Update on Molecular Diagnostics in Thyroid Pathology: A Review
Abstract
:1. Introduction
2. History of Molecular Tests in Thyroid Diagnostics
3. Fine Needle Aspiration and the Emergence of Molecular Testing Platforms
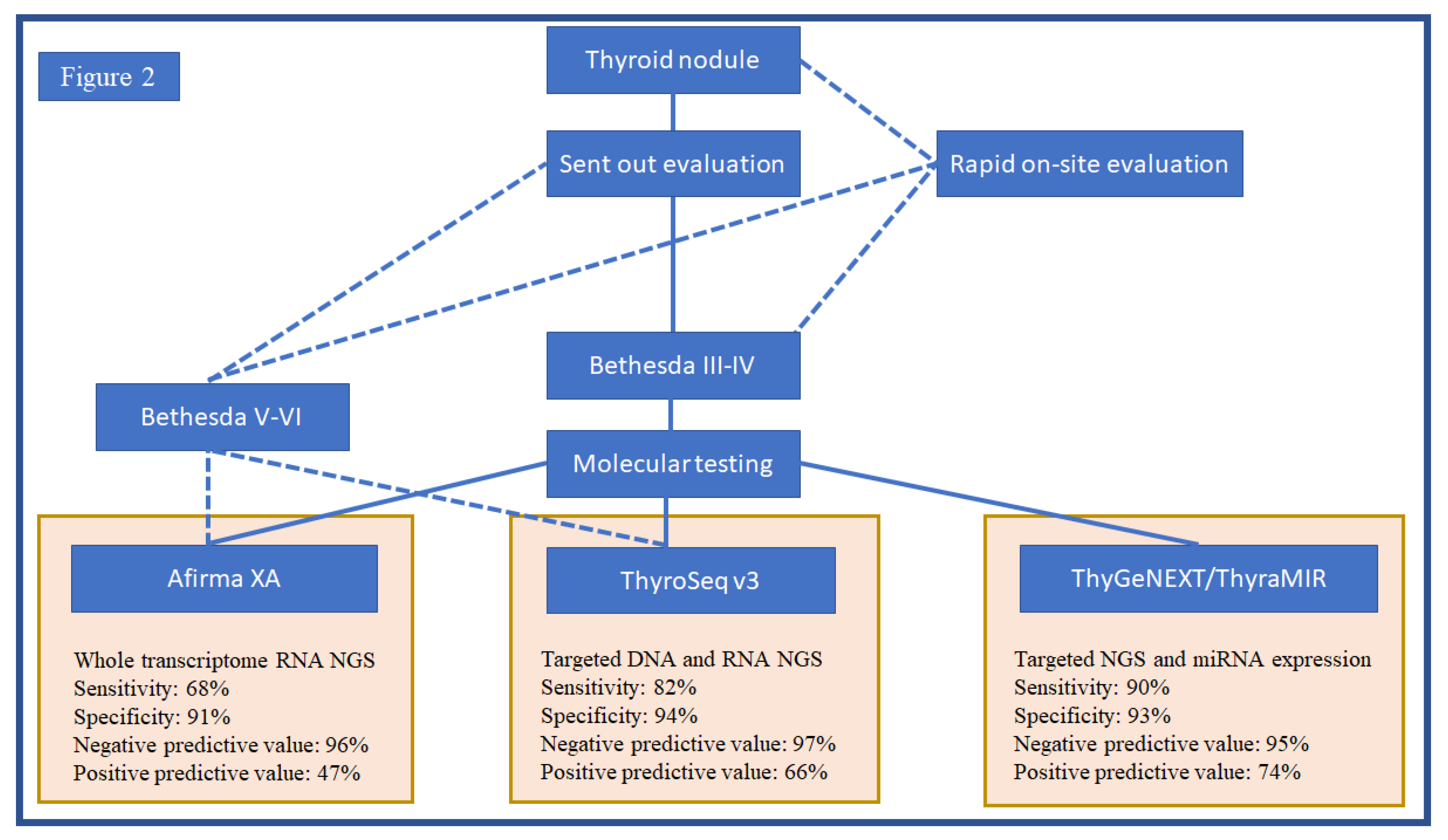
4. Molecular Tests and Clinical Practice
5. Molecular Tests in ITN
6. Cancer Prognosis in the Era of Molecular Testing
7. Molecular Identification of Targetable Alterations in Thyroid Cancer
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baloch, Z.W.; Asa, S.L.; Barletta, J.A.; Ghossein, R.A.; Juhlin, C.C.; Jung, C.K.; LiVolsi, V.A.; Papotti, M.G.; Sobrinho-Simoes, M.; Tallini, G.; et al. Overview of the 2022 WHO Classification of Thyroid Neoplasms. Endocr. Pathol. 2022, 33, 27–63. [Google Scholar] [CrossRef] [PubMed]
- Tirado, Y.; Williams, M.D.; Hanna, E.Y.; Kaye, F.J.; Batsakis, J.G.; El-Naggar, A.K. CRTC1/MAML2 fusion transcript in high grade mucoepidermoid carcinomas of salivary and thyroid glands and Warthin’s tumors: Implications for histogenesis and biologic behavior. Genes Chromosomes Cancer 2007, 46, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K.; Fridman, M. Characteristics of cribriform morular variant of papillary thyroid carcinoma in post-Chernobyl affected region. Hum. Pathol. 2018, 74, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Agaimy, A.; Witkowski, L.; Stoehr, R.; Cuenca, J.C.C.; Gonzalez-Muller, C.A.; Brutting, A.; Bahrle, M.; Mantsopoulos, K.; Amin, R.M.S.; Hartmann, A.; et al. Malignant teratoid tumor of the thyroid gland: An aggressive primitive multiphenotypic malignancy showing organotypical elements and frequent DICER1 alterations-is the term “thyroblastoma” more appropriate? Virchows Arch. 2020, 477, 787–798. [Google Scholar] [CrossRef]
- Saliba, M.; Mohanty, A.S.; Ho, A.L.; Drilon, A.; Dogan, S. Secretory Carcinoma of the Thyroid in a 49-Year-Old Man Treated with Larotrectinib: Protracted Clinical Course of Disease Despite the High-Grade Histologic Features. Head Neck Pathol. 2022, 16, 612–620. [Google Scholar] [CrossRef]
- Ding, Z.; Tao, X.; Deng, X.; Guo, B.; Kang, J.; Wu, B.; Yang, Z.; Chen, C.; Liu, P.; Zhang, Y.; et al. Genetic analysis and clinicopathologic features of locally advanced papillary thyroid cancers: A prospective observational study. J. Cancer Res. Clin. Oncol. 2023. [Google Scholar] [CrossRef]
- Elisei, R.; Grande, E.; Kreissl, M.C.; Leboulleux, S.; Puri, T.; Fasnacht, N.; Capdevila, J. Current perspectives on the management of patients with advanced RET-driven thyroid cancer in Europe. Front. Oncol. 2023, 13, 1141314. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014, 159, 676–690. [Google Scholar] [CrossRef] [Green Version]
- Asa, S.L.; Giordano, T.J.; LiVolsi, V.A. Implications of the TCGA genomic characterization of papillary thyroid carcinoma for thyroid pathology: Does follicular variant papillary thyroid carcinoma exist? Thyroid 2015, 25, 1–2. [Google Scholar] [CrossRef]
- Yoo, S.K.; Lee, S.; Kim, S.J.; Jee, H.G.; Kim, B.A.; Cho, H.; Song, Y.S.; Cho, S.W.; Won, J.K.; Shin, J.Y.; et al. Comprehensive Analysis of the Transcriptional and Mutational Landscape of Follicular and Papillary Thyroid Cancers. PLoS Genet. 2016, 12, e1006239. [Google Scholar] [CrossRef] [Green Version]
- Alexander, E.K.; Kennedy, G.C.; Baloch, Z.W.; Cibas, E.S.; Chudova, D.; Diggans, J.; Friedman, L.; Kloos, R.T.; LiVolsi, V.A.; Mandel, S.J.; et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N. Engl. J. Med. 2012, 367, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Randolph, G.W.; Sosa, J.A.; Hao, Y.; Angell, T.E.; Shonka, D.C., Jr.; LiVolsi, V.A.; Ladenson, P.W.; Blevins, T.C.; Duh, Q.Y.; Ghossein, R.; et al. Preoperative Identification of Medullary Thyroid Carcinoma (MTC): Clinical Validation of the Afirma MTC RNA-Sequencing Classifier. Thyroid 2022, 32, 1069–1076. [Google Scholar] [CrossRef]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J., Jr.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef]
