012, 14, 53–63. [Google Scholar] [CrossRef]
- Mwase, W.F.; Savill, P.S.; Hemery, G. Genetic parameter estimates for growth and form traits in common ash (Fraxinus excelsior, L.) in a breeding seedling orchard at Little Wittenham in England. New For. 2008, 36, 225–238. [Google Scholar] [CrossRef]
- Maluf, W.R.; Miranda, J.; Ferreira, P.E. Broad-sense heritabilities of root and vine traits in sweet potatoes (Ipomoea batatas (L.) Lam.). Revista brasileira de genetica. 1983, 6, 443–451. [Google Scholar]
- Stener, L.G.R.; Hedenberg, R. Genetic Parameters of Wood, Fibre, Stem Quality and Growth Traits in a Clone Test with Betula pendula. Scand. J. For. Res. 2003, 18, 103–110. [Google Scholar] [CrossRef]
- Liang, J. Survey and Analysis on Growth Variation and Cold Resistance for Half-Sib Progenies of Acacia crassicarpa. J. Anhui Agric. Sci. 2012, 18, 129. [Google Scholar] [CrossRef]
- Rytter, L.; Stener, L.G. Clonal variation in nutrient content in woody biomass of hybrid aspen (Populus tremula L. × P. tremuloides Michx.). Silva Fenn. 2003, 37, 313–324. [Google Scholar] [CrossRef]
- Pliura, A.; Suchockas, V.; Sarsekova, D.; Gudynaite, V. Genotypic variation and heritability of growth and adaptive traits, and adaptation of young poplar hybrids at northern margins of natural distribution of Populus nigra in Europe. Biomass Bioenerg. 2014, 70, 513–529. [Google Scholar] [CrossRef]
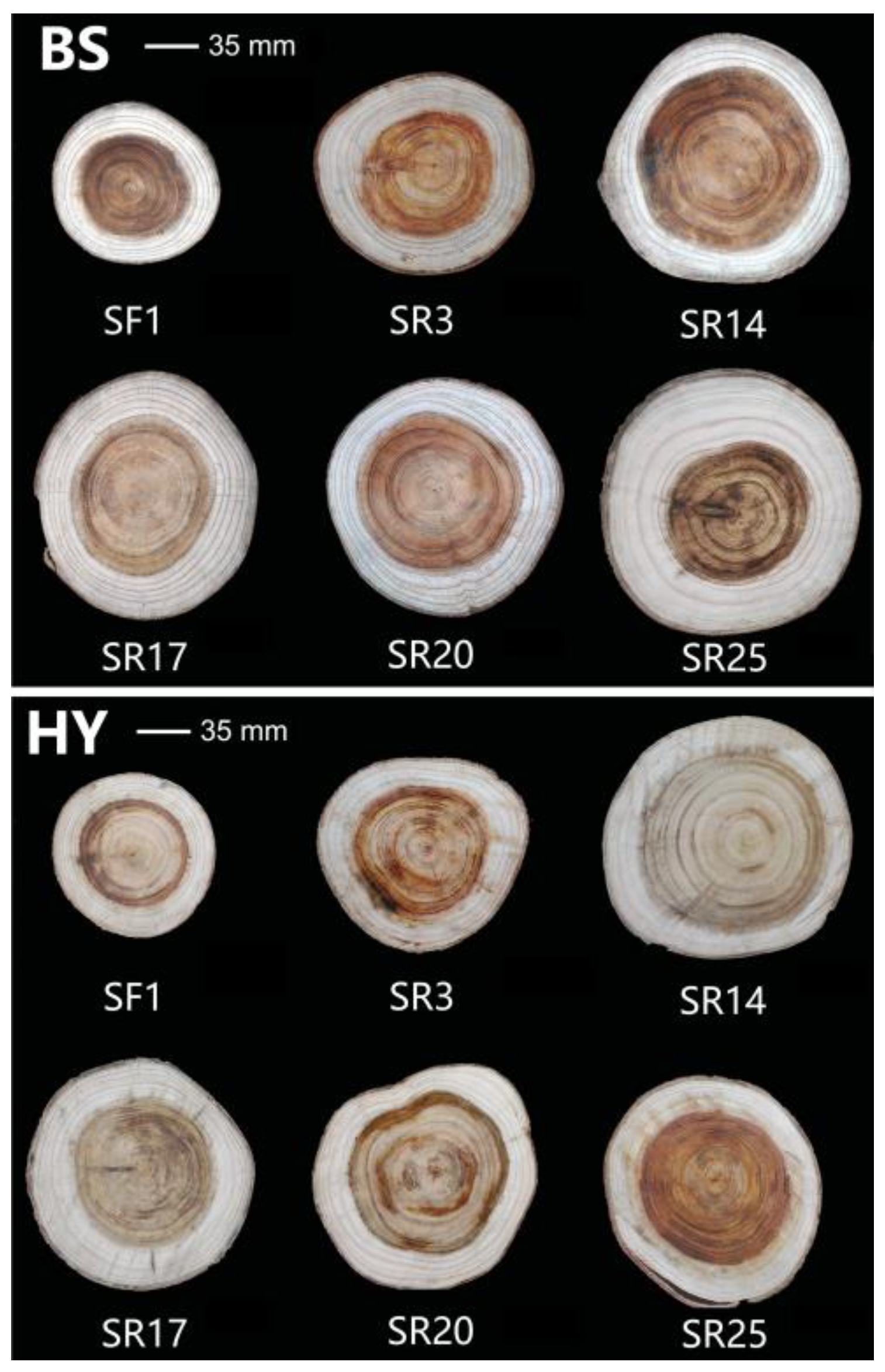
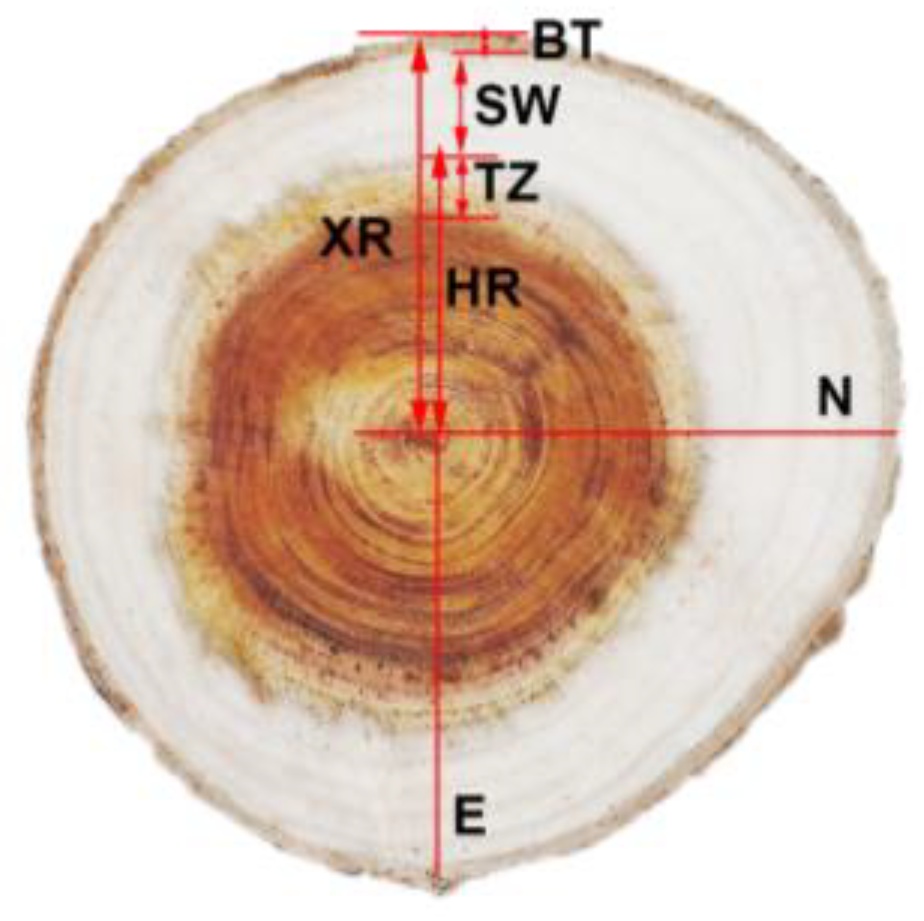

| Sites | Parameters | SF1 | SR3 | SR14 | SR17 | SR20 | SR25 |
|---|---|---|---|---|---|---|---|
| HY 1 | Latitude; Longitude | 23°40′ N; 15°19′ W | |||||
| Altitude (m) | 313–325 | 310–340 | 330–336 | 325–339 | 347–349 | 330–347 | |
