Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment
Abstract
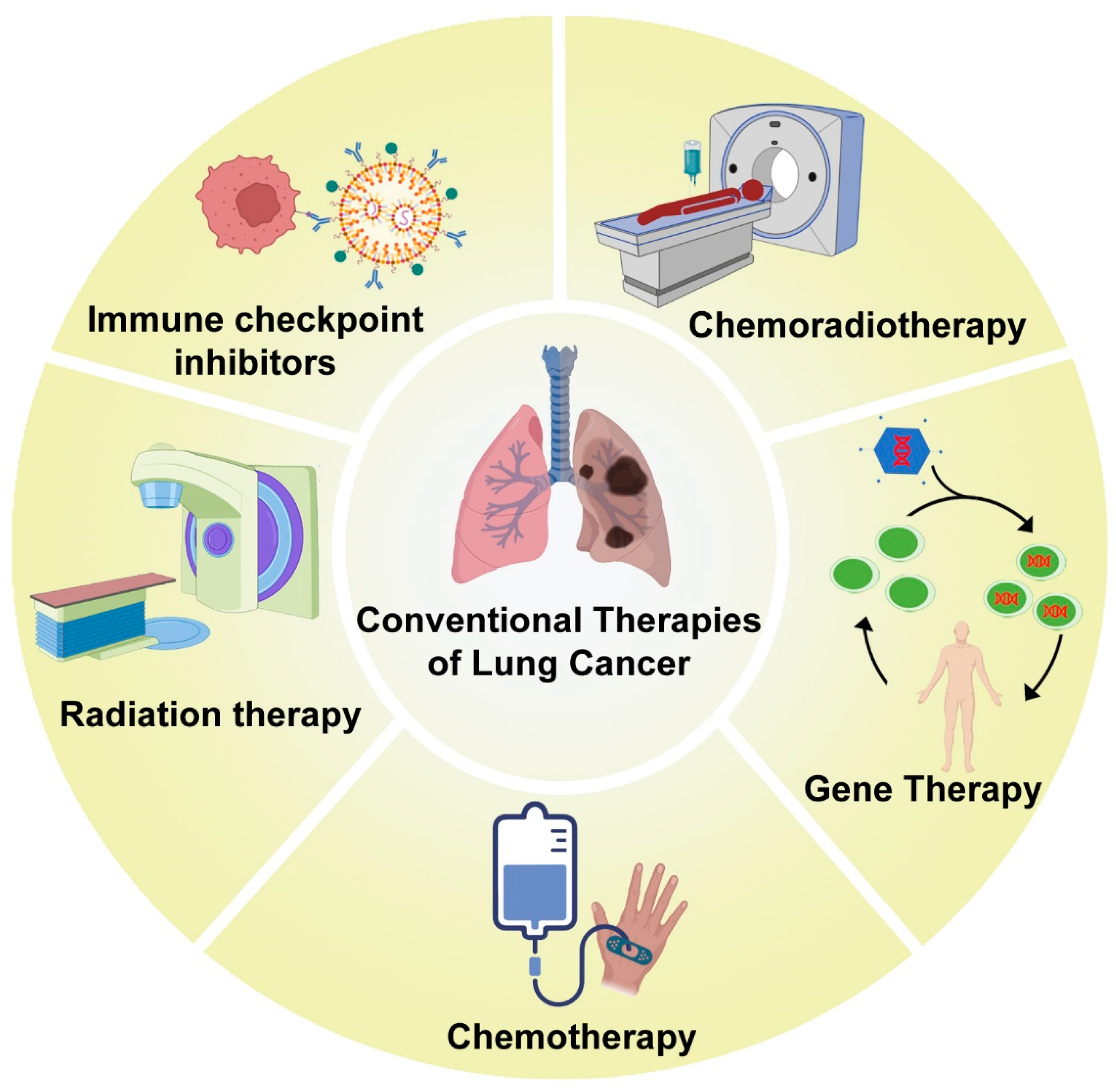
1. Introduction
2. Chemotherapy for Treatment of Lung Cancer
2.1. Chemoimmunotherapy
2.2. Tyrosine Kinase Inhibitor with Chemotherapy
3. Lung Cancer Management by Radiation Therapy
3.1. Stereotactic Body Radiation Therapy
3.2. Chemoradiation Therapy
4. Combination of Radiation and Chemotherapy for Lung Cancer Management
5. Other Treatment Strategies for Lung Cancer
5.1. Immune Checkpoint Inhibitors in Lung Cancer
5.2. Gene Therapy
6. Lung Cancer Chemotherapy Using Nano-Enabled Drug Delivery System
7. Lung Cancer Radiation Therapy in Combination with Nanoparticles
8. Use of Nanoparticles to Reduce the Risk of Normal Tissue Toxicity during Chemoradiation Therapy
- (i)
- The solubility of chemotherapeutic drugs is improved by using nanoparticles, and they also become stable in vivo.
- (ii)
- Nanoparticle-delivered chemotherapeutic drugs can be administered intravenously to increase biodistribution, extend circulation time, and decrease the adverse effects of reactions to chemotherapy.
- (iii)
- Since solid tumors have different internal dissection characteristics compared to normal healthy tissues, nanocarriers can be used to deliver chemotherapeutic drugs preferentially to the tumor site because of their enhanced permeability and retention (EPR) effects.
- (iv)
- As a result of the tortuous and abnormal nature of angiogenesis in tumors, most lymphatic vessels inside the tumors are compressed and folded with a gap size of 100 nm–2 μm. There is a pressure difference between the tissue at the center of the solid tumor and the tissue around it as a result of the valves that leak and the poorly functioning lymphatic drainage system inside the solid tumor. Due to this difference in pressure in the tumor, molecules with a size between 10 nm and 200 nm accumulate more efficiently and remain there for a long time. This EPR effect helps the nanocarriers to passively target the tumors by retaining their contents several times longer than unpackaged drugs. This is because the retention time of drugs contained in the nanoparticles is about 10 times as long as the retention time of unpackaged drugs [126].