- Tallini, G.; Tuttle, R.M.; Ghossein, R.A. The History of the Follicular Variant of Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2017, 102, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Fusco, A.; Grieco, M.; Santoro, M.; Berlingieri, M.T.; Pilotti, S.; Pierotti, M.A.; Della Porta, G.; Vecchio, G. A new oncogene in human thyroid papillary carcinomas and their lymph-nodal metastases. Nature 1987, 328, 170–172. [Google Scholar] [CrossRef]
- Santoro, M.; Carlomagno, F.; Hay, I.D.; Herrmann, M.A.; Grieco, M.; Melillo, R.; Pierotti, M.A.; Bongarzone, I.; Della Porta, G.; Berger, N.; et al. Ret oncogene activation in human thyroid neoplasms is restricted to the papillary cancer subtype. J. Clin. Investig. 1992, 89, 1517–1522. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, N.R.; Mayall, E.S.; Wyllie, F.S.; Williams, E.D.; Goyns, M.; Stringer, B.; Wynford-Thomas, D. High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene 1989, 4, 159–164. [Google Scholar]
- Namba, H.; Rubin, S.A.; Fagin, J.A. Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol. Endocrinol. 1990, 4, 1474–1479. [Google Scholar] [CrossRef] [Green Version]
- Kroll, T.G.; Sarraf, P.; Pecciarini, L.; Chen, C.J.; Mueller, E.; Spiegelman, B.M.; Fletcher, J.A. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science 2000, 289, 1357–1360. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Biddinger, P.W.; Caudill, C.M.; Kroll, T.G.; Nikiforov, Y.E. PAX8-PPARgamma rearrangement in thyroid tumors: RT-PCR and immunohistochemical analyses. Am. J. Surg. Pathol. 2002, 26, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Xing, M.; Mambo, E.; Guo, Z.; Wu, G.; Trink, B.; Beller, U.; Westra, W.H.; Ladenson, P.W.; Sidransky, D. BRAF mutation in papillary thyroid carcinoma. J. Natl. Cancer Inst. 2003, 95, 625–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiforov, Y.E.; Steward, D.L.; Robinson-Smith, T.M.; Haugen, B.R.; Klopper, J.P.; Zhu, Z.; Fagin, J.A.; Falciglia, M.; Weber, K.; Nikiforova, M.N. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J. Clin. Endocrinol. Metab. 2009, 94, 2092–2098. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Wald, A.I.; Roy, S.; Durso, M.B.; Nikiforov, Y.E. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J. Clin. Endocrinol. Metab. 2013, 98, E1852–E1860. [Google Scholar] [CrossRef] [Green Version]
- Nikiforov, Y.E.; Carty, S.E.; Chiosea, S.I.; Coyne, C.; Duvvuri, U.; Ferris, R.L.; Gooding, W.E.; LeBeau, S.O.; Ohori, N.P.; Seethala, R.R.; et al. Impact of the Multi-Gene ThyroSeq Next-Generation Sequencing Assay on Cancer Diagnosis in Thyroid Nodules with Atypia of Undetermined Significance/Follicular Lesion of Undetermined Significance Cytology. Thyroid 2015, 25, 1217–1223. [Google Scholar] [CrossRef]
- Wylie, D.; Beaudenon-Huibregtse, S.; Haynes, B.C.; Giordano, T.J.; Labourier, E. Molecular classification of thyroid lesions by combined testing for miRNA gene expression and somatic gene alterations. J. Pathol. Clin. Res. 2016, 2, 93–103. [Google Scholar] [CrossRef]
- Benjamin, H.; Schnitzer-Perlman, T.; Shtabsky, A.; VandenBussche, C.J.; Ali, S.Z.; Kolar, Z.; Pagni, F.; Rosetta Genomics, G.; Bar, D.; Meiri, E. Analytical validity of a microRNA-based assay for diagnosing indeterminate thyroid FNA smears from routinely prepared cytology slides. Cancer Cytopathol. 2016, 124, 711–721. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Mercurio, S.; Wald, A.I.; Barbi de Moura, M.; Callenberg, K.; Santana-Santos, L.; Gooding, W.E.; Yip, L.; Ferris, R.L.; Nikiforov, Y.E. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer 2018, 124, 1682–1690. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.N.; Angell, T.E.; Babiarz, J.; Barth, N.M.; Blevins, T.; Duh, Q.Y.; Ghossein, R.A.; Harrell, R.M.; Huang, J.; Kennedy, G.C.; et al. Performance of a Genomic Sequencing Classifier for the Preoperative Diagnosis of Cytologically Indeterminate Thyroid Nodules. JAMA Surg. 2018, 153, 817–824. [Google Scholar] [CrossRef]
- Hao, Y.; Duh, Q.Y.; Kloos, R.T.; Babiarz, J.; Harrell, R.M.; Traweek, S.T.; Kim, S.Y.; Fedorowicz, G.; Walsh, P.S.; Sadow, P.M.; et al. Identification of Hurthle cell cancers: Solving a clinical challenge with genomic sequencing and a trio of machine learning algorithms. BMC Syst. Biol. 2019, 13 (Suppl. S2), 27. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.I.; Waguespack, S.G.; Dosiou, C.; Ladenson, P.W.; Livhits, M.J.; Wirth, L.J.; Sadow, P.M.; Krane, J.F.; Stack, B.C.; Zafereo, M.E.; et al. Afirma Genomic Sequencing Classifier and Xpression Atlas Molecular Findings in Consecutive Bethesda III-VI Thyroid Nodules. J. Clin. Endocrinol. Metab. 2021, 106, 2198–2207. [Google Scholar] [CrossRef]