| Vegetation Situation | Rhaphiolepis indica, Pyracantha fortuneana, Carex spp., Smilax corbularia, Dicranopteris dichotoma, Dianella ensifolia, Phyllostachys glauca, Ardisia japonica, Spatholobus suberectus, Embelia laeta, Melastoma candidum, Rourea microphylla, Arthraxon hispidus, Schima superba. | ||||||
| BS 2 | Latitude; Longitude | 24°39′ N;105°46′ W | |||||
| Altitude (m) | 531–538 | 528–539 | 522–526 | 522–534 | 520–522 | 516–518 | |
| Vegetation Situation | Maesa japonica, Thysanolaena maxima, Dicranopteris dichotoma, Callicarpa bodinieri, Helicteres angustifolia, Lygodium japonicum, Callicarpa macrophylla, Millettia pulchra, Cipadessa baccifera, Glochidion puberum, Ohwia caudata, Oplismenus compositus, Eupatorium odoratum, Tetracera asiatica, Parthenocissus tricuspidata. | ||||||
| Sites | Parameters | SF1 | SR3 | SR14 | SR17 | SR20 | SR25 |
|---|---|---|---|---|---|---|---|
| BS | HR 2 (cm) | 4.42 (0.25) c 1 | 5.05 (0.21) b | 6.43 (0.1) a | 6.09 (0.59) ab | 5.9 (0.11) ab | 6.53 (0.41) a |
| SW 3 (cm) | 1.64 (0.15) c | 2.58 (0.25) b | 2.14 (0.3) b | 2.14 (0.39) b | 2.40 (0.11) b | 3.78 (0.86) a | |
| BT 4 (mm) | 2.78 (0.56) c | 2.62 (0.25) c | 3.10 (0.38) b | 3.17 (0.53) b | 3.47 (0.52) ab | 3.75 (0.20) a | |
| HA 5 (cm2) | 99.1 (22.83) c | 111.90 (9.31) c | 183.29 (19.39) a | 182.16 (34.63) a | 170.8 (21.49) a | 174.97 (1.38) a | |
| SA 6 (cm2) | 91.91 (15.63) c | 151.26 (24.45) b | 150.47 (23.38) b | 156.97 (35.93) b | 149.14 (36.31) b | 224.01 (9.05) a | |
| HV 7 (m3) | 0.06 (0.01) c | 0.07 (0.01) c | 0.13 (0.02) a | 0.10 (0.02) b | 0.12 (0.03) a | 0.10 (0.00) b | |
| HRP 8 | 0.73 (0.02) ab | 0.66 (0.02) c | 0.75 (0.03) ab | 0.74 (0.02) ab | 0.71 (0.05) b | 0.64 (0.07) c | |
| HAP 9 | 0.52 (0.03) ab | 0.43 (0.02) b | 0.55 (0.04) a | 0.54 (0.01) ab | 0.54 (0.03) ab | 0.44 (0.01) c | |
| HVP 10 | 0.52 (0.04) b | 0.42 (0.01) c | 0.56 (0.04) a | 0.52 (0.02) b | 0.54 (0.00) b | 0.42 (0.00) c | |