- (v)
- Stimuli-responsive drug delivery can also be executed for the delivery of cancer drugs specifically into the tumor tissues. It is known that the tumor microenvironment is acidic in nature, with a pH of nearly 6.3, whereas the normal tissue surroundings have a physiological pH of 7.2. When a chemotherapeutic drug is encapsulated in a nanostructure that will open up and release the drug at acidic pH, the drug is released near the tumor tissue, not in the normal tissues. Thus, targeted drug delivery can be executed without harming the normal cells. A similar strategy was also applied for delivering radiosensitizers, thereby targeting the cancerous tumors and not the benign cells [127,128].
9. Second Primary Cancers after Radiotherapy
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Doumat, G.; Daher, D.; Zerdan, M.B.; Nasra, N.; Bahmad, H.F.; Recine, M.; Poppiti, R. Drug Repurposing in Non-Small Cell Lung Carcinoma: Old Solutions for New Problems. Curr. Oncol. 2023, 30, 704–719. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, P.; Harini, K.; Crowder, S.; Ghosh, D.; Gowtham, P.; Girigoswami, K.; Girigoswami, A. Rhodamine-Conjugated Anti-Stokes Gold Nanoparticles with Higher ROS Quantum Yield as Theranostic Probe to Arrest Cancer and MDR Bacteria. Appl. Biochem. Biotechnol. 2023, 1–15. [Google Scholar] [CrossRef]
- Gowtham, P.; Girigoswami, K.; Pallavi, P.; Harini, K.; Gurubharath, I.; Girigoswami, A. Alginate-Derivative Encapsulated Carbon Coated Manganese-Ferrite Nanodots for Multimodal Medical Imaging. Pharmaceutics 2022, 14, 2550. [Google Scholar] [CrossRef]
- Soni, A.; Bhandari, M.P.; Tripathi, G.K.; Bundela, P.; Khiriya, P.K.; Khare, P.S.; Kashyap, M.K.; Dey, A.; Vellingiri, B.; Sundaramurthy, S. Nano-biotechnology in tumour and cancerous disease: A perspective review. J. Cell. Mol. Med. 2023, 27, 737–762. [Google Scholar] [CrossRef]
- Jagdale Swati, C.; HableAsawaree, A.; ChabukswarAnuruddha, R. Nanomedicine in lung cancer therapy. Adv. Nov. Formul. Drug Deliv. 2023, 433–448. [Google Scholar] [CrossRef]
- Ou, W.; Stewart, S.; White, A.; Kwizera, E.A.; Xu, J.; Fang, Y.; Shamul, J.G.; Xie, C.; Nurudeen, S.; Tirada, N.P. In-situ cryo-immune engineering of tumor microenvironment with cold-responsive nanotechnology for cancer immunotherapy. Nature Commun. 2023, 14, 392. [Google Scholar] [CrossRef]
- García-Fernández, C.; Fornaguera, C.; Borrós, S. Nanomedicine in non-small cell lung cancer: From conventional treatments to immunotherapy. Cancers 2020, 12, 1609. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Yu, X.; Hu, Y.; Sun, Y.; Wang, Z.; Zhao, J.; Yu, Y.; Hu, C.; Yang, K. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non–small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. 2021, 7, 709–717. [Google Scholar] [CrossRef] [PubMed]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.-G.; Daniel, D.; McCune, S.; Mekhail, T. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef] [PubMed]
- Shu, C.A.; Gainor, J.F.; Awad, M.M.; Chiuzan, C.; Grigg, C.M.; Pabani, A.; Garofano, R.F.; Stoopler, M.B.; Cheng, S.K.; White, A. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 786–795. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.-J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non–small cell lung cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef]
- Reck, M.; Ciuleanu, T.-E.; Dols, M.C.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O. Nivolumab (NIVO)+ ipilimumab (IPI)+ 2 cycles of platinum-doublet chemotherapy (chemo) vs 4 cycles chemo as first-line (1L) treatment (tx) for stage IV/recurrent non-small cell lung cancer (NSCLC): CheckMate 9LA. J. Clin. Oncol. 2020, 38 (Suppl. 15), 9501. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S. Five-year outcomes with pembrolizumab versus chemotherapy for metastatic non–small-cell lung cancer with PD-L1 tumor proportion score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; Carpeño, J.D.C. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Noronha, V.; Patil, V.M.; Joshi, A.; Menon, N.; Chougule, A.; Mahajan, A.; Janu, A.; Purandare, N.; Kumar, R.; More, S. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J. Clin. Oncol. 2020, 38, 124–136. [Google Scholar] [CrossRef]
- Hosomi, Y.; Morita, S.; Sugawara, S.; Kato, T.; Fukuhara, T.; Gemma, A.; Takahashi, K.; Fujita, Y.; Harada, T.; Minato, K. Gefitinib alone versus gefitinib plus chemotherapy for non–small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J. Clin. Oncol. 2020, 38, 115–123. [Google Scholar] [CrossRef]
- Kris, M.G.; Gaspar, L.E.; Chaft, J.E.; Kennedy, E.B.; Azzoli, C.G.; Ellis, P.M.; Lin, S.H.; Pass, H.I.; Seth, R.; Shepherd, F.A.; et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non–Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 2960–2974. [Google Scholar] [CrossRef]
- Simone II, C.B.; Bogart, J.A.; Cabrera, A.R.; Daly, M.E.; DeNunzio, N.J.; Detterbeck, F.; Faivre-Finn, C.; Gatschet, N.; Gore, E.; Jabbour, S.K. Radiation therapy for small cell lung cancer: An ASTRO clinical practice guideline. Radiat. Oncol. 2020, 10, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Vinod, S.K.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Videtic, G.M.; Paulus, R.; Singh, A.K.; Chang, J.Y.; Parker, W.; Olivier, K.R.; Timmerman, R.D.; Komaki, R.R.; Urbanic, J.J.; Stephans, K.L. Long-term follow-up on NRG Oncology RTOG 0915 (NCCTG N0927): A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Prezzano, K.M.; Ma, S.J.; Hermann, G.M.; Rivers, C.I.; Gomez-Suescun, J.A.; Singh, A.K. Stereotactic body radiation therapy for non-small cell lung cancer: A review. World J. Clin. Oncol. 2019, 10, 14. [Google Scholar] [CrossRef]