- Sistrunk, J.W.; Shifrin, A.; Frager, M.; Bardales, R.H.; Thomas, J.; Fishman, N.; Goldberg, P.; Guttler, R.; Grant, E. Clinical performance of multiplatform mutation panel and microRNA risk classifier in indeterminate thyroid nodules. J. Am. Soc. Cytopathol. 2020, 9, 232–241. [Google Scholar] [CrossRef]
- Jackson, S.; Kumar, G.; Banizs, A.B.; Toney, N.; Silverman, J.F.; Narick, C.M.; Finkelstein, S.D. Incremental utility of expanded mutation panel when used in combination with microRNA classification in indeterminate thyroid nodules. Diagn. Cytopathol. 2020, 48, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Finkelstein, S.D.; Sistrunk, J.W.; Malchoff, C.; Thompson, D.V.; Kumar, G.; Timmaraju, V.A.; Repko, B.; Mireskandari, A.; Evoy-Goodman, L.A.; Massoll, N.A.; et al. A Retrospective Evaluation of the Diagnostic Performance of an Interdependent Pairwise MicroRNA Expression Analysis with a Mutation Panel in Indeterminate Thyroid Nodules. Thyroid 2022, 32, 1362–1371. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, H.; Bardelli, A.; Lengauer, C.; Kinzler, K.W.; Vogelstein, B.; Velculescu, V.E. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 2002, 418, 934. [Google Scholar] [CrossRef]
- Singer, G.; Oldt, R., 3rd; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih Ie, M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar] [CrossRef] [Green Version]
- Rowe, L.R.; Bentz, B.G.; Bentz, J.S. Detection of BRAF V600E activating mutation in papillary thyroid carcinoma using PCR with allele-specific fluorescent probe melting curve analysis. J. Clin. Pathol. 2007, 60, 1211–1215. [Google Scholar] [CrossRef] [Green Version]
- Sadow, P.M.; Heinrich, M.C.; Corless, C.L.; Fletcher, J.A.; Nose, V. Absence of BRAF, NRAS, KRAS, HRAS mutations, and RET/PTC gene rearrangements distinguishes dominant nodules in Hashimoto thyroiditis from papillary thyroid carcinomas. Endocr. Pathol. 2010, 21, 73–79. [Google Scholar] [CrossRef]
- Krane, J.F.; Cibas, E.S.; Endo, M.; Marqusee, E.; Hu, M.I.; Nasr, C.E.; Waguespack, S.G.; Wirth, L.J.; Kloos, R.T. The Afirma Xpression Atlas for thyroid nodules and thyroid cancer metastases: Insights to inform clinical decision-making from a fine-needle aspiration sample. Cancer Cytopathol. 2020, 128, 452–459. [Google Scholar] [CrossRef]
- Santos, M.T.D.; Buzolin, A.L.; Gama, R.R.; Silva, E.; Dufloth, R.M.; Figueiredo, D.L.A.; Carvalho, A.L. Molecular Classification of Thyroid Nodules with Indeterminate Cytology: Development and Validation of a Highly Sensitive and Specific New miRNA-Based Classifier Test Using Fine-Needle Aspiration Smear Slides. Thyroid 2018, 28, 1618–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, C.W.; Kameyama, K.; Kitagawa, W.; Azar, N. Rapid on-site evaluation (ROSE) for fine needle aspiration of thyroid: Benefits, challenges and innovative solutions. Gland. Surg. 2020, 9, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Klopper, J.; Cottrill, E.E. Molecular diagnostics in the evaluation of thyroid nodules: Current use and prospective opportunities. Front. Endocrinol. 2023, 14, 1101410. [Google Scholar] [CrossRef] [PubMed]
- Nikiforova, M.N.; Lepe, M.; Tolino, L.A.; Miller, M.E.; Ohori, N.P.; Wald, A.I.; Landau, M.S.; Kaya, C.; Malapelle, U.; Bellevicine, C.; et al. Thyroid cytology smear slides: An untapped resource for ThyroSeq testing. Cancer Cytopathol. 2021, 129, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhou, X.; Huang, F.; Wang, W.; Qi, Y.; Xu, H.; Yang, S.; Shen, L.; Fei, X.; Xie, J.; et al. The genetic landscape of benign thyroid nodules revealed by whole exome and transcriptome sequencing. Nat. Commun. 2017, 8, 15533. [Google Scholar] [CrossRef] [Green Version]