| HY | HR (cm) | 4.36 (0.47) c | 4.5 (0.54) c | 7.51 (0.74) a | 7.48 (0.07) a | 6.71 (0.2) a | 5.66 (0.28) a |
| SW (cm) | 1.78 (0.2) c | 2.2 (0.87) b | 1.77 (0.31) c | 2.37 (0.06) b | 28.36 (4.62) a | 1.88 (0.3) c | |
| BT (mm) | 2.64 (0.53) c | 3.28 (0.46) b | 3.83 (1.01) a | 4.36 (0.36) a | 2.71 (0.4) c | 3.38 (0.54) b | |
| HA (cm2) | 97.02 (12.21) c | 112.57 (25.4) bc | 243.75 (21.67) a | 212.55 (1.55) a | 194.93 (31.18) a | 154.03 (19.72) b | |
| SA (cm2) | 97.84 (9.4) c | 125.52 (20.6) b | 147.03 (35.41) b | 167.35 (20.94) b | 209.35 (52.2) a | 207.02 a | |
| HV (m3) | 0.07 (0.01) c | 0.07 (0.01) c | 0.18 (0.02) a | 0.12 (0.03) a | 0.13 (0.02) a | 0.11 (0.02) ab | |
| HRP | 0.71 (0.02) b | 0.68 (0.10) c | 0.81 (0.01) a | 0.76 (0.01) a | 0.70 (0.04) b | 0.75 (0.02) ab | |
| HAP | 0.50 (0.02) ab | 0.47 (0.06) c | 0.63 (0.04) a | 0.55 (0.02) a | 0.49 (0.02) c | 0.49 (0.02) c | |
| HVP | 0.50 (0.01) b | 0.47 (0.01) c | 0.66 (0.03) a | 0.55 (0.07) ab | 0.51 (0.03) b | 0.56 (0.04) a |
| Sites | Traits | HR | SW | BT | HA | SA | HV | HRP | HAP | HVP | DBH | DGH | H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HY | HR | 1 | 0.65 | −0.29 | 0.99 ** 1 | 0.60 | 0.20 | 0.80 | 0.80 | −0.10 | 0.39 | 0.35 | 0.93 ** |
| SW | 0.53 | 1 | −0.09 | 0.63 | 0.84 ** | 0.06 | 0.54 | 0.51 | −0.42 | 0.51 | 0.43 | 0.44 | |
| BT | 0.91 * 2 | 0.32 | 1 | −0.36 | 0.23 | 0.55 | −0.72 | −0.73 | 0.52 | 0.57 | 0.58 | −0.19 | |
| HA | −0.91 * | −0.91 * | −0.26 | 1 | 0.59 | 0.22 | 0.84 * | 0.85 * | −0.06 | 0.37 | 0.34 | 0.89 * | |
| SA | 0.79 | 0.56 | 0.49 | 0.62 | 1 | 0.59 | 0.26 | 0.27 | 0.14 | 0.88 * | 0.84 * | 0.37 | |
| HV | 0.65 | −0.12 | 0.44 | 0.84 * | 0.51 | 1 | −0.24 | −0.19 | 0.89 * | 0.86 * | 0.90 * | 0.11 | |
| HRP | −0.38 | 0.26 | −0.78 | −0.45 | −0.03 | −0.43 | 1 | 0.99 * | −0.44 | −0.11 | −0.16 | 0.68 | |
| HAP | −0.03 | 0.54 | −0.65 | −0.12 | 0.33 | −0.21 | 0.23 | 1 | 0.38 | −0.09 | −0.13 | 0.67 | |
