L138ins Variant of the CFTR Gene in Russian Infertile Men
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dickinson, K.M.; Collaco, J.M. Cystic Fibrosis. Pediatr. Rev. 2021, 42, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Claustres, M. Molecular Pathology of the CFTR Locus in Male Infertility. Reprod. Biomed. Online 2005, 10, 14–41. [Google Scholar] [CrossRef] [PubMed]
- Bieth, E.; Hamdi, S.M.; Mieusset, R. Genetics of the Congenital Absence of the Vas Deferens. Hum. Genet. 2021, 140, 59–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bieniek, J.M.; Lapin, C.D.; Jarvi, K.A. Genetics of CFTR and Male Infertility. Transl. Androl. Urol. 2021, 10, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Chernykh, V.B.; Stepanova, A.A.; Beskorovainaya, T.S.; Sorokina, T.M.; Shileiko, L.V.; Kurilo, L.F.; Polyakov, A.V. The Frequency and Spectrum of Mutations and the IVS8-T Polymorphism of the CFTR Gene in Russian Infertile Men. Russ. J. Genet. 2010, 46, 750–757. [Google Scholar] [CrossRef]
- Solovyova, E.V.; Tataru, D.A.; Preda, O.G.; Artyukhova, V.G.; Sekira, A.G.; Derevjeva, V.Y.; Makhalova, N.A.; Novoseltseva, A.V.; Rendashkin, I.V.; Zaitseva, T.A.; et al. CFTR Mutations in Male Infertility. Med. Genet. 2018, 17, 28–38. [Google Scholar] [CrossRef]
- Sedova, A.; Shtaut, M.; Bragina, E.; Sorokina, T.; Shmarina, G.; Andreeva, M.; Kurilo, L.; Krasovskiy, S.; Polyakov, A.; Chernykh, V. Comprehensive Semen Examination in Patients with Pancreatic-Sufficient and Pancreatic-Insufficient Cystic Fibrosis. Asian J. Androl. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Dörk, T.; Dworniczak, B.; Aulehla-Scholz, C.; Wieczorek, D.; Böhm, I.; Mayerova, A.; Seydewitz, H.H.; Nieschlag, E.; Meschede, D.; Horst, J.; et al. Distinct Spectrum of CFTR Gene Mutations in Congenital Absence of Vas Deferens. Hum. Genet. 1997, 100, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wen, Q.; Li, H.; Zhao, L.; Zhang, X.; Wang, J.; Cheng, L.; Yang, J.; Chen, S.; Ma, X.; et al. Mutations in the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in Chinese Patients with Congenital Bilateral Absence of Vas Deferens. J. Cyst. Fibros. 