- Guan, H.; Matonis, D.; Toraldo, G.; Lee, S.L. Clinical Significance of Thyroid-Stimulating Hormone Receptor Gene Mutations and/or Sodium-Iodine Symporter Gene Overexpression in Indeterminate Thyroid Fine Needle Biopsies. Front. Endocrinol. 2018, 9, 566. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Nikitski, A.V.; Panebianco, F.; Kaya, C.; Yip, L.; Williams, M.; Chiosea, S.I.; Seethala, R.R.; Roy, S.; Condello, V.; et al. GLIS Rearrangement is a Genomic Hallmark of Hyalinizing Trabecular Tumor of the Thyroid Gland. Thyroid 2019, 29, 161–173. [Google Scholar] [CrossRef]
- Angell, T.E.; Wirth, L.J.; Cabanillas, M.E.; Shindo, M.L.; Cibas, E.S.; Babiarz, J.E.; Hao, Y.; Kim, S.Y.; Walsh, P.S.; Huang, J.; et al. Analytical and Clinical Validation of Expressed Variants and Fusions From the Whole Transcriptome of Thyroid FNA Samples. Front. Endocrinol. 2019, 10, 612. [Google Scholar] [CrossRef]
- Ciarletto, A.M.; Narick, C.; Malchoff, C.D.; Massoll, N.A.; Labourier, E.; Haugh, K.; Mireskandari, A.; Finkelstein, S.D.; Kumar, G. Analytical and clinical validation of pairwise microRNA expression analysis to identify medullary thyroid cancer in thyroid fine-needle aspiration samples. Cancer Cytopathol. 2021, 129, 239–249. [Google Scholar] [CrossRef]
- Corver, W.E.; Ruano, D.; Weijers, K.; den Hartog, W.C.; van Nieuwenhuizen, M.P.; de Miranda, N.; van Eijk, R.; Middeldorp, A.; Jordanova, E.S.; Oosting, J.; et al. Genome haploidisation with chromosome 7 retention in oncocytic follicular thyroid carcinoma. PLoS ONE 2012, 7, e38287. [Google Scholar] [CrossRef] [Green Version]
- Gopal, R.K.; Kubler, K.; Calvo, S.E.; Polak, P.; Livitz, D.; Rosebrock, D.; Sadow, P.M.; Campbell, B.; Donovan, S.E.; Amin, S.; et al. Widespread Chromosomal Losses and Mitochondrial DNA Alterations as Genetic Drivers in Hurthle Cell Carcinoma. Cancer Cell 2018, 34, 242–255.e5. [Google Scholar] [CrossRef] [Green Version]
- Ganly, I.; Makarov, V.; Deraje, S.; Dong, Y.; Reznik, E.; Seshan, V.; Nanjangud, G.; Eng, S.; Bose, P.; Kuo, F.; et al. Integrated Genomic Analysis of Hurthle Cell Cancer Reveals Oncogenic Drivers, Recurrent Mitochondrial Mutations, and Unique Chromosomal Landscapes. Cancer Cell 2018, 34, 256–270.e5. [Google Scholar] [CrossRef] [Green Version]
- Silaghi, C.A.; Lozovanu, V.; Georgescu, C.E.; Georgescu, R.D.; Susman, S.; Nasui, B.A.; Dobrean, A.; Silaghi, H. Thyroseq v3, Afirma GSC, and microRNA Panels Versus Previous Molecular Tests in the Preoperative Diagnosis of Indeterminate Thyroid Nodules: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 649522. [Google Scholar] [CrossRef]
- Lee, E.; Terhaar, S.; McDaniel, L.; Gorelik, D.; Gerhard, E.; Chen, C.; Ma, Y.; Joshi, A.S.; Goodman, J.F.; Thakkar, P.G. Diagnostic performance of the second-generation molecular tests in the assessment of indeterminate thyroid nodules: A systematic review and meta-analysis. Am. J. Otolaryngol. 2022, 43, 103394. [Google Scholar] [CrossRef]
- Livhits, M.J.; Zhu, C.Y.; Kuo, E.J.; Nguyen, D.T.; Kim, J.; Tseng, C.H.; Leung, A.M.; Rao, J.; Levin, M.; Douek, M.L.; et al. Effectiveness of Molecular Testing Techniques for Diagnosis of Indeterminate Thyroid Nodules: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 70–77. [Google Scholar] [CrossRef]
- Hu, Q.L.; Schumm, M.A.; Zanocco, K.A.; Yeh, M.W.; Livhits, M.J.; Wu, J.X. Cost analysis of reflexive versus selective molecular testing for indeterminate thyroid nodules. Surgery 2022, 171, 147–154. [Google Scholar] [CrossRef]
- Chen, D.W.; Haymart, M.R. Disparities Research in Thyroid Cancer: Challenges and Strategies for Improvement. Thyroid 2020, 30, 1231–1235. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, X.; Liang, L.; Li, D.; Yan, L.; Li, X.; Di, J.; Li, T. Application of biomarkers in the diagnosis of uncertain samples of core needle biopsy of thyroid nodules. Virchows Arch. 2021, 479, 961–974. [Google Scholar] [CrossRef]
- Alzumaili, B.A.; Krumeich, L.N.; Collins, R.; Kravchenko, T.; Ababneh, E.I.; Fisch, A.S.; Faquin, W.C.; Nose, V.; Martinez-Lage, M.; Randolph, G.W.; et al. A Comprehensive Study on the Diagnosis and Management of Noninvasive Follicular Thyroid Neoplasm with Papillary-Like Nuclear Features. Thyroid 2023, 33, 566–577. [Google Scholar] [CrossRef]
- Labourier, E.; Fahey, T.J., 3rd. Preoperative molecular testing in thyroid nodules with Bethesda VI cytology: Clinical experience and review of the literature. Diagn. Cytopathol. 2021, 49, E175–E180. [Google Scholar] [CrossRef]