| HVP | 0.15 | −0.15 | 0.09 | 0.31 | 0.55 | 0.78 | −0.24 | −0.24 | 1 | 0.54 | 0.61 | −0.08 | |
| DBH | 0.73 | −0.09 | 0.49 | 0.86 * | 0.26 | 0.55 | 0.26 | 0.26 | 0.84 * | 1 | 0.99 ** | 0.22 | |
| DGH | 0.73 | −0.09 | 0.49 | 0.86 * | 0.26 | 0.55 | 0.26 | 0.26 | 0.84 * | 0.43 | 1 | 0.19 | |
| H | 0.61 | −0.21 | 0.55 | 0.80 | 0.38 | 0.72 | 0.09 | 0.09 | 0.90 * | 0.49 | 0.61 | 1 | |
| BS | HR | 1 | −0.27 | −0.32 | 0.95 ** | −0.22 | −0.44 | 0.02 | −0.30 | 0.07 | −0.34 | −0.28 | −0.20 |
| SW | −0.86 * | 1 | 0.62 | −0.39 | 0.97 ** | 0.42 | −0.80 | −0.67 | −0.72 | 0.89 * | 0.84 * | 0.50 | |
| BT | −0.20 | 0.12 | 1 | −0.57 | 0.72 | 0.76 | −0.64 | −0.38 | 0.04 | 0.78 | 0.81 | 0.73 | |
| HA | −0.55 | 0.54 | 0.84 * | 1 | −0.36 | −0.55 | 0.12 | −0.24 | 0.05 | −0.47 | −0.43 | −0.33 | |
| SA | −0.85 * | 0.06 | 0.32 | −0.80 | 1 | 0.53 | −0.91 * | −0.76 | −0.59 | 0.93 ** | 0.90 * | 0.60 | |
| HV | −0.15 | −0.30 | 0.44 | −0.27 | 0.15 | 1 | −0.58 | −0.40 | 0.28 | 0.88 * | 0.82 * | 0.95 * | |
| HRP | −0.32 | 0.65 | 0.38 | −0.39 | 0.26 | 0.12 | 1 | 0.92 ** | 0.39 | −0.87 * | −0.86 * | −0.66 | |
| HAP | −0.32 | 0.65 | 0.38 | −0.39 | 0.26 | 0.12 | 0.14 | 1 | 0.40 | −0.72 | −0.72 | −0.58 | |
| HVP | 0.15 | 0.03 | 0.61 | −0.09 | 0.32 | 0.85 | −0.78 | −0.65 | 1 | −0.35 | −0.26 | 0.19 | |
| DBH | −0.73 | 0.03 | 0.55 | −0.80 | 0.56 | 0.56 | −0.32 | 0.03 | 0.45 | 1 | 0.99 ** | 0.83 * | |
| DGH | −0.50 | −0.06 | 0.44 | −0.56 | 0.44 | 0.65 | −0.49 | −0.21 | 0.51 | 0.49 | 1 | 0.88 * | |
| H | −0.44 | 0.48 | 0.84 * | −0.68 | 0.79 | 0.06 | −0.14 | 0.03 | −0.36 | 0.38 | 0.38 | 1 |
| Sites | Traits | HR | SW | BT | HA | SA | HV | HRP | HAP | HVP | DBH | DGH | H |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HY | HR | 17.24 | 10.21 | −5.03 | 17.21 | 9.26 | 3.47 | 13.74 | 13.87 | −1.69 | 6.69 | 6.00 | 16.01 |