- Cerullo, M.; Lee, H.-J.; Kelsey, C.; Farrow, N.E.; Scales, C.D.; Tong, B.C. Surgical evaluation in patients undergoing radiation therapy for early-stage lung cancer. Ann. Thorac. Surg. 2023, 115, 338–345. [Google Scholar] [CrossRef]
- Singh, A.K.; Gomez-Suescun, J.A.; Stephans, K.L.; Bogart, J.A.; Hermann, G.M.; Tian, L.; Groman, A.; Videtic, G.M. One versus three fractions of stereotactic body radiation therapy for peripheral stage I to II non-small cell lung cancer: A randomized, multi-institution, phase 2 trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 752–759. [Google Scholar] [CrossRef]
- Ladbury, C.J.; Rusthoven, C.G.; Camidge, D.R.; Kavanagh, B.D.; Nath, S.K. Impact of radiation dose to the host immune system on tumor control and survival for stage III non-small cell lung cancer treated with definitive radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 346–355. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Reddy, G.K. Systemic effects of radiation therapy-induced abscopal responses in patients with advanced lung cancer. Oncology 2021, 99, 1–14. [Google Scholar] [CrossRef]
- Finazzi, T.; Palacios, M.A.; Haasbeek, C.J.; Admiraal, M.A.; Spoelstra, F.O.; Bruynzeel, A.M.; Slotman, B.J.; Lagerwaard, F.J.; Senan, S. Stereotactic MR-guided adaptive radiation therapy for peripheral lung tumors. Radiother. Oncol. 2020, 144, 46–52. [Google Scholar] [CrossRef]
- Haslett, K.; Bayman, N.; Franks, K.; Groom, N.; Harden, S.V.; Harris, C.; Hanna, G.; Harrow, S.; Hatton, M.; McCloskey, P. Isotoxic intensity modulated radiation therapy in stage III non-small cell lung cancer: A feasibility study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 1341–1348. [Google Scholar] [CrossRef]
- Or, M.; Liu, B.; Lam, J.; Vinod, S.; Xuan, W.; Yeghiaian-Alvandi, R.; Hau, E. A systematic review and meta-analysis of treatment-related toxicities of curative and palliative radiation therapy in non-small cell lung cancer. Sci. Rep. 2021, 11, 5939. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Tepper, J.E. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA A Cancer J. Clin. 2021, 71, 437–454. [Google Scholar] [CrossRef] [PubMed]
- Conibear, J.; Limited, A.U. Rationale for concurrent chemoradiotherapy for patients with stage III non-small-cell lung cancer. Br. J. Cancer 2020, 123, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, P.; Branz, J.; Volpi, G.; Pancera, S.; Buraschi, R.; Bianchi, L.N.C.; Bonù, M.L.; Greco, D.; Facheris, G.; Tomasi, C. Home-based pulmonary rehabilitation in patients undergoing (chemo) radiation therapy for unresectable lung cancer: A prospective explorative study. Radiol. Med. 2022, 127, 1322–1332. [Google Scholar] [CrossRef]
- Diamond, B.H.; Verma, N.; Shukla, U.C.; Park, H.S.; Koffer, P.P. Consolidative thoracic radiation therapy after first-line chemotherapy and immunotherapy in extensive-stage small cell lung cancer: A multi-institutional case series. Adv. Radiat. Oncol. 2022, 7, 100883. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, G.J.; Khrizman, P.; Squillante, C.; Callahan, K.; Xu, Q.; Abouzgheib, W.; Boujaoude, Z.; Patel, A.; Hageboutros, A. Stereotactic body radiotherapy and systemic dose chemotherapy for locally advanced lung cancer: Single arm phase 2 study. Am. J. Clin. Oncol. 2022, 45, 129–133. [Google Scholar] [CrossRef]
- Mamdani, H.; Induru, R.; Jalal, S.I. Novel therapies in small cell lung cancer. Transl. Lung Cancer Res. 2015, 4, 533. [Google Scholar] [PubMed]
- Leonetti, A.; Wever, B.; Mazzaschi, G.; Assaraf, Y.G.; Rolfo, C.; Quaini, F.; Tiseo, M.; Giovannetti, E. Molecular basis and rationale for combining immune checkpoint inhibitors with chemotherapy in non-small cell lung cancer. Drug Resist. Updates 2019, 46, 100644. [Google Scholar] [CrossRef]
- Yang, K.; Li, J.; Bai, C.; Sun, Z.; Zhao, L. Efficacy of immune checkpoint inhibitors in non-small-cell lung cancer patients with different metastatic sites: A systematic review and meta-analysis. Front. Oncol. 2020, 10, 1098. [Google Scholar] [CrossRef]
- Ferrara, R.; Imbimbo, M.; Malouf, R.; Paget-Bailly, S.; Calais, F.; Marchal, C.; Westeel, V. Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2020, 12, CD013257. [Google Scholar] [CrossRef]
- Soh, J.; Hamada, A.; Fujino, T.; Mitsudomi, T. Perioperative therapy for non-small cell lung cancer with immune checkpoint inhibitors. Cancers 2021, 13, 4035. [Google Scholar] [CrossRef] [PubMed]
- Dafni, U.; Tsourti, Z.; Vervita, K.; Peters, S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer 2019, 134, 127–140. [Google Scholar] [CrossRef]
- Blumenthal, G.M.; Zhang, L.; Zhang, H.; Kazandjian, D.; Khozin, S.; Tang, S.; Goldberg, K.; Sridhara, R.; Keegan, P.; Pazdur, R. Milestone analyses of immune checkpoint inhibitors, targeted therapy, and conventional therapy in metastatic non–small cell lung cancer trials: A meta-analysis. JAMA Oncol. 2017, 3, e171029. [Google Scholar] [CrossRef]
- Swisher, S.G.; Roth, J.A.; Carbone, D.P. Genetic and immunologic therapies for lung cancer. Semin. Oncol. 2002, 29, 95–101. [Google Scholar] [CrossRef]
- Toloza, E.M.; Morse, M.A.; Lyerly, H.K. Gene therapy for lung cancer. J. Cell. Biochem. 2006, 99, 1–22. [Google Scholar] [CrossRef]