- Niciporuka, R.; Nazarovs, J.; Ozolins, A.; Narbuts, Z.; Miklasevics, E.; Gardovskis, J. Can We Predict Differentiated Thyroid Cancer Behavior? Role of Genetic and Molecular Markers. Medicina 2021, 57, 1131. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, R.; Shen, X.; Zhu, G.; Li, B.; Xing, M. The Genetic Duet of BRAF V600E and TERT Promoter Mutations Robustly Predicts Loss of Radioiodine Avidity in Recurrent Papillary Thyroid Cancer. J. Nucl. Med. 2020, 61, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skaugen, J.M.; Taneja, C.; Liu, J.B.; Wald, A.I.; Nikitski, A.V.; Chiosea, S.I.; Seethala, R.R.; Ohori, N.P.; Karslioglu-French, E.; Carty, S.E.; et al. Performance of a Multigene Genomic Classifier in Thyroid Nodules with Suspicious for Malignancy Cytology. Thyroid 2022, 32, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Hescot, S.; Al Ghuzlan, A.; Henry, T.; Sheikh-Alard, H.; Lamartina, L.; Borget, I.; Hadoux, J.; Baudin, E.; Dupuy, C.; Nikitski, A.V.; et al. Prognostic of recurrence and survival in poorly differentiated thyroid cancer. Endocr. Relat. Cancer 2022, 29, 625–634. [Google Scholar] [CrossRef]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef]
- Sherman, S.I. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin. Oncol. (R. Coll. Radiol.) 2010, 22, 464–468. [Google Scholar] [CrossRef]
- Kelly, L.M.; Barila, G.; Liu, P.; Evdokimova, V.N.; Trivedi, S.; Panebianco, F.; Gandhi, M.; Carty, S.E.; Hodak, S.P.; Luo, J.; et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 4233–4238. [Google Scholar] [CrossRef] [Green Version]
- Brose, M.S.; Nutting, C.M.; Jarzab, B.; Elisei, R.; Siena, S.; Bastholt, L.; de la Fouchardiere, C.; Pacini, F.; Paschke, R.; Shong, Y.K.; et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet 2014, 384, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.M.; Politti, U.; Spisni, R.; Materazzi, G.; Baldini, E.; Ulisse, S.; Miccoli, P.; Antonelli, A.; Fallahi, P. Sorafenib in the treatment of thyroid cancer. Expert. Rev. Anticancer Ther. 2015, 15, 863–874. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Elia, G.; Ragusa, F.; Paparo, S.R.; Mazzi, V.; Miccoli, M.; Galdiero, M.R.; Varricchi, G.; Foddis, R.; Guglielmi, G.; et al. Lenvatinib: An investigational agent for the treatment of differentiated thyroid cancer. Expert Opin. Investig. Drugs 2021, 30, 913–921. [Google Scholar] [CrossRef]
- Schlumberger, M.; Tahara, M.; Wirth, L.J.; Robinson, B.; Brose, M.S.; Elisei, R.; Habra, M.A.; Newbold, K.; Shah, M.H.; Hoff, A.O.; et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015, 372, 621–630. [Google Scholar] [CrossRef] [Green Version]
- Falchook, G.S.; Millward, M.; Hong, D.; Naing, A.; Piha-Paul, S.; Waguespack, S.G.; Cabanillas, M.E.; Sherman, S.I.; Ma, B.; Curtis, M.; et al. BRAF inhibitor dabrafenib in patients with metastatic BRAF-mutant thyroid cancer. Thyroid 2015, 25, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Brose, M.S.; Cabanillas, M.E.; Cohen, E.E.; Wirth, L.J.; Riehl, T.; Yue, H.; Sherman, S.I.; Sherman, E.J. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: A non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1272–1282. [Google Scholar] [CrossRef] [Green Version]
- Rothenberg, S.M.; McFadden, D.G.; Palmer, E.L.; Daniels, G.H.; Wirth, L.J. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin. Cancer Res. 2015, 21, 1028–1035. [Google Scholar] [CrossRef] [Green Version]
- Dunn, L.A.; Sherman, E.J.; Baxi, S.S.; Tchekmedyian, V.; Grewal, R.K.; Larson, S.M.; Pentlow, K.S.; Haque, S.; Tuttle, R.M.; Sabra, M.M.; et al. Vemurafenib Redifferentiation of BRAF Mutant, RAI-Refractory Thyroid Cancers. J. Clin. Endocrinol. Metab. 2019, 104, 1417–1428. [Google Scholar] [CrossRef]