| SW | 12.63 | 12.67 | −1.26 | 8.83 | 10.45 | 0.84 | 7.48 | 7.13 | −5.73 | 7.05 | 5.95 | 6.11 | |
| BT | 7.82 | 2.52 | 14.02 | −5.06 | 2.87 | 7.71 | −10.00 | −10.23 | 7.11 | 7.90 | 8.04 | −2.64 | |
| HA | −8.97 | −8.17 | −2.62 | 30.19 | 15.81 | 6.63 | 25.06 | 25.59 | −1.76 | 11.02 | 10.13 | 26.61 | |
| SA | 26.51 | 17.01 | 16.81 | 20.80 | 16.27 | 10.79 | 4.71 | 4.94 | 2.50 | 15.91 | 15.19 | 6.72 | |
| HV | 11.77 | −1.95 | 7.96 | 15.11 | 8.14 | 28.64 | −6.81 | −5.44 | 24.87 | 24.36 | 25.50 | 3.13 | |
| HRP | −1.67 | 1.06 | −3.49 | −1.95 | −0.12 | −1.94 | 5.29 | 5.28 | −0.42 | −0.58 | −0.85 | 3.60 | |
| HAP | −0.19 | 3.09 | −4.17 | −0.76 | 1.85 | −1.33 | 1.50 | 9.58 | 7.29 | −0.85 | −1.23 | 6.37 | |
| HVP | 1.18 | −1.08 | 0.71 | 2.38 | 3.82 | 6.19 | −1.88 | −1.90 | 8.41 | 4.61 | 5.20 | −0.68 | |
| DBH | 8.53 | −0.94 | 5.84 | 9.98 | 2.75 | 6.52 | 3.06 | 3.09 | 9.71 | 13.53 | 13.39 | 2.99 | |
| DGH | 7.77 | −0.85 | 5.32 | 9.10 | 2.50 | 5.95 | 2.79 | 2.81 | 8.86 | 4.65 | 12.64 | 2.41 | |
| H | 2.33 | −0.71 | 2.12 | 3.01 | 1.29 | 2.78 | 0.33 | 0.34 | 3.37 | 1.87 | 2.31 | 7.47 | |
| BS | HR | 6.30 | −2.00 | −2.19 | 5.07 | −1.63 | −3.03 | 0.14 | −2.09 | 0.50 | −2.44 | −2.00 | −1.26 |
| SW | −17.06 | 21.25 | 12.20 | −5.97 | 20.66 | 8.31 | −16.43 | −13.40 | −14.76 | 18.35 | 17.25 | 9.07 | |
| BT | −1.62 | 1.74 | 8.80 | −3.90 | 6.86 | 6.73 | −5.88 | −3.40 | 0.37 | 7.19 | 7.44 | 5.92 | |
| HA | −3.65 | 12.21 | 17.95 | 5.70 | −2.85 | −4.05 | 0.92 | −1.79 | 0.38 | −3.60 | −3.28 | −2.23 | |
| SA | −20.50 | 0.89 | 4.47 | −8.54 | 29.13 | 14.34 | −25.56 | −20.79 | −16.54 | 26.22 | 25.28 | 14.88 | |
| HV | −2.55 | −9.36 | 12.82 | −5.98 | 4.63 | 19.48 | −11.72 | −7.88 | 5.65 | 17.85 | 16.57 | 16.95 | |
| HRP | −1.22 | 3.44 | 1.85 | −1.44 | 1.39 | 0.58 | 4.43 | 3.97 | 1.72 | −3.87 | −3.81 | −2.58 | |
| HAP | −1.72 | 6.02 | 3.24 | −2.52 | 2.43 | 1.02 | 1.31 | 6.06 | 2.48 | −4.49 | −4.48 | −3.18 | |