- Lara-Guerra, H.; Roth, J.A. Gene therapy for lung cancer. Crit. Rev. Oncog. 2016, 21, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Prabavathy, D.; Swarnalatha, Y.; Ramadoss, N. Lung cancer stem cells—Origin, characteristics and therapy. Stem Cell Investig. 2018, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.l.; Xue, F.z.; Ge, B.s.; Zhao, F.r.; Shi, B.; Zhang, W. Electrochemical treatment of lung cancer. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 1997, 18, 8–13. [Google Scholar] [CrossRef]
- Xue, W.; Dahlman, J.E.; Tammela, T.; Khan, O.F.; Sood, S.; Dave, A.; Cai, W.; Chirino, L.M.; Yang, G.R.; Bronson, R. Small RNA combination therapy for lung cancer. Proc. Natl. Acad. Sci. USA 2014, 111, E3553–E3561. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Tian, H.; Wang, P.; Wang, Y.; Chen, X. The suppression of metastatic lung cancer by pulmonary administration of polymer nanoparticles for co-delivery of doxorubicin and Survivin siRNA. Biomater. Sci. 2016, 4, 1646–1654. [Google Scholar] [CrossRef]
- Dupuy, D.E.; DiPetrillo, T.; Gandhi, S.; Ready, N.; Ng, T.; Donat, W.; Mayo-Smith, W.W. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest 2006, 129, 738–745. [Google Scholar] [CrossRef]
- Rivera Díaz, M.; Vivas-Mejia, P.E. Nanoparticles as drug delivery systems in cancer medicine: Emphasis on RNAi-containing nanoliposomes. Pharmaceuticals 2013, 6, 1361–1380. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Farokhi, M.; Fatahi, Y.; Atyabi, F.; Dinarvand, R. New insights into designing hybrid nanoparticles for lung cancer: Diagnosis and treatment. J. Control. Release 2019, 295, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Gholami, L.; Ivari, J.R.; Nasab, N.K.; Oskuee, R.K.; Sathyapalan, T.; Sahebkar, A. Recent advances in lung cancer therapy based on nanomaterials: A review. Curr. Med. Chem. 2023, 30, 335–355. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, D.; Zuo, Y.Y. Nano-bio Interactions in the Lung. In Nanomedicine; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–31. [Google Scholar]
- Smith, L.; Byrne, H.L.; Waddington, D.; Kuncic, Z. Nanoparticles for MRI-guided radiation therapy: A review. Cancer Nanotechnol. 2022, 13, 38. [Google Scholar] [CrossRef]
- Sharmiladevi, P.; Akhtar, N.; Haribabu, V.; Girigoswami, K.; Chattopadhyay, S.; Girigoswami, A.J.N.-S. Excitation wavelength independent carbon-decorated ferrite nanodots for multimodal diagnosis and stimuli responsive therapy. ACS Appl. Bio Mater. 2019, 2, 1634–1642. [Google Scholar] [CrossRef]
- Sharma, U.; Jagannathan, N.R. Magnetic Resonance Imaging (MRI) and MR Spectroscopic Methods in Understanding Breast Cancer Biology and Metabolism. Metabolites 2022, 12, 295. [Google Scholar] [CrossRef]
- Haribabu, V.; Sharmiladevi, P.; Akhtar, N.; Farook, A.S.; Girigoswami, K.; Girigoswami, A.J.N.-S. Label free ultrasmall fluoromagnetic ferrite-clusters for targeted cancer imaging and drug delivery. Curr. Drug Deliv. 2019, 16, 233–241. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle delivery of cancer drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- Zamore, P.D.; Tuschl, T.; Sharp, P.A.; Bartel, D.P. RNAi: Double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.K.; Patil, S.D.; Burgess, D.J. Recent progress in non-viral nucleic acids delivery. Int. J. Pharm. 2012, 427, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Gencer, A.; Duraloglu, C.; Ozbay, S.; Ciftci, T.T.; Yabanoglu-Ciftci, S.; Arica, B. Recent advances in treatment of lung cancer: Nanoparticle-based drug and siRNA delivery systems. Curr. Drug Deliv. 2021, 18, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-D.; Park, T.-E.; Singh, B.; Maharjan, S.; Choi, Y.-J.; Choung, P.-H.; Arote, R.B.; Cho, C.-S. Nanoparticle-mediated delivery of siRNA for effective lung cancer therapy. Nanomedicine 2015, 10, 1165–1188. [Google Scholar] [CrossRef] [PubMed]
- Itani, R.; Al Faraj, A. SiRNA conjugated nanoparticles—A next generation strategy to treat lung cancer. Int. J. Mol. Sci. 2019, 20, 6088. [Google Scholar] [CrossRef] [PubMed]
- Amarzguioui, M.; Peng, Q.; Wiiger, M.T.; Vasovic, V.; Babaie, E.; Holen, T.; Nesland, J.M.; Prydz, H. Ex vivo and in vivo delivery of anti-tissue factor short interfering RNA inhibits mouse pulmonary metastasis of B16 melanoma cells. Clin. Cancer Res. 2006, 12, 4055–4061. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, N.; Liu, X.; Liang, W.; Xu, W.; Torchilin, V.P. siRNA-containing liposomes modified with polyarginine effectively silence the targeted gene. J. Control. Release 2006, 112, 229–239. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, E.; Liang, Z.; Zhao, Y.; Zhang, S.; Xu, H.; Wang, H.; Shu, X.; Kang, X.; Sun, L. Liposome-based co-delivery of 7-O-geranyl-quercetin and IGF-1R siRNA for the synergistic treatment of non-small cell lung cancer. J. Drug Deliv. Sci. Technol. 2019, 54, 101316. [Google Scholar] [CrossRef]
- Dong, Z.; Yin, Y.; Luo, J.; Li, B.; Lou, F.; Wang, Q.; Zhou, Q.; Ye, B.; Wang, Y. An FGFR1-Binding Peptide Modified Liposome for siRNA Delivery in Lung Cancer. Int. J. Mol. Sci. 2022, 23, 8380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, S.; Zhi, D.; Zhao, Y.; Cui, S.; Cui, J. Co-delivery of paclitaxel and survivin siRNA with cationic liposome for lung cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124054. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Petersen, G.H.; Alzghari, S.K.; Chee, W.; Sankari, S.S.; La-Beck, N.M. Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Control. Release 2016, 232, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Bhattacharyya, J.; Sagar, M.V.; Chaudhuri, A. Liposomally encapsulated CDC20 siRNA inhibits both solid melanoma tumor growth and spontaneous growth of intravenously injected melanoma cells on mouse lung. Drug Deliv. Transl. Res. 2013, 3, 224–234. [Google Scholar] [CrossRef]