- Liu, S.V.; Macke, L.A.; Colton, B.S.; Imran, S.S.; Christiansen, J.; Chow-Maneval, E.; Hornby, Z.; Multani, P.S. Response to Entrectinib in Differentiated Thyroid Cancer With a ROS1 Fusion. JCO Precis. Oncol. 2017, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.; Cabanillas, M.E.; Urbanowitz, G.; et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 2018, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Cho, J.Y.; Schellens, J.H.M.; Soria, J.C.; Wen, P.Y.; Zielinski, C.C.; Cabanillas, M.E.; Boran, A.; et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant anaplastic thyroid cancer: Updated analysis from the phase II ROAR basket study. Ann. Oncol. 2022, 33, 406–415. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef] [Green Version]
- Wirth, L.J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef]
- Brose, M.S.; Robinson, B.; Sherman, S.I.; Krajewska, J.; Lin, C.C.; Vaisman, F.; Hoff, A.O.; Hitre, E.; Bowles, D.W.; Hernando, J.; et al. Cabozantinib for radioiodine-refractory differentiated thyroid cancer (COSMIC-311): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Hu, M.I.; Wirth, L.J.; Schuler, M.; Mansfield, A.S.; Curigliano, G.; Brose, M.S.; Zhu, V.W.; Leboulleux, S.; Bowles, D.W.; et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): A multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021, 9, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Waguespack, S.G.; Drilon, A.; Lin, J.J.; Brose, M.S.; McDermott, R.; Almubarak, M.; Bauman, J.; Casanova, M.; Krishnamurthy, A.; Kummar, S.; et al. Efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma. Eur. J. Endocrinol. 2022, 186, 631–643. [Google Scholar] [CrossRef]
- Solomon, B.J.; Tan, L.; Lin, J.J.; Wong, S.Q.; Hollizeck, S.; Ebata, K.; Tuch, B.B.; Yoda, S.; Gainor, J.F.; Sequist, L.V.; et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-Driven Malignancies. J. Thorac. Oncol. 2020, 15, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Zhu, V.W.; Madison, R.; Schrock, A.B.; Ou, S.I. Emergence of High Level of MET Amplification as Off-Target Resistance to Selpercatinib Treatment in KIF5B-RET NSCLC. J. Thorac. Oncol. 2020, 15, e124–e127. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Jiang, Y.; Jiang, W.; Wang, H.; Liu, S.; Shao, Y.; Zhao, W.; Ning, R.; Yu, Q. STRN-ALK Fusion in Lung Adenocarcinoma with Excellent Response Upon Alectinib Treatment: A Case Report and Literature Review. OncoTargets Ther. 2020, 13, 12515–12519. [Google Scholar] [CrossRef]
- Genutis, L.K.; Tomsic, J.; Bundschuh, R.A.; Brock, P.L.; Williams, M.D.; Roychowdhury, S.; Reeser, J.W.; Frankel, W.L.; Alsomali, M.; Routbort, M.J.; et al. Microsatellite Instability Occurs in a Subset of Follicular Thyroid Cancers. Thyroid 2019, 29, 523–529. [Google Scholar] [CrossRef]
- Janz, T.A.; Neskey, D.M.; Nguyen, S.A.; Lentsch, E.J. Is the incidence of anaplastic thyroid cancer increasing: A population based epidemiology study. World J. Otorhinolaryngol. Head Neck Surg. 2019, 5, 34–40. [Google Scholar] [CrossRef]
- Lin, B.; Ma, H.; Ma, M.; Zhang, Z.; Sun, Z.; Hsieh, I.Y.; Okenwa, O.; Guan, H.; Li, J.; Lv, W. The incidence and survival analysis for anaplastic thyroid cancer: A SEER database analysis. Am. J. Transl. Res. 2019, 11, 5888–5896. [Google Scholar]
- Lang, M.; Longerich, T.; Anamaterou, C. Targeted therapy with vemurafenib in BRAF(V600E)-mutated anaplastic thyroid cancer. Thyroid Res. 2023, 16, 5. [Google Scholar] [CrossRef]
- Xu, B.; Fuchs, T.; Dogan, S.; Landa, I.; Katabi, N.; Fagin, J.A.; Tuttle, R.M.; Sherman, E.; Gill, A.J.; Ghossein, R. Dissecting Anaplastic Thyroid Carcinoma: A Comprehensive Clinical, Histologic, Immunophenotypic, and Molecular Study of 360 Cases. Thyroid 2020, 30, 1505–1517. [Google Scholar] [CrossRef]
- Maniakas, A.; Zafereo, M.; Cabanillas, M.E. Anaplastic Thyroid Cancer: New Horizons and Challenges. Endocrinol. Metab. Clin. North. Am. 2022, 51, 391–401. [Google Scholar] [CrossRef]
- Rosove, M.H.; Peddi, P.F.; Glaspy, J.A. BRAF V600E inhibition in anaplastic thyroid cancer. N. Engl. J. Med. 2013, 368, 684–685. [Google Scholar] [CrossRef]
- Smith, A.L.; Williams, M.D.; Stewart, J.; Wang, W.L.; Krishnamurthy, S.; Cabanillas, M.E.; Roy-Chowdhuri, S. Utility of the BRAF p.V600E immunoperoxidase stain in FNA direct smears and cell block preparations from patients with thyroid carcinoma. Cancer Cytopathol. 2018, 126, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Sandulache, V.C.; Williams, M.D.; Lai, S.Y.; Lu, C.; William, W.N.; Busaidy, N.L.; Cote, G.J.; Singh, R.R.; Luthra, R.; Cabanillas, M.E. Real-Time Genomic Characterization Utilizing Circulating Cell-Free DNA in Patients with Anaplastic Thyroid Carcinoma. Thyroid 2017, 27, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Khatami, F.; Tavangar, S.M. Liquid Biopsy in Thyroid Cancer: New Insight. Int. J. Hematol. Oncol. Stem Cell Res. 2018, 12, 235–248. [Google Scholar]
