The Responses of Alternative Splicing during Heat Stress in the Pacific White Shrimp Litopenaeus vannamei
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and AS Event Identification
2.2. Transcriptome Assembly and Differential Expression Analysis
2.3. Gene Function Enrichment Analysis
3. Results
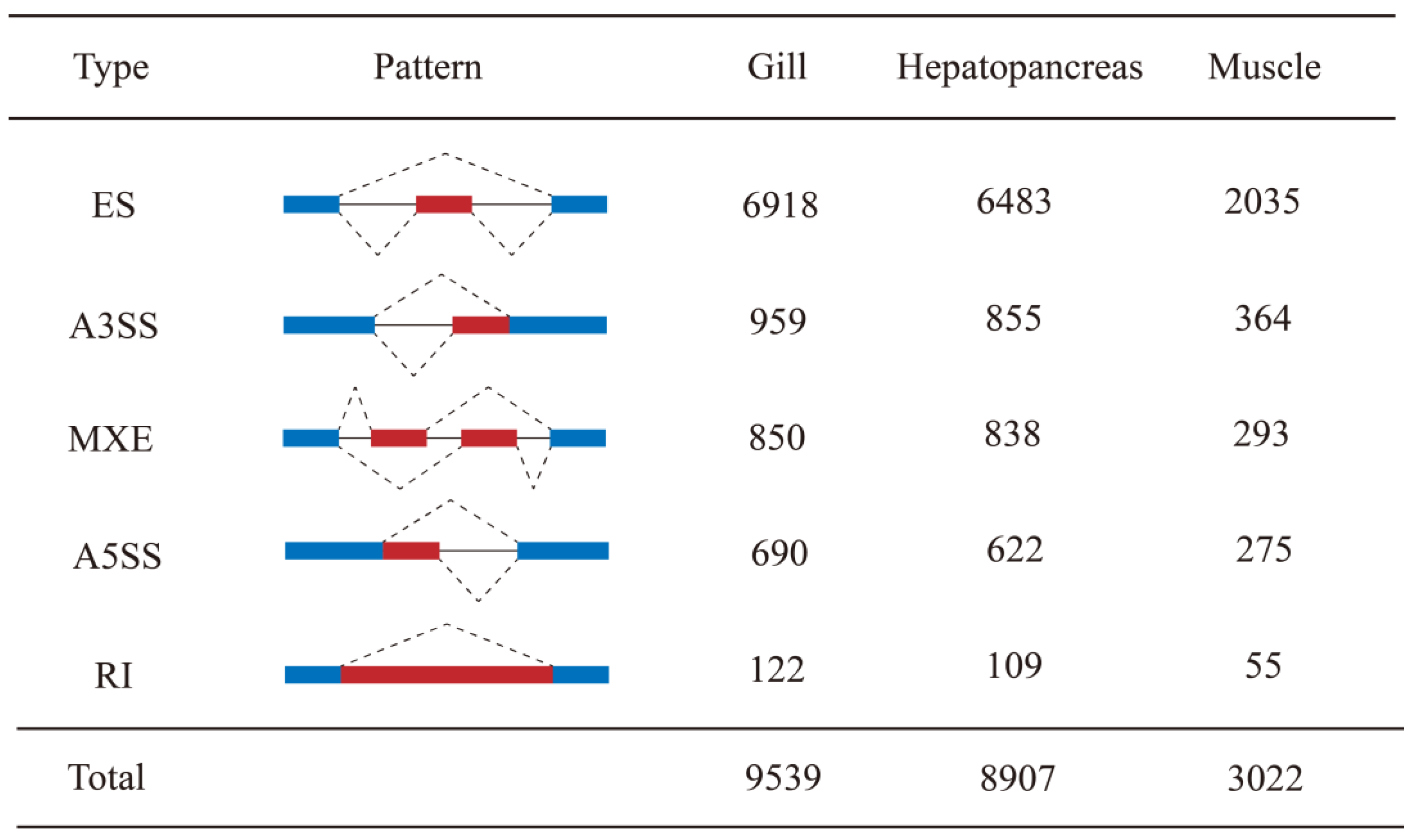
3.1. Overview of AS Events
3.2. Identification of DAS Events and Their Related Pathways under Heat Stress
3.3. Different Tissues Possessed Distinct AS Regulation Patterns under Heat Stress
3.4. Heat Stress Prefered Longer Isoforms Occurring A3SS
3.5. Regulation of AS and Transcription in Response to Heat Stress
3.6. The Effect of AS on Gene Function under Heat Shock
4. Discussion
4.1. The Effect of Heat Stress on the AS Profile of Shrimp
4.2. The Self-Alternative Splice of Splicing Factors and RNA-Binding Proteins Plays a Crucial Role in Conferring Heat Resistance
4.3. Separate Regulatory Mechanisms of AS and Transcription in Response to Heat Stress
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang, C.-Y.; Lin, W.-D.; Tu, S.-L. Genome-wide analysis of heat-sensitive alternative splicing in Physcomitrella patens. Plant Physiol. 2014, 165, 826–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Will, C.L.; Lührmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 2011, 3, a003707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Black, D.L. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 2003, 72, 291–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lareau, L.F.; Green, R.E.; Bhatnagar, R.S.; Brenner, S.E. The evolving roles of alternative splicing. Curr. Opin. Struct. Biol. 2004, 14, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Kelemen, O.; Convertini, P.; Zhang, Z.; Wen, Y.; Shen, M.; Falaleeva, M.; Stamm, S. Function of alternative splicing. Gene 2013, 514, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Syed, N.H.; Kalyna, M.; Marquez, Y.; Barta, A.; Brown, J.W.S. Alternative splicing in plants–coming of age. Trends Plant Sci. 2012, 17, 616–623. [Google Scholar] [CrossRef] [Green Version]
- Walters, B.; Lum, G.; Sablok, G.; Min, X.J. Genome-wide landscape of alternative splicing events in Brachypodium distachyon. DNA Res. 2013, 20, 163–171. [Google Scholar] [CrossRef]
- Pan, Q.; Shai, O.; Lee, L.J.; Frey, B.J.; Blencowe, B.J. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat. Genet. 2008, 40, 1413–1415. [Google Scholar] [CrossRef]
- Wang, E.T.; Sandberg, R.; Luo, S.; Khrebtukova, I.; Zhang, L.; Mayr, C.; Kingsmore, S.F.; Schroth, G.P.; Burge, C.B. Alternative isoform regulation in human tissue transcriptomes. Nature 2008, 456, 470–476. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yuan, J.; Zhang, X.; Liu, C.; Xiang, J.; Li, F. Genome-wide analysis of alternative splicing provides insights into stress response of the Pacific white shrimp Litopenaeus vannamei. Front. Genet. 2019, 10, 845. [Google Scholar] [CrossRef] [Green Version]