- Song, X.-L.; Ju, R.-J.; Xiao, Y.; Wang, X.; Liu, S.; Fu, M.; Liu, J.-J.; Gu, L.-Y.; Li, X.-T.; Cheng, L. Application of multifunctional targeting epirubicin liposomes in the treatment of non-small-cell lung cancer. Int. J. Nanomed. 2017, 12, 7433. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Mohammed, K.A.; Nasreen, N. Nanoparticle-based targeted gene therapy for lung cancer. Am. J. Cancer Res. 2016, 6, 1118. [Google Scholar]
- Sukumar, U.K.; Bhushan, B.; Dubey, P.; Matai, I.; Sachdev, A.; Packirisamy, G. Emerging applications of nanoparticles for lung cancer diagnosis and therapy. Int. Nano Lett. 2013, 3, 45. [Google Scholar] [CrossRef]
- Danhier, F.; Lecouturier, N.; Vroman, B.; Jérôme, C.; Marchand-Brynaert, J.; Feron, O.; Préat, V. Paclitaxel-loaded PEGylated PLGA-based nanoparticles: In vitro and in vivo evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar] [CrossRef]
- Derakhshandeh, K.; Erfan, M.; Dadashzadeh, S. Encapsulation of 9-nitrocamptothecin, a novel anticancer drug, in biodegradable nanoparticles: Factorial design, characterization and release kinetics. Eur. J. Pharm. Biopharm. 2007, 66, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Braden, A.R.; Kafka, M.T.; Cunningham, L.; Jones, H.; Vishwanatha, J.K. Polymeric nanoparticles for sustained down-regulation of annexin A2 inhibit prostate tumor growth. J. Nanosci. Nanotechnol. 2009, 9, 2856–2865. [Google Scholar] [CrossRef]
- Ogris, M.; Wagner, E. Tumor-targeted gene transfer with DNA polyplexes. Somat. Cell Mol. Genet. 2002, 27, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Xie, X.; Neu, M.; Dumitrascu, R.; Reul, R.; Sitterberg, J.; Bakowsky, U.; Schermuly, R.; Fink, L.; Schmehl, T. Effects of cell-penetrating peptides and pegylation on transfection efficiency of polyethylenimine in mouse lungs. J. Gene Med. A Cross-Discip. J. Res. Sci. Gene Transf. Its Clin. Appl. 2008, 10, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Koshkina, N.V.; Agoulnik, I.Y.; Melton, S.L.; Densmore, C.L.; Knight, V. Biodistribution and pharmacokinetics of aerosol and intravenously administered DNA–polyethyleneimine complexes: Optimization of pulmonary delivery and retention. Mol. Ther. 2003, 8, 249–254. [Google Scholar] [CrossRef]
- Gautam, A.; Densmore, C.L.; Melton, S.; Golunski, E.; Waldrep, J.C. Aerosol delivery of PEI–p53 complexes inhibits B16-F10 lung metastases through regulation of angiogenesis. Cancer Gene Ther. 2002, 9, 28–36. [Google Scholar] [CrossRef]
- Kimura, S.; Egashira, K.; Chen, L.; Nakano, K.; Iwata, E.; Miyagawa, M.; Tsujimoto, H.; Hara, K.; Morishita, R.; Sueishi, K. Nanoparticle-mediated delivery of nuclear factor κB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension 2009, 53, 877–883. [Google Scholar] [CrossRef]
- Ziady, A.-G.; Gedeon, C.R.; Muhammad, O.; Stillwell, V.; Oette, S.M.; Fink, T.L.; Quan, W.; Kowalczyk, T.H.; Hyatt, S.L.; Payne, J. Minimal toxicity of stabilized compacted DNA nanoparticles in the murine lung. Mol. Ther. 2003, 8, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Ziady, A.-G.; Gedeon, C.R.; Miller, T.; Quan, W.; Payne, J.M.; Hyatt, S.L.; Fink, T.L.; Muhammad, O.; Oette, S.; Kowalczyk, T. Transfection of airway epithelium by stable PEGylated poly-L-lysine DNA nanoparticles in vivo. Mol. Ther. 2003, 8, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Kaul, G.; Amiji, M. Tumor-targeted gene delivery using poly (ethylene glycol)-modified gelatin nanoparticles: In vitro and in vivo studies. Pharm. Res. 2005, 22, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.M.; Köping-Höggård, M.; Tømmeraas, K.; Vårum, K.M.; Christensen, B.E.; Strand, S.P.; Artursson, P. Targeted gene delivery with trisaccharide-substituted chitosan oligomers in vitro and after lung administration in vivo. J. Control. Release 2006, 115, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.J.; Souto, E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv. Drug Deliv. Rev. 2007, 59, 478–490. [Google Scholar] [CrossRef]
- Beck-Broichsitter, M.; Gauss, J.; Packhaeuser, C.B.; Lahnstein, K.; Schmehl, T.; Seeger, W.; Kissel, T.; Gessler, T. Pulmonary drug delivery with aerosolizable nanoparticles in an ex vivo lung model. Int. J. Pharm. 2009, 367, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Bivas-Benita, M.; Oudshoorn, M.; Romeijn, S.; van Meijgaarden, K.; Koerten, H.; van der Meulen, H.; Lambert, G.; Ottenhoff, T.; Benita, S.; Junginger, H. Cationic submicron emulsions for pulmonary DNA immunization. J. Control. Release 2004, 100, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Fu, S.; Peng, Q.; Han, Y.; Xie, J.; Zan, N.; Chen, Y.; Fan, J. Paclitaxel-loaded polymeric nanoparticles combined with chronomodulated chemotherapy on lung cancer: In vitro and in vivo evaluation. Int. J. Pharm. 2017, 516, 313–322. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Zeng, X.; Guo, W.; Jin, Y.; Wang, S.; Tian, R.; Han, Y.; Guo, L.; Han, J. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm. Sin. B 2019, 9, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Sivarajakumar, R.; Mallukaraj, D.; Kadavakollu, M.; Neelakandan, N.; Chandran, S.; Bhojaraj, S.; Karri, V.V.S.R. Nanoparticles for the treatment of lung cancers. J. Young Pharm. 2018, 10, 276. [Google Scholar] [CrossRef]