| HVP | 1.07 | 0.27 | 5.23 | −0.58 | 2.96 | 7.37 | −7.02 | −5.65 | 8.52 | −3.00 | −2.22 | 1.43 | |
| DBH | −7.39 | 0.40 | 6.96 | −7.69 | 7.54 | 7.11 | −4.20 | 0.38 | 5.89 | 11.79 | 11.63 | 8.61 | |
| DGH | −4.73 | −0.77 | 5.31 | −5.23 | 5.74 | 7.93 | −6.27 | −2.55 | 6.44 | 6.29 | 11.06 | 8.59 | |
| H | −1.31 | 3.22 | 5.33 | −3.29 | 5.36 | 0.37 | −0.96 | 0.19 | −2.36 | 2.50 | 2.49 | 3.07 |
| Paired Samples | Mean | Standard Deviation | Standard Error | t | Significance Level (p) |
|---|---|---|---|---|---|
| Observed–Predicted | 0.0054 | 0.01612 | 0.00806 | 0.670 | 0.551 |
| Character | Min | Max | Range | Mean | Standard Deviation | CV | F Values | Repeatability | Broad-Sense Heritability |
|---|---|---|---|---|---|---|---|---|---|
| HR (cm) | 3.94 | 8.13 | 4.19 | 5.75 | 0.19 | 0.03 | 9.10 | 0.90 | 0.98 |
| SW (cm) | 1.46 | 4.59 | 3.13 | 2.34 | 0.12 | 0.05 | 2.51 | 0.75 | 0.94 |
| HA (cm2) | 66.30 | 286.30 | 220.00 | 155.50 | 9.32 | 0.06 | 7.49 | 0.88 | 0.98 |
| HV (m3) | 0.08 | 0.33 | 0.25 | 0.18 | 0.01 | 0.06 | 21.37 | 0.75 | 0.99 |
| HRP | 0.55 | 0.82 | 0.27 | 0.71 | 0.01 | 0.01 | 2.82 | 0.75 | 0.95 |
| HAP | 0.33 | 0.67 | 0.34 | 0.51 | 0.01 | 0.03 | 3.31 | 0.75 | 0.95 |
| HVP | 0.41 | 0.69 | 0.28 | 0.52 | 0.01 | 0.02 | 7.33 | 0.90 | 0.98 |
| DBH (cm) | 11.95 | 22.63 | 10.68 | 16.92 | 0.52 | 0.03 | 7.21 | 0.88 | 0.96 |
| DGH (cm) | 13.3 | 24.73 | 11.43 | 18.86 | 0.56 | 0.03 | 7.04 | 0.88 | 0.96 |
| H (m) | 17.30 | 25.50 | 8.20 | 16.80 | 2.12 | 0.13 | 2.12 | 0.90 | 0.98 |
| Clones | Character | Range | Mean | Standard Deviation | CV | Clones | Character | Range | Mean | Standard Deviation | CV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SF1 | HR (cm) | 3.98–4.88 | 4.39 | 0.34 | 0.08 | SR3 | HR (cm) | 3.94–5.28 | 4.77 | 0.47 | 0.10 |