2012, 11, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levkova, M.; Chervenkov, T.; Hachmeriyan, M.; Angelova, L. CFTR Gene Variants as a Reason for Impaired Spermatogenesis: A Pilot Study and a Meta-Analysis of Published Data. Hum. Fertil. 2022, 25, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Kondratyeva, E.I.; Voronkova, A.Y.; Kashirskaya, N.Y.; Krasovsky, S.A.; Starinova, M.A.; Amelina, E.L.; Avdeev, S.N.; Kutsev, S.I. Russian Registry of Patients with Cystic Fibrosis: Lessons and Perspectives. Pulmonologiya 2023, 33, 171–181. [Google Scholar] [CrossRef]
- Stepanova, A.A.; Krasovsky, S.A.; Polyakov, A.V. Reliability of the Search for 19 Common Mutations in the CFTR Gene in Russian Cystic Fibrosis Patients and the Calculated Frequency of the Disease in Russian Federation. Russ. J. Genet. 2016, 52, 204–213. [Google Scholar] [CrossRef]
- Petrova, N.V.; Marakhonov, A.V.; Vasilyeva, T.A.; Kashirskaya, N.Y.; Ginter, E.K.; Kutsev, S.I.; Zinchenko, R.A. Comprehensive Genotyping Reveals Novel CFTR Variants in Cystic Fibrosis Patients from the Russian Federation. Clin. Genet. 2019, 95, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.V.; Kashirskaya, N.Y.; Vasilyeva, T.A.; Kondratyeva, E.I.; Zhekaite, E.K.; Voronkova, A.Y.; Sherman, V.D.; Galkina, V.A.; Ginter, E.K.; Kutsev, S.I.; et al. Analysis of CFTR Mutation Spectrum in Ethnic Russian Cystic Fibrosis Patients. Genes 2020, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Petrova, N.; Balinova, N.; Marakhonov, A.; Vasilyeva, T.; Kashirskaya, N.; Galkina, V.; Ginter, E.; Kutsev, S.; Zinchenko, R. Ethnic Differences in the Frequency of CFTR Gene Mutations in Populations of the European and North Caucasian Part of the Russian Federation. Front. Genet. 2021, 12, 678374. [Google Scholar] [CrossRef] [PubMed]
- McGinniss, M.J.; Chen, C.; Redman, J.B.; Buller, A.; Quan, F.; Peng, M.; Giusti, R.; Hantash, F.M.; Huang, D.; Sun, W.; et al. Extensive Sequencing of the CFTR Gene: Lessons Learned from the First 157 Patient Samples. Hum. Genet. 2005, 118, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Marnat, E.G.; Adyan, T.A.; Stepanova, A.A.; Beskorovainaya, T.S.; Polyakov, A.V.; Chernykh, V.B. CFTR Gene Variants and Genotypes in Russian Patients with CBAVD Syndrome. Russ. J. Genet. 2020, 56, 496–501. [Google Scholar] [CrossRef]