- Perepelyuk, M.; Sacko, K.; Thangavel, K.; Shoyele, S.A. Evaluation of MUC1-aptamer functionalized hybrid nanoparticles for targeted delivery of miRNA-29b to nonsmall cell lung cancer. Mol. Pharm. 2018, 15, 985–993. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Riely, G.J.; Azzoli, C.G.; Miller, V.A.; Ng, K.K.; Fiore, J.; Chia, G.; Brower, M.; Heelan, R.; Hawkins, M.J. Phase I/II trial of weekly intravenous 130-nm albumin-bound paclitaxel as initial chemotherapy in patients with stage IV non–small-cell lung cancer. J. Clin. Oncol. 2008, 26, 639–643. [Google Scholar] [CrossRef]
- Mukherjee, A.; Paul, M.; Mukherjee, S. Recent progress in the theranostics application of nanomedicine in lung cancer. Cancers 2019, 11, 597. [Google Scholar] [CrossRef]
- Knights, O.B.; McLaughlan, J.R. Gold nanorods for light-based lung cancer theranostics. Int. J. Mol. Sci. 2018, 19, 3318. [Google Scholar] [CrossRef]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef]
- Sarkar, S.; Osama, K.; Mohammad Sajid Jamal, Q.; Amjad Kamal, M.; Sayeed, U.; Khan, K.A.; Siddiqui, H.; Akhtar, S. Advances and implications in nanotechnology for lung cancer management. Curr. Drug Metab. 2017, 18, 30–38. [Google Scholar] [CrossRef]
- Madni, A.; Batool, A.; Noreen, S.; Maqbool, I.; Rehman, F.; Kashif, P.M.; Tahir, N.; Raza, A. Novel nanoparticulate systems for lung cancer therapy: An updated review. J. Drug Target. 2017, 25, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Pandey, A.; Vighetto, V.; Di Marzio, N.; Ferraro, F.; Hirsch, M.; Ferrante, N.; Mitra, S.; Grattoni, A.; Filgueira, C.S. Gold Nanoparticles Radio-Sensitize and Reduce Cell Survival in Lewis Lung Carcinoma. Nanomaterials 2020, 10, 1717. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, X.; Wang, Y.; Liu, Z.; Fu, L.; Hu, L. Enhancement of radiation effect and increase of apoptosis in lung cancer cells by thio-glucose-bound gold nanoparticles at megavoltage radiation energies. J. Nanoparticle Res. 2013, 15, 1–12. [Google Scholar] [CrossRef]
- Zhuang, M.; Jiang, S.; Gu, A.; Chen, X.; Mingyan, E. Radiosensitizing effect of gold nanoparticle loaded with small interfering RNA-SP1 on lung cancer: AuNPs-si-SP1 regulates GZMB for radiosensitivity. Transl. Oncol. 2021, 14, 101210. [Google Scholar] [CrossRef]
- Boateng, F.; Ngwa, W. Delivery of nanoparticle-based radiosensitizers for radiotherapy applications. Int. J. Mol. Sci. 2019, 21, 273. [Google Scholar] [CrossRef]
- Iyer, R.; Ramachandramoorthy, H.; Nguyen, T.; Xu, C.; Fu, H.; Kotadia, T.; Chen, B.; Hong, Y.; Saha, D.; Nguyen, K.T. Lung Cancer Targeted Chemoradiotherapy via Dual-Stimuli Responsive Biodegradable Core-Shell Nanoparticles. Pharmaceutics 2022, 14, 1525. [Google Scholar] [CrossRef]
- Hao, Y.; Altundal, Y.; Moreau, M.; Sajo, E.; Kumar, R.; Ngwa, W. Potential for enhancing external beam radiotherapy for lung cancer using high-Z nanoparticles administered via inhalation. Phys. Med. Biol. 2015, 60, 7035. [Google Scholar] [CrossRef]
- Hauser, A.K.; Mitov, M.I.; Daley, E.F.; McGarry, R.C.; Anderson, K.W.; Hilt, J.Z. Targeted iron oxide nanoparticles for the enhancement of radiation therapy. Biomaterials 2016, 105, 127–135. [Google Scholar] [CrossRef]
- Jiang, J.; Mao, Q.; Li, H.; Lou, J. Apigenin stabilized gold nanoparticles increased radiation therapy efficiency in lung cancer cells. Int. J. Clin. Exp. Med. 2017, 10, 13298–13305. [Google Scholar]
- Cruz, L.Y.; Wang, D.; Liu, J. Biosynthesis of selenium nanoparticles, characterization and X-ray induced radiotherapy for the treatment of lung cancer with interstitial lung disease. J. Photochem. Photobiol. B Biol. 2019, 191, 123–127. [Google Scholar] [CrossRef]
- Menon, J.U.; Kuriakose, A.; Iyer, R.; Hernandez, E.; Gandee, L.; Zhang, S.; Takahashi, M.; Zhang, Z.; Saha, D.; Nguyen, K.T. Dual-drug containing core-shell nanoparticles for lung cancer therapy. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reda, M.; Ngamcherdtrakul, W.; Gu, S.; Bejan, D.S.; Siriwon, N.; Gray, J.W.; Yantasee, W. PLK1 and EGFR targeted nanoparticle as a radiation sensitizer for non-small cell lung cancer. Cancer Lett. 2019, 467, 9–18. [Google Scholar] [CrossRef]
- Du, Y.; Sun, H.; Lux, F.; Xie, Y.; Du, L.; Xu, C.; Zhang, H.; He, N.; Wang, J.; Liu, Y. Radiosensitization effect of AGuIX, a gadolinium-based nanoparticle, in nonsmall cell lung cancer. ACS Appl. Mater. Interfaces 2020, 12, 56874–56885. [Google Scholar] [CrossRef]
- Tian, J.; Wei, X.; Zhang, W.; Xu, A. Effects of selenium nanoparticles combined with radiotherapy on lung cancer cells. Front. Bioeng. Biotechnol. 2020, 8, 598997. [Google Scholar] [CrossRef]
- Li, Y.; Yun, K.-H.; Lee, H.; Goh, S.-H.; Suh, Y.-G.; Choi, Y. Porous platinum nanoparticles as a high-Z and oxygen generating nanozyme for enhanced radiotherapy in vivo. Biomaterials 2019, 197, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Werner, M.E.; Cummings, N.D.; Sethi, M.; Wang, E.C.; Sukumar, R.; Moore, D.T.; Wang, A.Z. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 463–468. [Google Scholar] [CrossRef]
- Hu, Y.; Paris, S.; Barsoumian, H.; Abana, C.O.; He, K.; Wasley, M.; Younes, A.I.; Masrorpour, F.; Chen, D.; Yang, L. Radiation therapy enhanced by NBTXR3 nanoparticles overcomes anti-PD1 resistance and evokes abscopal effects. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 647–657. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, W.; Wang, R.; Hopkins, S.P.; Spagnoli, J.C.; Racin, M.; Bai, L.; Li, L.; Jiang, W.; Yang, X. Nanoparticles encapsulating nitrosylated maytansine to enhance radiation therapy. ACS Nano 2020, 14, 1468–1481. [Google Scholar] [CrossRef]