| SW (cm) | 1.53–1.94 | 1.71 | 0.17 | 0.10 | SW (cm) | 1.58–3.19 | 2.39 | 0.61 | 0.26 | ||
| HA (cm2) | 76.84–122.46 | 98.06 | 16.41 | 0.17 | HA (cm2) | 66.30–122.07 | 105.57 | 26.10 | 0.25 | ||
| SA (cm2) | 73.89–105.81 | 94.88 | 11.99 | 0.13 | SA (cm2) | 83.93–178.30 | 135.06 | 30.82 | 0.23 | ||
| HV (m3) | 0.09–0.12 | 0.10 | 0.01 | 0.11 | HV (m3) | 0.08–0.14 | 0.11 | 0.02 | 0.18 | ||
| HRP | 0.68–0.75 | 0.72 | 0.02 | 0.03 | HRP | 0.55–0.74 | 0.67 | 0.07 | 0.10 | ||
| HAP | 0.47–0.55 | 0.51 | 0.03 | 0.05 | HAP | 0.38–0.53 | 0.44 | 0.05 | 0.11 | ||
| HVP | 0.47–0.56 | 0.51 | 0.03 | 0.06 | HVP | 0.41–0.48 | 0.45 | 0.03 | 0.07 | ||
| DBH (cm) | 11.95–14.30 | 12.74 | 0.92 | 0.07 | DBH (cm) | 12.80–16.46 | 14.92 | 1.26 | 0.08 | ||
| DGH (cm) | 13.30–16.22 | 14.78 | 1.11 | 0.08 | DGH (cm) | 14.25–18.60 | 16.26 | 1.51 | 0.09 | ||
| H (m) | 17.80–19.80 | 12.74 | 0.92 | 0.07 | H (m) | 17.30–20.30 | 14.92 | 1.26 | 0.08 | ||
| SR14 | HR (cm) | 6.32–8.13 | 6.97 | 0.76 | 0.11 | SR17 | HR (cm) | 4.49–7.55 | 6.01 | 1.23 | 0.21 |
| SW (cm) | 1.49–2.46 | 1.95 | 0.34 | 0.17 | SW (cm) | 2.11–2.86 | 2.49 | 0.26 | 0.10 | ||
| HA (cm2) | 164.09–257.39 | 210.19 | 38.21 | 0.18 | HA (cm2) | 93.77–211.00 | 161.79 | 55.34 | 0.34 | ||
| SA (cm2) | 98.28–182.86 | 146.45 | 30.94 | 0.21 | SA (cm2) | 113.65–218.35 | 160.50 | 38.88 | 0.24 | ||
| HV (m3) | 0.20–0.33 | 0.26 | 0.05 | 0.20 | HV (m3) | 0.14–0.23 | 0.20 | 0.04 | 0.18 | ||
| HRP | 0.72–0.82 | 0.78 | 0.04 | 0.05 | HRP | 0.66–0.76 | 0.70 | 0.04 | 0.05 | ||
| HAP | 0.51–0.67 | 0.59 | 0.06 | 0.10 | HAP | 0.44–0.58 | 0.49 | 0.05 | 0.10 | ||
| HVP | 0.53–0.65 | 0.61 | 0.06 | 0.10 | HVP | 0.47–0.60 | 0.54 | 0.05 | 0.10 | ||
| DBH (cm) | 17.00–19.50 | 18.54 | 1.67 | 0.09 | DBH (cm) | 13.77–20.57 | 18.30 | 3.06 | 0.17 | ||
| DGH (cm) | 19.12–23.32 | 21.01 | 1.69 | 0.08 | DGH (cm) | 15.63–24.73 | 20.13 | 3.43 | 0.17 | ||