- Graveley, B.R.; Brooks, A.N.; Carlson, J.W.; Duff, M.O.; Landolin, J.M.; Yang, L.; Artieri, C.G.; van Baren, M.J.; Boley, N.; Booth, B.W.; et al. The developmental transcriptome of Drosophila melanogaster. Nature 2011, 471, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Ramani, A.K.; Calarco, J.A.; Pan, Q.; Mavandadi, S.; Wang, Y.; Nelson, A.C.; Lee, L.J.; Morris, Q.; Blencowe, B.J.; Zhen, M.; et al. Genome-wide analysis of alternative splicing in Caenorhabditis elegans. Genome Res. 2011, 21, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.; Zhang, L.; Tang, X.; Zhang, G.; Li, L. Genome-wide analysis of alternative splicing provides insights into stress adaptation of the Pacific oyster. Mar. Biotechnol. 2016, 18, 598–609. [Google Scholar] [CrossRef]
- Martin Anduaga, A.; Evantal, N.; Patop, I.; Bartok, O.; Weiss, R.; Kadener, S. Thermosensitive alternative splicing senses and mediates temperature adaptation in Drosophila. eLife 2019, 8, e44642. [Google Scholar] [CrossRef]
- Liu, M.; Guo, X. A novel and stress adaptive alternative oxidase derived from alternative splicing of duplicated exon in oyster Crassostrea virginica. Sci. Rep. 2017, 7, 10785. [Google Scholar] [CrossRef]
- Suresh, S.; Crease, T.; Cristescu, M.; Chain, F. Alternative splicing is highly variable among Daphnia pulex lineages in response to acute copper exposure. BMC Genom. 2020, 21, 433. [Google Scholar] [CrossRef]
- Jakšić, A.M.; Schlötterer, C. The interplay of temperature and genotype on patterns of alternative splicing in Drosophila melanogaster. Genetics 2016, 204, 315–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.; Wang, W.; Tian, C.; Niu, D.; Zhou, T.; Jin, Y.; Yang, Y.; Gao, D.; Dunham, R.; Liu, Z. Heat stress induced alternative splicing in catfish as determined by transcriptome analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 29, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Nevo, Y.; Sperling, J.; Sperling, R. Heat shock activates splicing at latent alternative 5′ splice sites in nematodes. Nucleus 2015, 6, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yuan, J.; Zhang, X.; Yu, Y.; Li, F. Comparative transcriptomic analysis unveils a network of energy reallocation in Litopenaeus vannamei responsive to heat-stress. Ecotoxicol. Environ. Saf. 2022, 238, 113600. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Jia, G. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, J.; Sun, Y.; Li, S.; Gao, Y.; Yu, Y.; Liu, C.; Wang, Q.; Lv, X.; Zhang, X.; et al. Penaeid shrimp genome provides insights into benthic adaptation and frequent molting. Nat. Commun. 2019, 10, 356. [Google Scholar] [CrossRef] [Green Version]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Shen, S.; Park, J.W.; Lu, Z.-x.; Lin, L.; Henry, M.D.; Wu, Y.N.; Zhou, Q.; Xing, Y. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl. Acad. Sci. USA 2014, 111, E5593. [Google Scholar] [CrossRef]