- Mokwena, M.G.; Kruger, C.A.; Ivan, M.-T.; Heidi, A. A review of nanoparticle photosensitizer drug delivery uptake systems for photodynamic treatment of lung cancer. Photodiagnosis Photodyn. Ther. 2018, 22, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, P.; Sharmiladevi, P.; Haribabu, V.; Girigoswami, K.; Girigoswami, A. A Nano Approach to Formulate Photosensitizers for Photodynamic Therapy. Curr. Nanosci. 2022, 18, 675–689. [Google Scholar]
- Chen, M.-H.; Hanagata, N.; Ikoma, T.; Huang, J.-Y.; Li, K.-Y.; Lin, C.-P.; Lin, F.-H. Hafnium-doped hydroxyapatite nanoparticles with ionizing radiation for lung cancer treatment. Acta Biomater. 2016, 37, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, H.; Ding, L.; Fan, R.; Kato, T.; Lee, D.; Fujino, K.; Kinoshita, T.; Lee, C.Y.; Waddell, T.K.; Keshavjee, S. Porphyrin–high-density lipoprotein: A novel photosensitizing nanoparticle for lung cancer therapy. Ann. Thorac. Surg. 2019, 107, 369–377. [Google Scholar] [CrossRef]
- Yan, L.; Shen, J.; Wang, J.; Yang, X.; Dong, S.; Lu, S. Nanoparticle-based drug delivery system: A patient-friendly chemotherapy for oncology. Dose-Response 2020, 18, 1559325820936161. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, J.; Lu, X.; He, X. Nanoparticle systems reduce systemic toxicity in cancer treatment. Nanomedicine 2016, 11, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Sharmiladevi, P.; Girigoswami, K.; Haribabu, V.; Girigoswami, A. Nano-enabled theranostics for cancer. Mater. Adv. 2021, 2, 2876–2891. [Google Scholar] [CrossRef]
- Amsaveni, G.; Farook, A.S.; Haribabu, V.; Murugesan, R.; Girigoswami, A. Engineered multifunctional nanoparticles for DLA cancer cells targeting, sorting, MR imaging and drug delivery. Adv. Sci. Eng. Med. 2013, 5, 1340–1348. [Google Scholar] [CrossRef]
- Dracham, C.B.; Shankar, A.; Madan, R. Radiation induced secondary malignancies: A review article. Radiat. Oncol. J. 2018, 36, 85. [Google Scholar] [CrossRef]
- Han, C.; Wu, Y.; Kang, K.; Wang, Z.; Liu, Z.; Zhang, F. Long-term radiation therapy-related risk of second primary malignancies in patients with lung cancer. J. Thorac. Dis. 2021, 13, 5863. [Google Scholar] [CrossRef]
- Wennstig, A.-K.; Wadsten, C.; Garmo, H.; Johansson, M.; Fredriksson, I.; Blomqvist, C.; Holmberg, L.; Nilsson, G.; Sund, M. Risk of primary lung cancer after adjuvant radiotherapy in breast cancer—A large population-based study. NPJ Breast Cancer 2021, 7, 71. [Google Scholar] [CrossRef] [PubMed]
- Shruthi, N.; Samatha Jain, M.; Ganesan, H.; Banerjee, A.; Zhang, H.; Sun, X.-F.; Pathak, S. Drug Repurposing in Cancer. In Drug Repurposing for Emerging Infectious Diseases and Cancer; Springer: Berlin/Heidelberg, Germany, 2023; pp. 159–179. [Google Scholar]




| Nanomaterials Used | Drugs Formulated | Company | Disease Indications | Route | Status |
|---|---|---|---|---|---|
| Liposomes | Dox | Ortho Biotech | Antineoplastic | IV | Approved/1995 |
| Daunorubicin | Diatos | Antineoplastic | IV | Approved/1996 | |
| Cisplatin | Alza | Lung cancer | IV | Phase-III trail | |
| Paclitaxel | Neopharma | Lung cancer | IV | Phase-II trial | |
| BLP 25 | Lung cancer | IV | Phase-II trial | ||
| Polymer | Interferon α-2a | Genentech | Hepatitis C | IV | Approved/2002 |
| Interferon α-2b | Merck | Hepatitis C | IV | Approved/2001 | |
| Leuprolide acetate | Fierce Parma | Prostate cancer | IV | Approved/2002 | |
| Dox | Breast cancer, adenocarcinoma of the esophagus | IV | Phase-III/I trial | ||
| Metallic | Ferumoxtran-10 | Advanced Mag. | Tumor imaging | IV | Filed |
| TNF-α | Cyt Immune Sci. | Solid tumor | IV | Phase-I trial | |
| Porfimer | Concordia Labs | Lung cancer | IV | Approved/1995 | |
| Dox | - | Solid tumor | IV | Phase-I trial | |
| Paclitaxel | - | Gastric and colon cancer | IV | Phase-I trial |
| Nanoparticle Used | Cell Line/Cancer Model Used | Summary of the Study | Reference |
|---|---|---|---|
| Cisplatin nanoparticles (CNPs), gold nanoparticles, (GNPs), and carboplatin nanoparticles (CBNPs) | An analytical method was used to estimate the dose enhancement to lung tumors due to radiation-induced photoelectrons generated by NPs administered via inhalation (IR) versus intravenous (IV) administration, based on Monte Carlo-generated megavoltage energy spectra. In this model, the tumor voxel is sized 10 μm × 10 μm × 10 μm. It was assumed that nanoparticles were distributed evenly within the tumor subvolume, as indicated by this model. Under the conditions of different drug concentrations, the number of nanoparticles or concentrations was determined. | A range of nanoparticle concentrations and tumor sizes were considered in order to calculate the dose enhancement factor (DEF), which was defined as the ratio of the radiotherapy dose with and without nanoparticles. A comparison was then made between the DEF for IR and IV. The results of these experimental studies indicated that IR could deliver 3.5–14.6 times higher NP concentrations to the lungs than IV. Based on the results of this study, IR administration of targeted high-Z CNPs/CBNPs/GNPs could significantly reduce lung tumor growth compared with IV administration during external beam radiotherapy. | [110] |