- WHO. Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO: Geneva, Switzerland, 2021.
- Petrova, N.V.; Kashirskaya, N.Y.; Vasilyeva, T.A.; Voronkova, A.Y.; Kondratieva, E.I.; Sherman, V.D.; Novoselova, O.G.; Krasovskiy, S.A.; Chernyak, A.V.; Amelina, E.L.; et al. Phenotypic Features in Patients with Cystic Fibrosis with L138ins (p.Leu138dup) Mutation. Pediatr.—Zhurnal Im. G.N. Speranskogo 2017, 96, 64–72. [Google Scholar] [CrossRef]
- Shadrina, V.; Krasovsky, S.; Kondratyeva, E.; Furman, E. P033 Epidemiological Features “Middle—Ural” Variant L138ins in the CFTR Gene at Cystic Fibrosis in Russia. J. Cyst. Fibros. 2020, 19, S64. [Google Scholar] [CrossRef]
- Kiseleva, A.; Klimushina, M.; Sotnikova, E.; Skirko, O.; Divashuk, M.; Kurilova, O.; Ershova, A.; Khlebus, E.; Zharikova, A.; Efimova, I.; et al. Cystic Fibrosis Polymorphic Variants in a Russian Population. Pharmgenom. Pers. Med. 2020, 13, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, A.V.; Klimushina, M.V.; Sotnikova, E.A.; Divashuk, M.G.; Ershova, A.I.; Skirko, O.P.; Kurilova, O.V.; Zharikova, A.A.; Khlebus, E.Y.; Efimova, I.A.; et al. A Data-Driven Approach to Carrier Screening for Common Recessive Diseases. J. Pers. Med. 2020, 10, 140. [Google Scholar] [CrossRef] [PubMed]
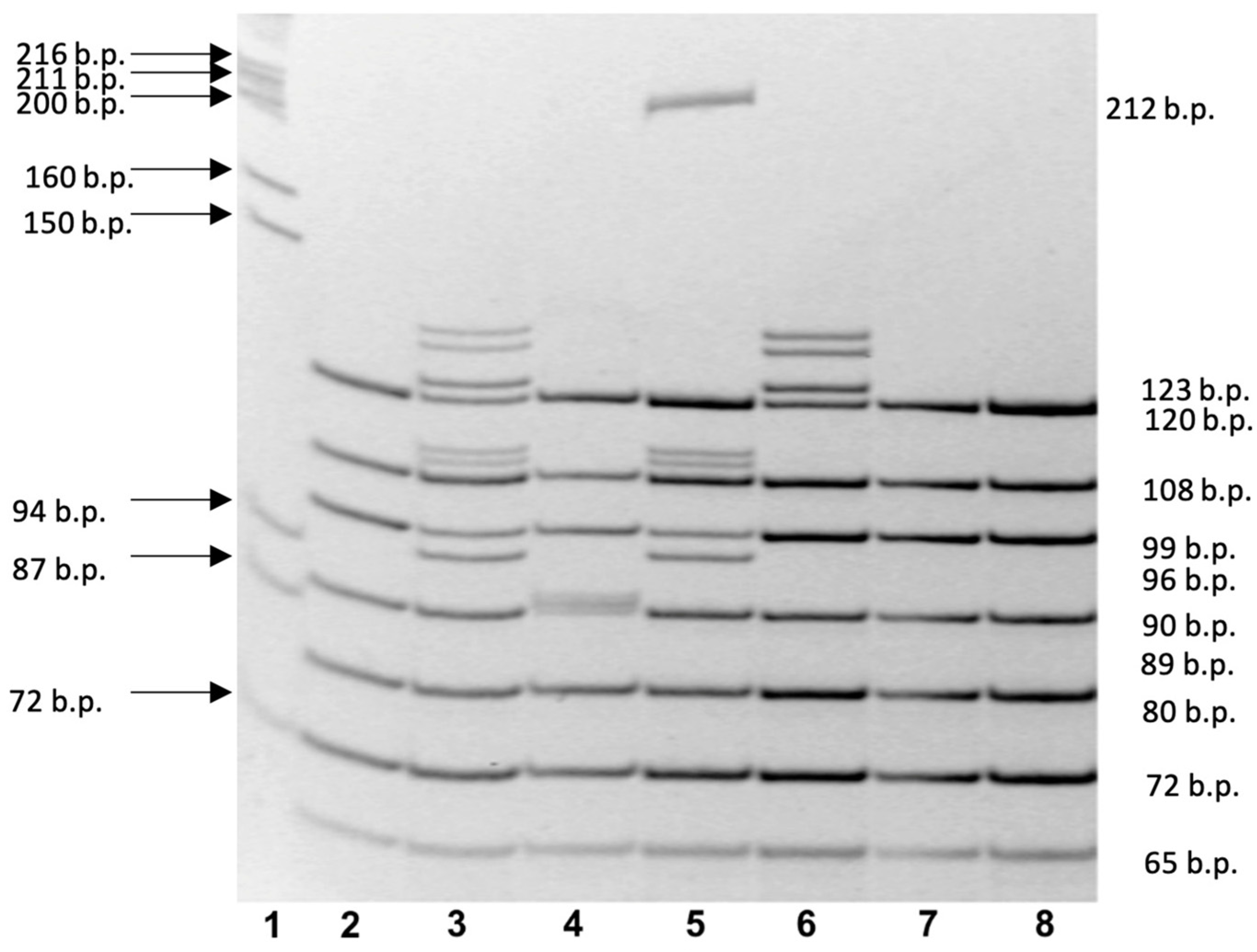
| CFTR Gene Variant * | Length of Normal DNA Fragment (b.p.) | Length of Abnormal DNA Fragment in the CFTR Gene Variant (b.p.) |
|---|---|---|
| c.54-5940_273+10250del21kb (CFTRdele2,3) * | No fragment | 212 |
| c.413_415dupTAC (L138ins) | 120 | 123 |
| c.3816_3817delGT (3944delGT) | 108 | 106 |
| c.1521_1523delCTT (F508del) c.1545_1546delTA (1677delTA) | 99 | 96 97 |
| c.2012delT (2143delT), c.2051_2052delAAinsG (2183AA>G) (c.2052dupA (2184insA)) | 89 | 88 90 |
| c.262_263delTT (394delTT) | 80 | 78 |
| c.3691delT (3821delT) | 72 | 71 |
| c.472_473insA (604insA) | 65 | 66 |
| CFTR Gene Variant * | Number of Alleles, Carrying This Variant, n | Allele Frequency (AF) | The Percentage of Variant among All Detected CFTR Gene Variants, % |
|---|---|---|---|
| F508del | 183 1 | 0.01517 | 61.00 |
| CFTRdele2.3(21kb) | 20 | 0.00166 | 6.67 |
| L138ins | 18 2 | 0.00149 | 6.00 |
| W1282X | 16 | 0.00133 | 5.33 |
| 1677delTA | 8 | 0.00066 | 2.67 |
| 3849+10kbC>T | 8 3 | 0.00066 | 2.67 |