- Yeo, M.K.; Liang, Z.L.; Oh, T.; Moon, Y.; An, S.; Kim, M.K.; Kim, K.S.; Shong, M.; Kim, J.M.; Jo, Y.S. Pyrosequencing cut-off value identifying BRAFV600E mutation in fine needle aspiration samples of thyroid nodules. Clin. Endocrinol. 2011, 75, 555–560. [Google Scholar] [CrossRef]
- Huber, F.; Lang, H.P.; Glatz, K.; Rimoldi, D.; Meyer, E.; Gerber, C. Fast Diagnostics of BRAF Mutations in Biopsies from Malignant Melanoma. Nano Lett. 2016, 16, 5373–5377. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.R.; Zafereo, M.E.; Dadu, R.; Ferrarotto, R.; Busaidy, N.L.; Lu, C.; Ahmed, S.; Gule-Monroe, M.K.; Williams, M.D.; Sturgis, E.M.; et al. Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAF(V600E)-Mutated Anaplastic Thyroid Carcinoma. Thyroid 2019, 29, 1036–1043. [Google Scholar] [CrossRef] [Green Version]
- Gild, M.L.; Bullock, M.; Tsang, V.; Clifton-Bligh, R.J.; Robinson, B.G.; Wirth, L.J. Challenges and Strategies to Combat Resistance Mechanisms in Thyroid Cancer Therapeutics. Thyroid 2023, 33, 682–690. [Google Scholar] [CrossRef]
- Ceolin, L.; Duval, M.; Benini, A.F.; Ferreira, C.V.; Maia, A.L. Medullary thyroid carcinoma beyond surgery: Advances, challenges, and perspectives. Endocr. Relat. Cancer 2019, 26, R499–R518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzumaili, B.; Xu, B.; Spanheimer, P.M.; Tuttle, R.M.; Sherman, E.; Katabi, N.; Dogan, S.; Ganly, I.; Untch, B.R.; Ghossein, R.A. Grading of medullary thyroid carcinoma on the basis of tumor necrosis and high mitotic rate is an independent predictor of poor outcome. Mod. Pathol. 2020, 33, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Boichard, A.; Croux, L.; Al Ghuzlan, A.; Broutin, S.; Dupuy, C.; Leboulleux, S.; Schlumberger, M.; Bidart, J.M.; Lacroix, L. Somatic RAS mutations occur in a large proportion of sporadic RET-negative medullary thyroid carcinomas and extend to a previously unidentified exon. J. Clin. Endocrinol. Metab. 2012, 97, E2031–E2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moura, M.M.; Cavaco, B.M.; Pinto, A.E.; Domingues, R.; Santos, J.R.; Cid, M.O.; Bugalho, M.J.; Leite, V. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br. J. Cancer 2009, 100, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Degrauwe, N.; Sosa, J.A.; Roman, S.; Deshpande, H.A. Vandetanib for the treatment of metastatic medullary thyroid cancer. Clin. Med. Insights Oncol. 2012, 6, 243–252. [Google Scholar] [CrossRef]
- Nagilla, M.; Brown, R.L.; Cohen, E.E. Cabozantinib for the treatment of advanced medullary thyroid cancer. Adv. Ther. 2012, 29, 925–934. [Google Scholar] [CrossRef]
- Frisco, N.A.; Gunn, A.H.; Thomas, S.M.; Stang, M.T.; Scheri, R.P.; Kazaure, H.S. Medullary thyroid cancer with RET V804M mutation: More indolent than expected? Surgery 2023, 173, 260–267. [Google Scholar] [CrossRef]

| Year Authors | Molecular Alterations or Genetic Testing | Diagnosis |
|---|---|---|
| 1987 Fusco et al. [16] | TRK and RET rearrangements | PTC |
| 1989 Lemoine et al. [18] | NRAS p.Q61R and HRAS p.Q61R | Thyroid neoplastic process (FTA, FTC and ATC) |
| 1990 Namba et al. [19] | NRAS, HRAS and KRAS at codons 12 and 13 | Benign and neoplastic thyroid nodules |
| 1992 Santoro et al. [17] | RET aberrations | PTC |
| 2000 Kroll et al. [20] | PAX8::PPARG fusion | FTC |
| 2002 Nikiforova et al. [21] | PAX8::PPARG fusion | FTC and FTA |
| 2003 Cohen et al. [22] | BRAF p.T1796A point mutation | PTC |
| 2009 Nikiforov et al. [23] | BRAF (p.V600E, p.K601E), NRAS, KRAS and HRAS and RET::PTC1, RET::PTC3 and PAX8::PPARG gene fusion | Bethesda III and IV |
| 2012 Alexander et al. [12] | Afirma GEC: (using machine learning to interpret the expression of mRNA of 167 genes through microarray platforms) | Bethesda III and IV |
| 2013 Nikiforova et al. [24] | ThyroSeq V1: NGS (BRAF, RET, NRAS, KRAS, HRAS, PIK3CA, TP53, TSHR, PTEN, GNAS, CTNNB1 and AKT1) | Bethesda III and IV |