- Grantham, M.E.; Brisson, J.A. Extensive differential splicing underlies phenotypically plastic aphid morphs. Mol. Biol. Evol. 2018, 35, 1934–1946. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Zheng, J.; Cui, Z. Alternative splicing derived invertebrate variable lymphocyte receptor displays diversity and specificity in immune system of crab Eriocheir sinensis. Front. Immunol. 2023, 13, 1105318. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, C.; Dai, X.; Zhang, R.; Cao, X.; Wang, K.; Huang, X.; Ren, Q. Two relish isoforms produced by alternative splicing participate in the regulation of antimicrobial peptides expression in Procambarus clarkii intestine. Fish Shellfish Immunol. 2020, 99, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhan, A. Highly dynamic transcriptional reprogramming and shorter isoform shifts under acute stresses during biological invasions. RNA Biol. 2020, 18, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Shalgi, R.; Hurt, J.A.; Lindquist, S.; Burge, C.B. Widespread inhibition of posttranscriptional splicing shapes the cellular transcriptome following heat shock. Cell Rep. 2014, 7, 1362–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.-D.; Ares, M. Context-dependent control of alternative splicing by RNA-binding proteins. Nat. Rev. Genet. 2014, 15, 689–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glisovic, T.; Bachorik, J.L.; Yong, J.; Dreyfuss, G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008, 582, 1977–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, S.K.N.; Suresh, S.; Munday, P.; Ravasi, T.; Bernal, M.A.; Schunter, C. The alternative splicing landscape of a coral reef fish during a marine heatwave. Ecol. Evol. 2022, 12, e8738. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Z.; Quan, J.; Li, L.; Zhao, G.; Lu, J. RNA-seq analysis reveals alternative splicing under heat stress in rainbow trout (Oncorhynchus mykiss). Mar. Biotechnol. 2022, 24, 5–17. [Google Scholar] [CrossRef]
- Staiger, D.; Brown, J.W.S. Alternative splicing at the intersection of biological timing, development, and stress responses. Plant Cell 2013, 25, 3640–3656. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Rio, D.C. Mechanisms and regulation of alternative pre-mRNA splicing. Annu. Rev. Biochem. 2015, 84, 291–323. [Google Scholar] [CrossRef] [Green Version]
- Bourgeois, C.F.; Lejeune, F.; Stévenin, J. Broad specificity of SR (Serine/Arginine) proteins in the regulation of alternative splicing of pre-messenger RNA. Prog. Nucleic Acid Res. Mol. Biol. 2004, 78, 37–88. [Google Scholar]
- Verta, J.-P.; Jacobs, A. The role of alternative splicing in adaptation and evolution. Trends Ecol. Evol. 2022, 37, 299–308. [Google Scholar] [CrossRef]
- Gehring, N.H.; Roignant, J.-Y. Anything but ordinary–emerging splicing mechanisms in eukaryotic gene regulation. Trends Genet. 2021, 37, 355–372. [Google Scholar] [CrossRef]
- Jacobs, A.; Elmer, K.R. Alternative splicing and gene expression play contrasting roles in the parallel phenotypic evolution of a salmonid fish. Mol. Ecol. 2021, 30, 4955–4969. [Google Scholar] [CrossRef]