| E92K | 8 4 | 0.00066 | 2.67 |
| 2143delT | 7 | 0.00058 | 2.33 |
| G542X | 6 | 0.00049 | 2.00 |
| 2184insA | 6 5 | 0.00049 | 2.00 |
| N1303K | 4 6 | 0.00033 | 1.33 |
| R334W | 4 | 0.00033 | 1.33 |
| 3821delT | 2 | 0.00017 | 0.67 |
| 2183AA>G (Lys684Ser) | 2 | 0.00017 | 0.67 |
| 3242-26A>G | 1 | 0.00008 | 0.33 |
| 4015delA | 1 | 0.00008 | 0.33 |
| 604insA | 1 | 0.00008 | 0.33 |
| 621+1G>T | 1 | 0.00008 | 0.33 |
| G551D | 1 | 0.00008 | 0.33 |
| G85E | 1 | 0.00008 | 0.33 |
| dup 7-11 | 1 | 0.00008 | 0.33 |
| S1196X | 1 | 0.00008 | 0.33 |
| CFTR Genotype * | Number of Patients, n | CBAVD, Obstructive Azoospermia | Extragenital Signs of CF ** |
|---|---|---|---|
| L138ins/N, 5T/7T | 1 | + | - |
| L138ins/N, 7T/7T | 8 | - | - |
| L138ins/N, 7T/9T | 2 | - | - |
| L138ins/L138ins, 7T/7T | 1 | + | + |
| L138ins/N1303K, 7T/9T | 1 | + | + |
| F508del/L138ins, 9T/7T | 4 | + | + |
| CFTR Gene Variant * | Coding DNA Name | Protein Name | rsID | Allele Frequency (AF) ** |
|---|---|---|---|---|
| F508del | c.1521_1523delCTT | p.(Phe508del) | rs113993960 | 0.5261 |
| CFTRdele2,3 | c.54-5940_273+10250 del21kb | p.(Ser18Argfs*16) | not found | 0.0615 |
| E92K | c.274G>A | p.(Glu92Lys) | rs121908751 | 0.0325 |
| 1677delTA | c.1545_1546delTA | p.(Tyr515*) | rs121908776 | 0.0212 |
| 3849+10kbC>T | c.3718-2477C>T | No protein name | rs75039782 | 0.0211 |
| 2143delT | c.2012delT | p.(Leu671*) | rs121908812 | 0.0202 |
| 2184insA | c.2052_2053insA*(c.2052dupA) | p.(Gln685Thrfs*4) | rs121908786 | 0.0193 |
| W1282X | c.3846G>A | p.(Trp1282*) | rs77010898 | 0.0173 |
| L138ins | c.413_415dupTAC | p.(Leu138dup) | rs397508679 | 0.0153 |
| N1303K | c.3909C>G | p.(Asn1303Lys) | rs80034486 | 0.0153 |
| G542X | c.1624G>T | p.(Gly542*) | rs113993959 | 0.0146 |
| 394delTT | c.262_263delTT | p.(Leu88Ilefs*22) | rs121908769 | 0.0085 |
| R334W | c.1000C>T | p.(Arg334Trp) | rs121909011 | 0.0074 |
| *S466X | c.1397C>G | p.(Ser466*) | rs121908805 | 0.0059 |
| W1282R | c.3844T>C | p.(Trp1282Arg) | rs397508616 | 0.0055 |
| 3821delT | c.3691delT | p.(Ser1231Profs*4) | rs121908783 | 0.0051 |
| S1196X | c.3587C>G | p.(Ser1196*) | rs121908763 | 0.0045 |
| 1367del5 | c.1240_1244delCAAAA (c.1243_1247delAACAA) | p.(Asn415*) | rs397508184 | 0.0040 |