| 2014 The Cancer Genome Atlas (TCGA) [9] | BRAFV600E-like or RAS-like phenotype | PTC including follicular variant |
| 2015 Yoo et al. [11] | BRAF-like, RAS-like and non-BRAF-/non-RAS-like (NBNR) | FTA and minimally invasive FTC |
| 2015 Nikiforov et al. [25] | ThyroSeq V2.1: ThyroSeq V1 and EIF1AX and BRAFV601K and 40 gene fusions including THADA, ALK, PAX8::PPARG, TRK1 and TRK3 | Bethesda III and IV |
| 2016 Wylie et al. [26] | RosettaGX Reveal (miRNA classifier) | Bethesda III and IV |
| 2016 Benjamin et al. [27] | Asuragen (miRNA and somatic gene mutational platform) | Bethesda III and IV |
| 2018 Nikiforova et al. [28] | ThyroSeq V3: NGS of 112 thyroid cancer-related genes | Bethesda III and IV |
| 2018 Patel et al. [29] | Afirma GSC (describing RNA transcriptome with additional sequencing of nuclear and mitochondrial genes, changes in genomic copy number including loss of heterozygosity) | Bethesda III and IV |
| 2019 Hao et al. [30] | Afirma GSC (NGS of whole transcriptome RNA sequencing) | Oncocytic cell neoplasms |
| 2019/2020 Hu et al. [31] | Afirma Xpression Atlas (enumerates mutations in 593 genes informing 905 variants and 235 fusions in suspicious Afirma GSC) | Bethesda III, IV, V and VI |
| 2020 Sistrunk et al. [32] Jackson et al. [33] Finkelstein et al. [34] | MPTX (ThyGeNEXT/ThyraMIR): NGS (ALK, BRAF, GNAS, HRAS, KRAS, NRAS, PIK3CA, PTEN, RET and TERT promoter genes) as well as mRNA fusion genes (ALK, BRAF, NTRK, PPARG, RET, PAX8, TBP, USP33 and THADA) and miRNA- (21, 29, 31, 138, 139, 155, 146, 204, 222, 375, 551) | Bethesda III and IV |
| Medication | Molecular Target | Mechanism of Action | Year | Reference |
|---|---|---|---|---|
| Vandetanib | Inhibits RET in MTC | Selective RET TKI | 2012 | Degrauwe et al. [10] |
| Alectinib * | ALK1 fusion-positive thyroid carcinoma | ALK-TKI | 2014 | Kelly et al. [67] |
| Sorafenib | Aggressive RAI-resistant PTC or FTC | Anti-angiogenic multi-targeted kinase inhibitor (aaMKI) | 2014 2015 | Brose and Nutting et al. [68] Ferrari, Politti et al. [69] |
| Lenvatinib | Aggressive RAI-resistant PTC or FTC | Anti-angiogenic multi-targeted kinase inhibitor (aaMKI) | 2015 2021 | Ferrari and Elia et al. [70] Schlumberger et al. [71] |
| Dabrafenib | BRAF p.V600E-mutated PTC | BRAF inhibitor | 2015 2016 | Falchook et al. [72] Brose and Cabanillas et al. [73] |
| Dabrafenib and Vemurafenib | BRAF p.V600E-mutated PTC | BRAF inhibitor | 2015 2019 | Rothenberg et al. [74] Dunn et al. [75] |
| Entrectinib | ROS1 fusion-positive thyroid carcinoma | Multikinase inhibitor (NTRK1/2/3, ROS1 and ALK) | 2017 | Liu et al. [76] |
| Dabrafenib/ Trametinib | BRAF p.V600E-mutated ATC | BRAF/ MEK inhibitors | 2018 2022 | Subbiah and Kreitman et al. [77] Subbiah and Kreitman et al. [78] |
| Pembrolizumab | Solid tumors including thyroid tumors | Programmed death (PD-1) inhibitors | 2019 | Marcus et al. [79] |
| Selpercatinib | -Inhibits RET in MTC -RET fusion-positive thyroid carcinoma | Selective RET TKI | 2020 | Wirth et al. [80] |
| Cabozantinib | Second line for treatment of aggressive RAI-resistant PTC or FTC | Antiangiogenic multi-targeted kinase inhibitor (aaMKIs) | 2021 | Brose and Robinson et al. [81] |
| Pralsetinib | -Inhibits RET in MTC -RET fusion-positive thyroid carcinoma | TKI | 2021 | Subbiah and Hu et al. [82] |
| Larotrectinib | TRK fusion-positive thyroid carcinoma | Selective TRK inhibitor | 2022 | Waguespack et al. [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzumaili, B.; Sadow, P.M. Update on Molecular Diagnostics in Thyroid Pathology: A Review. Genes 2023, 14, 1314. https://doi.org/10.3390/genes14071314
Alzumaili B, Sadow PM. Update on Molecular Diagnostics in Thyroid Pathology: A Review. Genes. 2023; 14(7):1314. https://doi.org/10.3390/genes14071314
Chicago/Turabian StyleAlzumaili, Bayan, and Peter M. Sadow. 2023. "Update on Molecular Diagnostics in Thyroid Pathology: A Review" Genes 14, no. 7: 1314. https://doi.org/10.3390/genes14071314
APA StyleAlzumaili, B., & Sadow, P. M. (2023). Update on Molecular Diagnostics in Thyroid Pathology: A Review. Genes, 14(7), 1314. https://doi.org/10.3390/genes14071314