| H (m) | 19.80–25.50 | 23.12 | 1.87 | 0.08 | H (m) | 21.30–23.50 | 22.87 | 0.84 | 0.04 | ||
| SR20 | HR (cm) | 4.75–7.94 | 1.78 | 2.60 | 1.46 | SR25 | HR (cm) | 5.56–6.87 | 6.07 | 0.45 | 0.07 |
| SW (cm) | 2.28–3.12 | 1.09 | 1.14 | 1.05 | SW (cm) | 1.46–4.59 | 2.86 | 1.37 | 0.48 | ||
| HA (cm2) | 144.55–286.30 | 43.97 | 54.89 | 1.25 | HA (cm2) | 131.39–214.35 | 167.84 | 27.49 | 0.16 | ||
| SA (cm2) | 134.01–230.74 | 55.37 | 70.69 | 1.28 | SA (cm2) | 79.71–351.49 | 204.98 | 109.33 | 0.53 | ||
| HV (m3) | 0.16–0.25 | 0.10 | 0.08 | 0.77 | HV (m3) | 0.15–0.22 | 0.19 | 0.03 | 0.13 | ||
| HRP | 0.65–0.75 | 0.28 | 0.34 | 1.20 | HRP | 0.57–0.80 | 0.69 | 0.10 | 0.14 | ||
| HAP | 0.41–0.57 | 0.20 | 0.21 | 1.05 | HAP | 0.33–0.56 | 0.48 | 0.12 | 0.26 | ||
| HVP | 0.49–0.55 | 0.18 | 0.23 | 1.28 | HVP | 0.41–0.60 | 0.49 | 0.08 | 0.16 | ||
| DBH (cm) | 15.14–22.63 | 18.47 | 2.45 | 0.13 | DBH (cm) | 14.95–122.29 | 18.57 | 3.24 | 0.17 | ||
| DGH (cm) | 15.64–24.21 | 20.42 | 2.79 | 0.14 | DGH (cm) | 16.35–24.29 | 20.56 | 3.15 | 0.15 | ||
| H (m) | 19.80–23.80 | 5.42 | 8.25 | 0.08 | H (m) | 19.80–24.00 | 21.70 | 1.70 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Zeng, B.; Chen, T.; Hu, B. Genotype–Environment Interaction and Horizontal and Vertical Distributions of Heartwood for Acacia melanoxylon R.Br. Genes 2023, 14, 1299. https://doi.org/10.3390/genes14061299
Zhang R, Zeng B, Chen T, Hu B. Genotype–Environment Interaction and Horizontal and Vertical Distributions of Heartwood for Acacia melanoxylon R.Br. Genes. 2023; 14(6):1299. https://doi.org/10.3390/genes14061299
Chicago/Turabian StyleZhang, Ruping, Bingshan Zeng, Tianxiao Chen, and Bing Hu. 2023. "Genotype–Environment Interaction and Horizontal and Vertical Distributions of Heartwood for Acacia melanoxylon R.Br" Genes 14, no. 6: 1299. https://doi.org/10.3390/genes14061299