| 2789+5G>A | c.2657+5G>A | No protein name | rs80224560 | 0.0038 |
| R1066C | c.3196C>T | p.(Arg1066Cys) | rs78194216 | 0.0038 |
| 3272-16T>A | c.3140-16T>A | No protein name | rs767232138 | 0.0033 |
| W1310X | c.3929G>A | p.(Trp1310*) | not found | 0.0032 |
| 3944delGT | c.3816_3817delGT | p.(Ser1273Leufs*28) | rs397508612 | 0.0029 |
| 712-1G>T | c.580-1G>T | No protein name | rs121908793 | 0.0022 |
| 621+1G>T | c.489+1G>T | No protein name | rs78756941 | 0.0020 |
| R553X | c.1657C>T | p.(Arg553*) | rs74597325 | 0.0020 |
| 4015delA | c.3883delA | p.(Ile1295Phefs*33) | rs397508630 | 0.0017 |
| L1335P | c.4004T>C | p.(Leu1335Pro) | rs397508658 | 0.0017 |
| R785X | c.2353C>T | p.(Arg785*) | rs374946172 | 0.0017 |
| R1162X | c.3484C>T | p.(Arg1162*) | rs74767530 | 0.0016 |
| 1898+1G>C | c.1766+1G>A | No protein name | rs121908748 | 0.0014 |
| CFTRdup7-11 (6b-10*) | c.(743+1_744-1)_(1584+1_1585-1)dup | No protein name | not found | 0.0014 |
| 1898+1G>A | c.1766+1G>C | No protein name | rs121908748 | 0.0013 |
| R347P | c.1040G>C | p.Arg347Pro | rs77932196 | 0.0013 |
| 3849G>A | c.3717G>A | No protein name | rs144781064 | 0.0012 |
| G85E | c.254G>A | p.Gly85Glu | rs75961395 | 0.0012 |
| S1159F | c.3476C>T | p.(Ser1159Phe) | rs397508573 | 0.0012 |
| 3667ins4 | c.3535_3536insTCAA (c.3532_3535dupTCAA) | p.(Thr1179Ilefs*17) | rs387906378 | 0.0012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernykh, V.; Sorokina, T.; Sedova, A.; Shtaut, M.; Solovova, O.; Marnat, E.; Adyan, T.; Beskorovaynaya, T.; Stepanova, A.; Shchagina, O.; et al. L138ins Variant of the CFTR Gene in Russian Infertile Men. Genes 2023, 14, 1407. https://doi.org/10.3390/genes14071407
Chernykh V, Sorokina T, Sedova A, Shtaut M, Solovova O, Marnat E, Adyan T, Beskorovaynaya T, Stepanova A, Shchagina O, et al. L138ins Variant of the CFTR Gene in Russian Infertile Men. Genes. 2023; 14(7):1407. https://doi.org/10.3390/genes14071407
Chicago/Turabian StyleChernykh, Vyacheslav, Tatyana Sorokina, Anna Sedova, Maria Shtaut, Olga Solovova, Ekaterina Marnat, Tagui Adyan, Tatyana Beskorovaynaya, Anna Stepanova, Olga Shchagina, and et al. 2023. "L138ins Variant of the CFTR Gene in Russian Infertile Men" Genes 14, no. 7: 1407. https://doi.org/10.3390/genes14071407