- Healy, T.M.; Schulte, P.M. Patterns of alternative splicing in response to cold acclimation in fish. J. Exp. Biol. 2019, 222, jeb193516. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Huang, Z.; Shen, Y.; Gan, Y.; Wang, Y.; Gong, S.; Lu, Y.; Luo, X.; You, W.; Ke, C. Transcriptome analysis reveals the molecular mechanisms of heterosis on thermal resistance in hybrid abalone. BMC Genom. 2021, 22, 650. [Google Scholar] [CrossRef]
- Li, B.J.; Zhu, Z.X.; Qin, H.; Meng, Z.N.; Lin, H.R.; Xia, J.H. Genome-wide characterization of alternative splicing events and their responses to cold stress in Tilapia. Front. Genet. 2020, 11, 244. [Google Scholar] [CrossRef] [Green Version]





| Gene ID | Description | Function |
|---|---|---|
| LOC113823825 | Nuclear transcription factor Y subunit alpha-like | Transcription; post-transcriptional regulation; pre-mRNA splicing |
| LOC113823374 | Nuclear transcription factor Y subunit gamma-like | Transcription; post-transcriptional regulation; pre-mRNA splicing |
| LOC113819830 | RNA binding protein fox-1 homolog 1-like | Pre-mRNA splicing |
| LOC113825931 | RNA-binding protein 25-like | Pre-mRNA splicing |
| LOC113803561 | RNA-binding protein squid-like | Pre-mRNA splicing |
| LOC113803611 | SAFB-like transcription modulator | Transcription; pre-mRNA splicing; apoptosis |
| LOC113820949 | Serine/arginine-rich splicing factor 2 | Pre-mRNA splicing |
| LOC113816496 | Serine/arginine-rich splicing factor 3-like | Pre-mRNA splicing |
| LOC113802307 | Serine/arginine-rich splicing factor 7-like | Pre-mRNA splicing |
| LOC113820651 | Alternative splicing factor ASF/SF2 | Pre-mRNA splicing |
| LOC113811523 | HnRNP protein | Transcription; Post-transcriptional modification |
| LOC113813300 | Transcription initiation factor TFIID subunit 1 | Transcription |
| LOC113827782 | Transcriptional repressor p66-beta | Transcription |
| LOC113808730 | Upstream activation factor subunit spp27-like | Transcription |
| AS Type | DAS Number | ORF Maintained | % ORF Maintained |
|---|---|---|---|
| A3SS | 382 | 331 | 86.65% |
| A5SS | 275 | 191 | 69.45% |
| MXE | 295 | 222 | 75.25% |
| RI | 39 | 8 | 20.51% |
| ES | 1393 | 770 | 55.28% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, X.; Yuan, J.; Li, F. The Responses of Alternative Splicing during Heat Stress in the Pacific White Shrimp Litopenaeus vannamei. Genes 2023, 14, 1473. https://doi.org/10.3390/genes14071473
Zhang X, Zhang X, Yuan J, Li F. The Responses of Alternative Splicing during Heat Stress in the Pacific White Shrimp Litopenaeus vannamei. Genes. 2023; 14(7):1473. https://doi.org/10.3390/genes14071473
Chicago/Turabian StyleZhang, Xiaoxi, Xiaojun Zhang, Jianbo Yuan, and Fuhua Li. 2023. "The Responses of Alternative Splicing during Heat Stress in the Pacific White Shrimp Litopenaeus vannamei" Genes 14, no. 7: 1473. https://doi.org/10.3390/genes14071473
APA StyleZhang, X., Zhang, X., Yuan, J., & Li, F. (2023). The Responses of Alternative Splicing during Heat Stress in the Pacific White Shrimp Litopenaeus vannamei. Genes, 14(7), 1473. https://doi.org/10.3390/genes14071473