| Iron oxide nanoparticles, TAT (transactivator of transcription)-conjugated iron oxide nanoparticles | A549 cells (lung cancer cells) | A cell penetrating peptide, TAT, was conjugated to iron oxide nanoparticles in this project to escape lysosomal encapsulation after internalization by cancer cells and catalyze hydroxyl radical production. A TAT functionalized iron oxide nanoparticle as well as an uncoated iron oxide nanoparticle permeabilized lysosomal membranes. TAT-functionalized nanoparticles and radiation also compromised mitochondrial integrity in A549 cells. A significant increase in ROS generation was also observed when TAT-functionalized nanoparticles were pre-treated with radiation. The combination of TAT-functionalized nanoparticles and radiation had a synergistic effect on long-term viability. Since the nanoparticles alone did not cause significant toxicity, it is likely that TAT functionalized nanoparticles sensitized the cells to radiation therapy. | [111] |
| Apigenin stabilized gold nanoparticles (AuNPs) | A549 cells (lung cancer cells) | In this study, gold nanoparticles stabilized by apigenin were used for in vitro cancer treatment with chemotherapy and enhanced radiotherapy. Cell apoptosis, proliferation inhibition, and arrest in G0/G1 phases were observed as a result of nanoparticle interaction with lung cancer cells (A549). In a study using X-rays and nanoparticles together, it was found to generate an additive anti-cancer effect as a result of the chemotherapeutic functions of apigenin, as well as the enhanced radiation killing effect caused by the interaction between the nanoparticles and the X-rays. | [112] |
| Selenium nanoparticles (SeNPs) | A549 cells (lung cancer cells) and IMR-90 normal fibroblast cells | Biosynthesized and characterized selenium nanoparticles were applied to the treatment of cancer cells (A549 cells) and normal cells (IMR-90 cells). Under the influence of X-rays, selenium nanoparticles were tested for their radio-sensitizing effect against cancer as well as healthy cells. A combination of SeNPs and X-rays was found to be cytotoxic to lung cancer cells in this study. | [113] |
| A folate receptor-targeting multifunctional dual drug-loaded nanoparticle (MDNP) containing a poly(N-isopropylacrylamide)-carboxymethyl chitosan shell and poly lactic-coglycolic acid (PLGA) core | A549 and H460 lung cancer cells | To treat lung cancer effectively, researchers developed a multifunctional dual drug-loaded nanoparticle (MDNP) that targets folate receptors with a poly(N-isopropylacrylamide)-carboxymethyl chitosan shell and polylactic-coglycolic acid (PLGA) core containing a poly(N-isopropylacrylamide)-carboxymethyl chitosan shell. This formulation provided controlled release of the encapsulated radiosensitizer NU7441 and the FDA-approved chemotherapy drug gemcitabine, which is used in lung cancer chemoradiation therapy. According to these results, MDNPs have the potential to be used as nanovehicles for the chemoradiation sensitization of lung cancer at the same time. | [114] |
| Mesoporous silica nanoparticle (MSNP) with surface functionalization. | NSCLC cells A549 (CCL-185) and H460 (HTB-177) | The researchers developed a new targeted therapy for NSCLC based on cetuximab-conjugated nanoparticles that deliver small interfering RNA (siRNA) against polo-like kinase 1 (PLK1). PLK1 is a key mitotic regulator whose inhibition improves radiation sensitivity, while EGFR is overexpressed in 50% of lung cancer patients. In this study, a nanoparticle construct called C-siPLK1-NP was used to target EGFR+ NSCLC cells and reduce the expression of PLK1, resulting in cell death and G2/M arrest. They found that C-siPLK1-NP is an effective targeted therapy as well as a potent radiation sensitizer for NSCLC. | [115] |
| Gadolinium-based nanoparticle, AGuIX | H1299 NSCLC cell line | An efficient radiosensitizer based on gadolinium, called AGuIX, was developed for magnetic resonance imaging-guided radiotherapy. It appears that low-energy photoelectrons and Auger electron interactions are responsible for the amplified radiation effects of AGuIX nanoparticles. This study in H1299 NSCLC cells demonstrated that AGuIX nanoparticles enhanced radiation-induced DNA double-strand breaks and slowed DNA repair. In addition, researchers found that the AGuIX nanoparticles significantly exacerbated tumor cell damage, under radiation therapy, in an H1299 mouse xenograft model. | [116] |
| Selenium nanoparticles (nano-Se) | A549 and NCI-H23 cells | A549 and NCI-H23 cells were treated with selenium nanoparticles (nano-Se) and radiotherapy to study the effects on proliferation, invasion, migration, and apoptosis. Increased nano-Se concentration increased the uptake of nano-Se in lung cancer cells. In combination with radiotherapy, nano-Se decreased the proliferation activity of NSCLC cell lines A549 and NCI-H23 (all p < 0.05). The outcome of this study indicated that nano-Se might also be used in clinical lung cancer treatment as a radiosensitizer. | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girigoswami, A.; Girigoswami, K. Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment. Genes 2023, 14, 1370. https://doi.org/10.3390/genes14071370
Girigoswami A, Girigoswami K. Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment. Genes. 2023; 14(7):1370. https://doi.org/10.3390/genes14071370
Chicago/Turabian StyleGirigoswami, Agnishwar, and Koyeli Girigoswami. 2023. "Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment" Genes 14, no. 7: 1370. https://doi.org/10.3390/genes14071370
APA StyleGirigoswami, A., & Girigoswami, K. (2023). Potential Applications of Nanoparticles in Improving the Outcome of Lung Cancer Treatment. Genes, 14(7), 1370. https://doi.org/10.3390/genes14071370






